Lumbar Bone Mineral Density in Patients With Major Depression: Evidence of Increased Bone Loss at Follow-Up
Abstract
OBJECTIVE: This study investigated whether an increase in the loss of bone mineral density occurs with major depression. METHOD: The authors measured lumbar bone density with the use of quantitative computerized tomography in a longitudinal assessment of 18 depressed patients older than 40 years on hospital admission and 21 healthy women and men. The follow-up period lasted at least 24 months. RESULTS: A repeated measures analysis of variance model with depression and gender as factors showed that bone loss was greater for the depressed patients than for the comparison subjects and greater for men than for women. CONCLUSIONS: Major depression may be associated with increased bone loss, especially for men.
Several studies have described low bone mineral density in individuals with different psychiatric disorders.
1. The first (1) looked at 80 women and men with major depression and 57 comparison subjects by using quantitative computerized tomography (CT) of the lumbar spine (a method that selectively assesses the density of trabecular bone).
2. Another (2) studied 24 women with major depression and 24 comparison subjects by using dual-energy X-ray absorptiometry (a method that assesses both cortical and trabecular bone) of the lumbar spine, the femoral neck, Ward’s triangle, and the trochanter.
3. Another (3) compared a mixed group of 68 male and female inpatients with major depressive disorder, schizophrenia, schizoaffective disorder, mania, or adjustment disorder compared to reference data by using dual-energy X-ray absorptiometry of the lumbar spine and the femoral neck.
With the exception of age, these studies found no other clinical parameter that correlated with low bone density in depression (gender, duration of illness, number of episodes, smoking, or duration of treatment with antidepressants). Lumbar bone density and plasma testosterone concentrations correlated negatively in men with psychiatric disorders (3). Indicators of bone turnover, such as osteocalcin and the ratio of urinary deoxypyridinoline cross-links to creatinine, were low in women with major depression (2).
The identification of depression as a risk factor for osteoporosis has important public health implications. Major depression has a high lifetime prevalence, approximately 5%–10% of the population. Moreover, an age-corrected decrease in bone density of about 10%–15% may result in a relevant increase of morbidity and mortality due to fractures in this population.
Furthermore, bone density in depression is of high theoretical interest. Major depression is associated with a characteristic neuroendocrine syndrome, particularly with an activation of the hypothalamic-pituitary-adrenal system. However, there is very limited information about the antecedents and the time course of this syndrome. Loss of bone density may constitute an endpoint of the cumulative intensity of endocrine alterations during depression.
Until now, only cross-sectional data on bone mineral density in depression were available. Low bone density in women and men may be due to a subnormal apposition of bone during childhood and young adulthood, due to abnormal bone loss, or due to both. Therefore, a longitudinal follow-up design seemed appropriate in order to examine the hypothesis of abnormal bone loss in major depression.
METHOD
Women and men older than 40 years who had participated in an earlier cross-sectional study of bone density in depression (1) were approached for a follow-up study. The patients had been consecutively admitted to our hospital and diagnosed with DSM-III-R major depression through the use of standardized diagnostic checklists. The patients were included in the follow-up assessment if a minimum of 24 months had passed since their initial bone density measurements and if they had not used any medication for the prevention of bone loss (e.g., estrogens or fluorides) during this time. Psychiatric treatment during this interval was on an outpatient basis and was not standardized. All patients were treated with antidepressants for various amounts of time. The study protocol had been approved by the local ethics committee. After a complete description of the study to the subjects, written informed consent was obtained.
Similarly, healthy individuals who had participated in the initial study were included after a minimum of 24 months since their initial bone density measurements if they had not used any medication for the prevention of bone loss in the preceding interval.
Bone density of the first to third lumbar vertebrae was measured by single-energy quantitative CT by using a General Electric CT 9800 scanner at 80 kV and 70 mA. A mineral calibration phantom was compared with an ellipsoid region of interest in the center of the vertebral bodies. Bone loss per year was calculated as the difference between measurement 1 and measurement 2 divided by the interval between measurements.
Data were analyzed by using analyses of variance (ANOVAs) with repeated measurements and analyses of covariance (ANCOVAs); p values below 0.05 were considered significant.
RESULTS
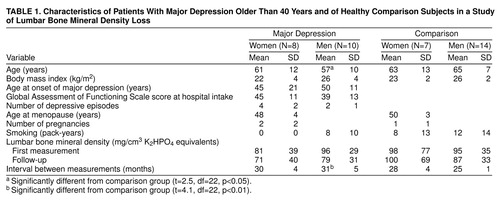
table 1 summarizes the demographic and clinical data and the mean lumbar spine bone mineral densities at intake and follow-up in the depressed women and men and healthy comparison subjects. Bone loss per year tended to be higher in the men with depression (mean=6.9 mg/cm, SD=5.5) and women with depression (mean=3.8 mg/cm, SD=4.3) than in the comparison men (mean=3.9 mg/cm, SD=4.2) and women (mean=–0.9 mg/cm, SD=8.2). Bone loss per year was not correlated with age (r=0.13, N=40) or initial bone density (r=0.27, N=40).
A two-way ANOVA model for the effects of depression and gender on bone loss per year yielded significant main effects for bone loss but no interaction effects. For gender (male/female) the sum of squares was 144.63 (F=4.85, df=1, 35, p=0.03); for depression (yes/no) the sum of squares was 130.86 (F=4.39, df=1, 35, p=0.04); and for the gender-by-depression interaction the sum of squares was 7.26 (F=0.24, df=1, 35, p=0.62). The sum of squares for the error term was 1,043.40 (df=35). Post hoc testing yielded trends for the effects of gender (p=0.05, Fisher’s protected least significant difference test [PLSD]) and depression (p=0.08, Fisher’s PLSD).
Bone density at follow-up was significantly lower in the depressed group after adjustment for the effects of initial bone density, age, and gender in an ANCOVA model. For initial bone density the sum of squares was 21,660.86 (F=192.03, df=1, 33, p<0.01); for age at follow-up (years) the sum of squares was 616.99 (F=5.47, df=1, 33, p=0.03); for gender (male/female) the sum of squares was 591.08 (F=5.24, df=1, 33, p=0.03); and for depression (yes/no) the sum of squares was 1,602.63 (F=14.21, df=1, 33, p<0.01). The sum of squares for the error term was 3,722.25 (df=33). None of the higher interactions in this model was significant. Post hoc testing yielded a significant effect of depression (p<0.001, Fisher’s PLSD) but not of gender.
DISCUSSION
In summary, patients with major depression suffer from low bone density and increased bone loss after correction for age, gender, and initial bone density when assessed after a follow-up period of approximately 2 years. Surprisingly, men showed a greater bone density loss than women.
The study has major limitations. With respect to the small group size and the inclusion of hospitalized patients only, the rate of bone loss detected in our patients cannot be considered representative of all patients with major depression at present. The small group size limited statistical power and therefore did not allow for testing of the effects of treatment modality and outcome.
The finding of greater bone loss in the women and men with major depression is in accordance with the hypothesis that the neuroendocrine alterations of depression have an effect on bone metabolism. Similar changes have also been described for other tissues, such as visceral adipose tissue (4), that are sensitive to the effects of altered adrenal, gonadal, thyroid, or other hormonal secretions. These alterations of body composition may constitute an important link in explaining the increased mortality from all causes that is observed in women and particularly in men with major depression (5).
Received Feb. 17, 1998; revisions received Jan. 4 and June 29, 1999; accepted July 29, 1999. From the Max Planck Institute of Psychiatry, Munich. Address reprint requests to Dr. Schweiger, Klinik für Psychiatrie, Medizinische Universität zu Lübeck, Ratzeburger Allee 160, D-23538 Lübeck, Germany; [email protected] (e-mail)
 |
1. Schweiger U, Deuschle M, Körner A, Lammers CH, Schmider J, Gotthardt U, Holsboer F, Heuser I: Low lumbar bone mineral density in patients with major depression. Am J Psychiatry 1994; 151:1691–1693Google Scholar
2. Michelson D, Stratakis C, Hill L, Reynolds J, Galliven E, Chrousos G, Gold P: Bone mineral density in women with depression. N Engl J Med 1996; 335:1176–1181Google Scholar
3. Halbreich U, Rojansky N, Palter S, Hreshchyshyn M, Kreeger J, Bakhai Y, Rosan R: Decreased bone mineral density in medicated psychiatric patients. Psychosom Med 1995; 57:485–491Crossref, Medline, Google Scholar
4. Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG: Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry 1997; 41:1140–1142Google Scholar
5. Zheng D, Macera CA, Croft JB, Giles WH, Davis D, Scott WK: Major depression and all-cause mortality among white adults in the United States. Ann Epidemiol 1997; 7:213–218Crossref, Medline, Google Scholar