Gender-Specific Effects of the Catechol- O -Methyltransferase Val 108/158 Met Polymorphism on Cognitive Function in Children
Abstract
Objective: The study aimed to determine the cognitive effect of the Val108/158Met polymorphism in the catechol- O -methyltransferase (COMT) gene in children before and during puberty. This polymorphism affects cognitive function in healthy adults and may contribute to risk for schizophrenia. Method: COMT genotype was determined for 8,707 children from the Avon Longitudinal Study of Parents and Children (ALSPAC), a geographically defined general population cohort of children born between April 1, 1991, and Dec. 31, 1992, in the southwest of England. Fourteen measures of cognitive function—including working memory, verbal and motor inhibition, attentional control, and IQ—were assessed at ages 8 and 10 years. Any pubertal development at age 9 years was reported by parents. Effects of COMT genotype on cognition and interactions with gender and puberty were assessed using general linear models. Results: In boys, genotype significantly affected executive function and explained up to 10 points normal variation in verbal IQ. The effects on IQ were significantly greater in pubertal than in prepubertal boys. In girls, there were no significant effects of genotype on cognition. Conclusions: This common polymorphism may be one of the genes of small effect that contribute to normal variation in IQ. The gender-specific nature of the effect and its possible interaction with puberty may be relevant to both normal cognitive and brain development and to abnormal development in disorders such as schizophrenia.
Genetic risk for complex neuropsychiatric disorders such as schizophrenia is probably conferred by multiple genes, each of which has a relatively small additive effect (1) . In healthy populations, the effects of these common genetic variants are largely unknown, but it is likely that they will prove important in explaining natural variation in biological and psychological processes and in vulnerability for psychiatric disorders.
Catechol- O -methyltransferase (COMT) catalyzes the first step in a major degradation pathway of the catecholamine neurotransmitters, including dopamine. The COMT gene on chromosome 22q11 contains a functional polymorphism ( Val108/158Met , rs4680) that affects the enzyme’s temperature sensitivity. Met alleles result in a fourfold decrease in enzyme activity at body temperature (2) leading to slower inactivation of released dopamine within the brain, notably in the prefrontal cortex where COMT is responsible for 60% of dopamine degradation (3) . As a result, COMT knockout mice show a threefold increase in concentrations of dopamine in the prefrontal cortex but not in other areas with strong dopaminergic connections, such as the striatum (4) .
Prefrontal dopaminergic dysfunction is a widely accepted feature of schizophrenia (5) . Many studies have now assessed the association between the Val158/108Met polymorphism and schizophrenia (6) . One recent meta-analysis found Val to be a small but reliable risk factor for schizophrenia in people of European ancestry (7) , although others have not supported this result (8) .
Regardless of any association with schizophrenia, the Val allele is common among the general population (9) in which it affects cognitive functions that rely on the prefrontal cortex. Healthy adults with the Val allele perform more poorly on cardinal tests of executive function such as the Wisconsin Card Sorting Test than those with Met alleles (6 , 10 , 11) . Some observational studies have failed to replicate the association (12 , 13) , but experimental studies showing that COMT inhibitors improve working memory in rats (14) and humans (15) offer potent support.
The cognitive effects of COMT genotype in children may be different from those in adults because children’s prefrontal cortices are not yet structurally mature, experiencing a gradual plateau and decrease in synaptic density throughout late childhood and adolescence (16) . The dorsolateral prefrontal cortex does not reach adult dimensions until the early 20s (17) . Age-related changes during adolescence involve reductions in gray matter (18) and increases in white matter volume, especially in the dorsolateral prefrontal cortex (19) .
Functional changes also occur in the frontal cortex during adolescence. While the majority of executive functions become operational at around age 8 (20) , many continue to develop until early adulthood (21) . Moreover, functional MRI studies show that children show activation in alternative neuronal circuits (22) or more widespread areas (23) of the prefrontal cortex when compared with adults performing the same tasks. If the frontal lobes continue to mature during adolescence, the cognitive functions performed by prefrontal cortex circuitry in adults may be performed by different or more diffuse circuits in prepubertal children. If the effect of COMT genotype is particularly important in the prefrontal cortex, then COMT genotype may have little effect on prepubertal cognitive performance.
Two studies exploring COMT genotype and cognition in healthy children have been published, with predominantly negative results. The first involved 39 subjects; children with Met alleles performed better on one executive function task (24) . The second study included an historical cohort of 460 children and found no difference in scores on a measure of general cognitive function at age 11, although an association much later in life was suggested (25) .
Large studies with specific neuropsychological measures are needed to determine the cognitive, or other, effects of candidate genes for psychiatric disorders in the general population. These have the potential to map genetic contributions to both normal and abnormal development and to detect what are likely to be small effects of individual variants. We assessed the effect of the COMT Val158/108Met polymorphism on cognitive function in a large birth cohort. We hypothesized that effects of the COMT genotype on cognition would be found in tasks that rely on frontal lobe function and that the magnitude of effect would be larger in children who were further through puberty.
Method
Cohort
The Avon Longitudinal Study of Parents and Children (ALSPAC) cohort consisted of more than 14,500 pregnant women in a geographically defined region in the southwest of England who were due to give birth between April 1, 1991, and Dec. 31, 1992. These pregnancies led to 14,062 live births and 13,971 infants still alive at 12 months (26) . From age 7, all children were invited annually for assessment; around 8,000 children have attended each time. Assessments included a wide range of physical, social, neuropsychological, and educational measures. Parents who enrolled their children into ALSPAC gave written informed consent at the time of enrollment, and they or the child are free to withdraw at any time.
Cognitive Assessments
We examined the effects of COMT genotype on measures of IQ and executive function. These tasks were completed at the age 8 assessment (IQ and attentional control tasks; mean subject age: 8 years, 8 months [SD=3.1 months]) and the age 10 assessment (working memory and inhibition tasks; mean subject age: 10 years, 8 months [SD=3.0 months]). We selected the following cognitive measures to test our hypotheses.
The WISC 3rd U.K. Edition (given at age 8 assessment)
Alternate items of the WISC were given for all tests except the coding subtest, which was given in its entirety. Age-scaled verbal, performance, and total IQ scores were calculated in accordance with standard procedures whenever four subscale scores were available. Forward and backward digit span and block design subtests were also considered individually because of their relevance to executive function.
The Test of Everyday Attention for Children (27) (given at age 8 assessment)
These tests of attentional control are designed to detect variation within the healthy population and in children with attentional problems. Three subtests were used: the Sky Search tasks of selective and divided attention and the Opposite Worlds verbal inhibition test. These produced four measures: a selective attention score reflecting the time taken per picture to select and circle identical pictures; a dual task decrement score reflecting the difference in time taken to do this task while simultaneously counting a number of noises; a “same worlds” trial time reflecting the time taken to read aloud a random string of the digits 1 and 2; and an “opposite worlds” trial time reflecting the time taken to make the opposite response to a similar string (i.e., saying “one” for the digit 2).
Counting Span Working Memory Task (28) (given at age 10 assessment)
Children were presented with a number of red and blue dots on a white screen and asked to count the red dots out loud. After each set of screens, the child was asked to recall the number of red dots seen on each screen within that set. After two practice sets, there were three sets each of two, three, four, and five screens. Children received a span score on the basis of the number of correctly recalled sets, with a maximum score of 5 in increments of 0.5. A global score was also calculated from the total number of trials correct, with a maximum of 42.
Stop Signal Motor Inhibition Task (29) (given at age 10 assessment)
This measures a child’s ability to inhibit a prerequested motor response. During primary trials, the child sees an O or X appear on the screen and must press the corresponding button. After 30 primary trials, a mean response time for that child is calculated. During subsequent stop signal trials, a bleep is heard randomly on 16 out of 48 trials after the visual stimulus appears, and the subject is told to not press the button if the bleep is heard. The bleep is either 150 msec (difficult condition) or 250 msec (easy condition) before the mean response time calculated from primary trials. Main outcome variables from this task are the relative finishing times of the inhibitory and primary task process for the two conditions, which reflect the probability of inhibiting the response (30) .
Assessment of Puberty
Puberty was assessed by questionnaires when the subjects were a mean age of 9 years, 8 months (SD=1.6 months), halfway between the two cognitive assessments. Pictures of the development of pubic hair according to Tanner’s five stages of sexual development (31) were shown, with descriptions of the differences between stages. Parents were asked to indicate which stage matched their child’s development. Since few children were showing signs of puberty at this young age, children were coded simply as “prepubertal” (Tanner stage 1) or “pubertal” (Tanner stage 2 or above).
Genotyping
DNA, obtained from blood and mouthwash samples, was extracted and processed as described previously (32) . COMT Val108/158Met genotyping was generated using a 5′-nuclease fluorescence assay. Primer/probe combinations were designed using Primer Express software (2.0) (ABI, Foster City, Calif.). DNA sequences were obtained from GenBank and the Celera Discovery System. Allele discrimination was performed using a Taqman 7900 machine. Four genotyping signal clusters were identified by SDS software version 1.7 (ABI, Foster City, Calif.). These clusters represent Val/Val homozygotes, Met/Met homozygotes, Val/Met heterozygotes, and no-DNA template controls. Two standards for each genotype ( Val/Val , Met/Met , and Val/Met ) were confirmed by sequencing six genomic DNA samples using a 377 sequencer (ABI, Foster City, Calif.).
Analysis
To allow parametric analysis, the following variables were transformed: selective attention score, divided attention (dual task decrement) (log 10 transforms), Same Worlds trial time, and Opposite Worlds trial time (1/square root of transforms). All other variables were normally distributed according to one-sample Kolmogorov-Smirnov test.
Deviation from Hardy-Weinberg equilibrium was assessed using a chi-square test. The effects of genotype on cognition were assessed in three steps. First, cognitive performance was compared between genotypes ( Val/Val , Val/Met , and Met/Met ) using a one-way ANOVA. Main effects of genotype and gender and a genotype-by-gender interaction were then assessed in a general linear model. Finally, main effects of genotype and puberty and a genotype-by-puberty interaction were assessed with separate models for each gender.
Each stage of analysis was completed on all available data. For example, children with no puberty data were excluded from puberty models but included in all other models.
Results
Data Availability
Val108/158Met genotype was available for 8,707 children from the cohort ( Val / Val =2,126; Val / Met =4,294; Met/Met =2,287). The overall frequency of the Met allele was 50.9%. This distribution was consistent with Hardy-Weinberg equilibrium (χ 2 =1.55, df=1, p>0.10).
Pubertal report was available for 4,821 of these children. The majority of children of both genders were in Tanner stage 1 (boys=83.4%; girls=81.5%), with a few girls (3.2%) and almost no boys (<0.01%) in stages 3 or above.
Biases were present in the availability of genotype data. Children for whom parental permission was granted to undertake genotyping scored more highly than the remainder on verbal IQ (t=–4.22, df=6991, p<0.001), performance IQ (t=–2.12, df=6514, p<0.05), and total IQ (t=–3.50, df=6454, p<0.001). They did not differ on any of the measures of executive function. There were no differences in cognitive performance between children who were successfully genotyped and those where genotyping failed.
There was no effect of gender on Val158/108Met genotype (χ 2 =0.27, df=2, p=0.87). There were no associations between pubertal development and COMT genotype in girls (χ 2 =0.91, df=2, p=0.63) or boys (χ 2 =1.83, df=2, p=0.40).
Ethnicity was known for 8,602 children. Only 401 children (4.7%) were described as having a nonwhite ethnic background, which we refer to as black and minority ethnic. Met alleles were underrepresented among the black and minority ethnic children (42.0%) relative to the reference group (51.4%) (χ 2 =27.0, df=1, p<0.001). Pubertal data availability was also lower for the black and minority ethnic children (girls χ 2 =28.1, df=1, p<0.001; boys χ 2 =32.4, df=1, p<0.001). Because of these biases, and to increase the ethnic homogeneity of the cohort, we excluded the black and minority ethnic children from all subsequent analyses.
Comparison of COMT Genotypes
One-way ANOVA was used to compare mean cognitive scores between genotypes across the cohort. Three cognitive measures showed an effect of genotype. Working memory count span was significantly associated with increasing Met alleles (F=5.166, df=2, 5046, p<0.05), and the same effect was seen in the global score (F=3.371, df=2, 5046, p≤0.05). In addition, verbal IQ showed an improvement of 0.8 IQ points for each Met allele (F=3.452, df=2, 5342, p<0.05). Post hoc comparisons of performance on each WISC subtest by genotype revealed no significant differences on any individual test.
COMT and Gender
The second analyses included gender, genotype, and gender-by-genotype interaction terms. There was a large effect of gender on almost every measure except total IQ scores, such that girls outperformed boys on all measures except block design and verbal IQ.
As seen in Figure 1 , there was a significant gender-by-genotype interaction for selective attention, and gender-by-genotype interactions approached significance for divided attention and verbal IQ (the gender-by-genotype interaction also approached significance for total IQ [F=2.66, df=2, 4947, p<0.10]). The IQ effects were such that boys showed a larger effect of genotype on cognition, however in selective and divided attention the interaction was nonlinear; heterozygotes appeared to be at an advantage in boys but a disadvantage in girls ( Figure 1 ).
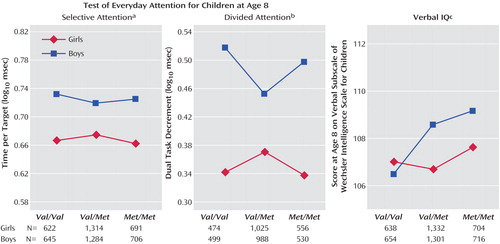
a Significant gender effect (F=249.9, df=1, 5256, p<0.001) and gender-by-genotype interaction (F=3.80, df=2, 5256, p<0.05).
b Main effect of gender (F=17.6, df=1, 4066, p<0.001); gender-by-genotype interaction (F=2.32, df=2, 4066, p<0.10).
c Significant main effects of gender (F=4.19, df=1, 5339, p<0.05), and genotype (F=3.42, df=2, 5339, p<0.05); gender-by-genotype interaction (F=2.40, df=2, 5539, p<0.10).
Significant main effects of genotype were found for working memory count span (F=5.24, df=2, 5043, p<0.01) and global score (F=3.44, df=2, 5043, p<0.05). There was also a main effect of genotype on verbal IQ (F=3.42, df=2, 5339, p<0.05) and a tendency toward an effect of genotype on the Opposite Worlds task (F=2.42, df=2, 5276, p<0.10). Inspection of the data showed that the effect on IQ was not caused by outliers; rather, in boys there appeared to be a population shift toward higher verbal IQ with increasing Met allele dose.
COMT and Puberty
The final stage of analyses assessed main effects of genotype and pubertal stage, and a genotype-by-puberty interaction term, separately for girls and boys.
In girls, models comprising these terms did not fit the data adequately, implying no significant effects on cognition of either genotype or pubertal stage. The only exception to this was selective attention, where puberty showed some effect on performance (F=3.80, df=1, 2050, p=0.05).
In boys, there were significant modeled effects in six of the cognitive measures. There was a main effect of genotype on verbal IQ (F=9.97, df=2, 1763, p<0.001) and total IQ (F=4.28, df=2, 1639, p<0.05), working memory count span (F=3.09, df=2, 1666, p<0.05) and global score (F=3.86, df=2, 1666, p<0.05) and the Opposite Worlds verbal inhibition task (F=3.02, df=2, 1734, p<0.05; see Figure 2 ). In addition, there was a nearly significant effect of genotype on selective attention (F=2.91, df=2,1735, p=0.05). In all cases effects were in the predicted direction, with improved performance in children with one or more Met alleles relative to those with two Val alleles. For verbal IQ, there was a genotype-by puberty interaction effect ( Figure 3 and Figure 4 ), with a larger effect of genotype in pubertal children when compared with prepubertal children (F=2.97, df=2, 1763, p=0.05). No main effects of puberty were found.

a SD=0.90 ( Val/Val ), 0.89 ( Val/Met ), and 0.87 ( Met/Met ). Significant main effect of COMT genotype (F=3.09, df=2, 1666, p<0.05).
b SD=7.8 ( Val/Val ), 8.1 ( Val/Met ), and 8.1 ( Met/Met ). Significant main effect of COMT genotype (F=3.86, df=2, 1666, p<0.05).
c SD=0.024 ( Val/Val ), 0.024 ( Val/Met ), and 0.026 ( Met/Met ). Significant main effect of COMT genotype (F=3.02, df=2, 1734, p<0.05).
d SD=0.14 ( Val/Val ), 0.13 ( Val/Met ), and 0.12 ( Met/Met ). Significant main effect of COMT genotype (F=2.92, df=2, 1735, p=0.05).

a Significant main effect of genotype (F=9.97, df=2, 1763, p<0.001) and genotype-by-puberty interaction (F=2.97, df=2, 1763, p=0.05).
b Significant main effect of genotype (F=4.28, df=2, 1639, p<0.05).
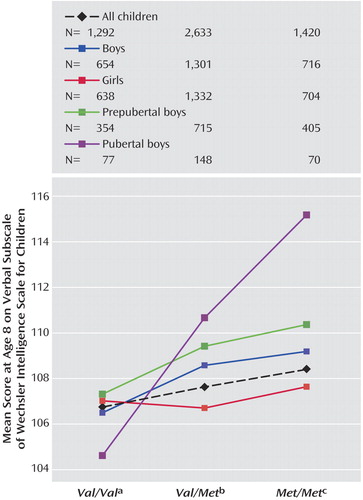
a SD=16.24 (all children), 17.10 (boys), 15.33 (girls), 17.32 (prepubertal boys), and 18.61 (pubertal boys).
b SD=16.50 (all children), 17.14 (boys), 15.82 (girls), 17.00 (prepubertal boys), and 16.84 (pubertal boys).
c SD=16.60 (all children), 17.29 (boys), 15.84 (girls), 16.79 (prepubertal boys), and 17.42 (pubertal boys).
Discussion
This large study of the effect of COMT genotype on children’s cognition found little effect of genotype on girls but widespread effects in boys on measures of executive function and IQ, especially when pubertal development was considered.
The gender distinction has not previously been reported, but few previous studies have been large enough to detect such effects. However, there is evidence that COMT’s effects may be gender-specific. Dopamine levels in the frontal cortex of COMT knockout mice are affected only in male mice, and while male knockout mice show increased aggression, females show decreased emotional reactivity (4) . In humans, COMT activity is lower in female subjects than in male subjects in dorsolateral prefrontal cortex tissue, and in lymphocytes, genotype affects enzyme activity in male subjects more than in female subjects (33) .
It is not yet known whether human COMT expression differs in the brains of prepubescent boys and girls, but the cortical organizational changes that occur during adolescence do differ between the genders, with boys showing apparently greater age-related synaptic pruning and myelination than girls (34) . These changes may conceivably be triggered by differences in the nature or level of gene expression. There are also significant gender differences in the incidence of neuropsychiatric disorders associated with COMT genotype such as schizophrenia and ADHD, both of which are more common in male subjects.
The peak onset of schizophrenia in male subjects is around late adolescence or early adulthood (35) , with cases before puberty being extremely rare. The cognitive deficit present in children before illness onset also worsens around the onset of puberty (36) . Therefore, the biology of schizophrenia is probably related to those of puberty and brain development, and genes that interact with these processes may be crucial.
Puberty was measured here by parent rating of the child’s development according to Tanner stage. This approach was pragmatic given the large sample but remains imperfect when compared with expert examination of the children. However, these results appear to be within the range of normal development reported in samples from the United States (37) and Italy (38) .
The effect of genotype on cognition was increased in boys when pubertal stage was included in the model. One possibility is that boys at the same developmental stage are more homogenous, so subtle effects can be detected that tend to be masked when prepubertal and pubertal children are considered together. Our hypothesis suggested that puberty would interact with the gene-cognition relationship such that genotype would have a stronger effect post-puberty. Although the children were too young for us to investigate the effects of COMT post-puberty, we assessed its effects in children prior to, versus already in, puberty. We suggest that the increase in effect size in the puberty models, and the fact that with the exception of IQ and working memory the effects cannot be detected unless pubertal stage is included, suggests neurobiological development is an important factor.
The possible interaction between gene and puberty in verbal IQ supports our hypothesis regarding the increasing effect of COMT on cognition as the frontal lobes come online. We did not find a clear interaction between gene and puberty in the majority of tests studied. This may be due to the numbers involved: the effect of COMT genotype on verbal IQ was the most robust result in the group as a whole; it may be that weaker genotype-by-puberty interactions were present but not detectable given the small numbers of children in the pubertal group.
Modeled COMT effects explained around 1% of the variance in childhood cognitive performance compared with around 4% in adult studies (6 , 10) . This may reflect increased specialization in the adult brain, or developmental differences in the importance of COMT versus other enzymes in prefrontal cortex, such as the monoamine oxidases. On the other hand, the size of the present study was sufficient to detect small effects that may be missed by smaller studies with limited power. The ALSPAC cohort represents the largest study to date of the cognitive effects of COMT in patients, healthy adults, or children. Even with this large group size, effects would not have been considered significant if strict corrections for multiple comparisons (e.g., Bonferroni corrections) were adopted. We did not follow this approach because of the hypothesis-driven nature of our strategy, the restricted phenotype that we considered, the intercorrelation between cognitive outcomes, and the increased probability of type II errors if such corrections had been applied. We report all the statistical tests that we undertook, and we were reassured that all main effects were in the hypothesized direction, suggesting that type I errors did not account for the results. Nevertheless, independent replication will be essential, as it is for all genetic studies.
Demonstrating an effect of COMT on IQ scores is important for several reasons. Performances on diverse tests of cognitive function tend to correlate; this underlying covariance represents general cognitive ability, or ‘ g ’. Neuroimaging studies have demonstrated that in adults, ‘high- g ’ tasks do not show diffuse recruitment from multiple brain systems but instead are associated with relatively selective recruitment of the lateral frontal cortex (39) . It is therefore logical that COMT would affect general intelligence as well as executive tasks.
Intelligence, like risk for neuropsychiatric disorders, doubtless involves multiple genes of small effect. Research has consistently found g to be strongly heritable (40) , but population-based studies have generally failed to establish genetic effects on cognitive development in children (41 , 42) . COMT may be the first gene that shows a plausible biological mechanism and has a relatively large effect across the normal range of IQ in children.
In schizophrenia, cognitive impairments present before the onset of psychosis reflect ongoing, aberrant neurodevelopmental processes that may worsen around the onset of puberty (36) . The increased prevalence of the Val allele in patients with schizophrenia (7) may explain a proportion of the IQ decrement in children who will later develop schizophrenia. This is estimated to be around 5–10 points and is more pronounced in male subjects (43) ; here we found a difference of 2–10 points in males attributable to COMT genotype.
It is possible that the effects attributed to the Val158/108Met polymorphism are in fact due to some other factor. There is now substantial evidence that haplotypes spanning the COMT gene, including other single nucleotide polymorphisms (SNPs) as well as Val158/108Met , are stronger predictors of risk for schizophrenia than that polymorphism alone (44 , 45) . These may reflect the importance of other sites that are in strong linkage disequilibrium with the Val158/108Met SNP, or regulatory factors such as promoter regions (45) .
The true complexity of COMT expression and function remains obscure but for the moment, we assume that Val158/108Met genotype does affect catecholamine degradation in human prefrontal cortex. This is probably reasonable: Chen et al. (33) studied the effects of Val158/108Met and three other SNPs on mRNA levels, protein levels, and enzyme activity in human prefrontal cortex tissue and in lymphocytes and concluded that Val is the predominant factor determining COMT activity in the prefrontal cortex. While other polymorphisms may still prove important, Val158/108Met is the most well-characterized polymorphism, with a plausible biological mechanism. Moreover, the relatively restricted cognitive phenotype that we have demonstrated to be associated with this SNP has a strong relevance for the cognitive deficits prominent in schizophrenia.
1. Owen MJ, Williams NM, O’Donovan MC: The molecular genetics of schizophrenia: new findings promise new insights. Mol Psychiatry 2004; 9:14–27Google Scholar
2. Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM: Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 1996; 6:243–250Google Scholar
3. Karoum F, Chrapusta SJ, Egan MF: 3-methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem 1994; 63:972–979Google Scholar
4. Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M: Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A 1998; 95:9991–9996Google Scholar
5. Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE: Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 2001; 50:825–844Google Scholar
6. Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR: Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A 2001; 98:6917–6922Google Scholar
7. Glatt SJ, Faraone SV, Tsuang MT: Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry 2003; 160:469–476Google Scholar
8. Munafo MR, Bowes L, Clark TG, Flint J: Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Mol Psychiatry 2005; 10:765–770Google Scholar
9. Palmatier MA, Kang AM, Kidd KK: Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry 1999; 46:557–567Google Scholar
10. Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D: A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry 2002; 159:652–654Google Scholar
11. Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martinez-Larrea A, Fananas L: New evidence of association between COMT gene and prefrontal neurocognitive function in healthy individuals from sibling pairs discordant for psychosis. Am J Psychiatry 2004; 161:1110–1112Google Scholar
12. Tsai SJ, Yu YW, Chen TJ, Chen JY, Liou YJ, Chen MC, Hong CJ: Association study of a functional catechol-O-methyltransferase-gene polymorphism and cognitive function in healthy females. Neurosci Lett 2003; 338:123–126Google Scholar
13. Stefanis NC, Van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, Stefanis CN: Variation in catechol-o-methyltransferase Val158Met genotype associated with schizotypy but not cognition: a population study in 543 young men. Biol Psychiatry 2004; 56:510–515Google Scholar
14. Liljequist R, Haapalinna A, Ahlander M, Li YH, Mannisto PT: Catechol O-methyltransferase inhibitor tolcapone has minor influence on performance in experimental memory models in rats. Behav Brain Res 1997; 82:195–202Google Scholar
15. Gasparini M, Fabrizio E, Bonifati V, Meco G: Cognitive improvement during tolcapone treatment in Parkinson’s disease. J Neural Transm 1997; 104:887–894Google Scholar
16. Bourgeois JP, Goldman-Rakic PS, Rakic P: Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex 1994; 4:78–96Google Scholar
17. Giedd JN: Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci 2004; 1021:77–85Google Scholar
18. Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW: In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci 1999; 2:859–861Google Scholar
19. Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB: Brain development, gender and IQ in children: a volumetric imaging study. Brain 1996; 119:1763–1774Google Scholar
20. Luciana M, Nelson CA: The functional emergence of prefrontally-guided working memory systems in four- to eight-year-old children. Neuropsychologia 1998; 36:273–293Google Scholar
21. De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C: Normative data from the CANTAB, I: development of executive function over the lifespan. J Clin Exp Neuropsychol 2003; 25:242–254Google Scholar
22. Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET: Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev 2000; 24:13–19Google Scholar
23. Casey BJ, Giedd JN, Thomas KM: Structural and functional brain development and its relation to cognitive development. Biol Psychol 2000; 54:241–257Google Scholar
24. Diamond A, Briand L, Fossella J, Gehlbach L: Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry 2004; 161:125–132Google Scholar
25. Harris SE, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ: The functional COMT polymorphism, Val158Met, is associated with logical memory and the personality trait intellect/imagination in a cohort of healthy 79 year olds. Neurosci Lett 2005; 385:1–6Google Scholar
26. Golding J, Pembrey M, Jones R: ALSPAC: the Avon Longitudinal Study of Parents and Children, I: study methodology. Paediatr Perinat Epidemiol 2001; 15:74–87Google Scholar
27. Manly T, Anderson V, Nimmo-Smith I, Turner A, Watson P, Robertson IH: The differential assessment of children’s attention: the Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. J Child Psychol Psychiatry 2001; 42:1065–1081Google Scholar
28. Case R, Kurland DM, Goldberg J: Operational efficiency and the growth of short-term-memory span. J Experimental Child Psychol 1982; 33:386–404Google Scholar
29. Logan GD, Cowan WB, Davis KA: On the ability to inhibit simple and choice reaction-time responses - a model and a method. J Experimental Psychol-Human Perception and Performance 1984; 10:276–291Google Scholar
30. Logan GD, Cowan WB: On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev 1984; 91:295–327Google Scholar
31. Tanner JM: Growth of Adolescents. Oxford, England, Blackwell Scientific Publications, 1962Google Scholar
32. Jones RW, Ring S, Tyfield L, Hamvas R, Simmons H, Pembrey M, Golding J, Team AS: A new human genetic resource: a DNA bank established as part of the Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC). Eur J Hum Genet 2000; 8:653–660Google Scholar
33. Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR: Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 2004; 75:807–821Google Scholar
34. De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM: Sex differences in brain maturation during childhood and adolescence. Cereb Cortex 2001; 11:552–557Google Scholar
35. Hafner H, Maurer K, Loffler W, Riecher-Rossler A: The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry 1993; 162:80–86Google Scholar
36. Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC: Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry 2002; 159:1183–1189Google Scholar
37. Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, Himes JH, Ryan AS: National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics 2002; 110:911–919Google Scholar
38. Danubio ME, De Simone M, Vecchi F, Amicone E, Altobelli E, Gruppioni G: Age at menarche and age of onset of pubertal characteristics in 6–14-year-old girls from the province of L’Aquila (Abruzzo, Italy). Am J Hum Biol 2004; 16:470–478Google Scholar
39. Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, Newell FN, Emslie H: A neural basis for general intelligence. Science 2000; 289:457–460Google Scholar
40. Plomin R: Genetics and general cognitive ability. Nature 1999; 402(6761 Suppl):C25–C29Google Scholar
41. Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Carothers A, Whalley LJ: Cognitive change and the APOE epsilon 4 allele. Nature 2002; 418:932Google Scholar
42. Deary IJ, Harris SE, Fox HC, Hayward C, Wright AF, Starr JM, Whalley LJ: KLOTHO genotype and cognitive ability in childhood and old age in the same individuals. Neurosci Lett 2005; 378:22–27Google Scholar
43. Aylward E, Walker E, Bettes B: Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull 1984; 10:430–459Google Scholar
44. Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, Schiffer R, Kotler M, Strous RD, Swartz-Vanetik M, Knobler HY, Shinar E, Beckmann JS, Yakir B, Risch N, Zak NB, Darvasi A: A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet 2002; 71:1296–1302Google Scholar
45. Palmatier MA, Pakstis AJ, Speed W, Paschou P, Goldman D, Odunsi A, Okonofua F, Kajuna S, Karoma N, Kungulilo S, Grigorenko E, Zhukova OV, Bonne-Tamir B, Lu RB, Parnas J, Kidd JR, DeMille MM, Kidd KK: COMT haplotypes suggest P2 promoter region relevance for schizophrenia. Mol Psychiatry 2004; 9:859–870Google Scholar



