Symptom Correlates of Prepulse Inhibition Deficits in Male Schizophrenic Patients
Abstract
OBJECTIVE: Information processing, inhibitory, and gating deficits in human and animal model studies of schizophrenia are demonstrated by using prepulse inhibition of the startle reflex. Prepulse inhibition deficits in schizophrenic patients correlate with core cognitive symptoms, such as thought disorder and distractibility, but their relationship to positive and negative symptoms of schizophrenia is less clear. METHOD: Fifty-one male schizophrenic patients and 26 male normal comparison subjects were tested for prepulse inhibition of the eyeblink component of the startle reflex measured by electromyogram recording. Startling stimuli (118 dB) were presented alone (pulse only) or were preceded 60 msec by discrete prepulse stimuli of 2, 4, 8, or 16 dB above the background 70-dB noise level. In addition, patients were assessed for demographic variables, generalized symptoms (Brief Psychiatric Rating Scale), and positive and negative symptoms. RESULTS: Schizophrenic and comparison groups differed significantly in the amount of prepulse inhibition produced by the 16-dB prepulse, with schizophrenic patients showing the expected deficient prepulse inhibition. Latency of the eyeblink response was generally slower for the schizophrenic patients, but the prepulse-induced latency facilitation for schizophrenic patients and comparison subjects did not differ significantly. The pattern of prepulse inhibition deficits in schizophrenic patients remained, with age and education controlled, in an analysis of covariance and subgroup matching. Deficient prepulse inhibition correlated with both positive and negative symptoms of schizophrenia. CONCLUSIONS: Under these experimental conditions, schizophrenia-linked deficits in prepulse inhibition detected with a relatively strong prepulse are correlated with both positive and negative symptoms of schizophrenia. The level of correlation, while significant in this cohort, is not as robust as that in previous reports linking prepulse inhibition deficits with other measures, such as thought disorder. Future work should probably focus on the relationship of prepulse inhibition deficits to measures such as thought disorder rather than positive and negative symptoms.
Information processing disturbances are viewed as central elements of the cognitive deficits that characterize schizophrenia (1–3). A major feature of information processing deficits in schizophrenic patients is deficient sensorimotor gating. Theoretically, gating allows for the screening or filtering of trivial or unimportant stimuli and the corresponding apportionment of attentional resources to salient environmental cues (4). Under most conditions, normal individuals can navigate efficiently in the stimulus-laden world because of their ability to automatically “gate out” much redundant, unessential information. Gating deficits may cause schizophrenic patients to become overloaded with excessive exteroceptive and, perhaps, enteroceptive (self-generated) stimuli, leading to the collapse of normal cognitive integrity with subsequent cognitive fragmentation (4, 5).
All mammals respond to sudden, intense stimuli in most sensory modalities with a startle reflex that consists of a series of flexion and extension responses mediated by a simple, pontine-based neural circuit (6). In humans, the startle response to a sudden (rapid-onset) strong sensory stimulus is typically assessed by measuring the electromyographic (EMG) response of the orbicularis oculi muscles surrounding the eye (7). If a “weak” prepulse (e.g., 5–15 dB over a 70-dB background) precedes the startling stimulus by about 100 msec, startle reflex magnitude is diminished; this process, called “prepulse inhibition,” is regulated by forebrain cortico-striato-pallido-pontine neural circuitry (8). On the basis of an initial report (9), there have been numerous reports of prepulse inhibition deficits in schizophrenic patients (10–12), schizotypal patients (13), and individuals who may be at risk for schizophrenia spectrum disorders (14, 15). The finding of prepulse inhibition deficits in individuals with schizophrenia spectrum disorders is consistent with the idea that these subjects have a quantifiable loss of brain-based inhibitory functioning.
The functional implications of deficient prepulse inhibition in schizophrenic patients are suggested by the significant correlation between prepulse inhibition deficits and both increased distractibility (16) and an operational measure of thought disorder (17). These findings indicate that in schizophrenia, a relative inability to gate exteroceptive stimuli (the startle pulse) may reflect neural processes that also contribute to a process culminating in cognitive fragmentation such as thought disorder (17). No studies to date have examined the relationship between sensorimotor gating deficits in schizophrenia, as assessed by prepulse inhibition, and positive and negative symptoms of this disorder.
This study was designed with several hypotheses in mind. We examined the symptomatic correlates of prepulse inhibition deficits in a large group of male schizophrenic patients in order to determine whether the reported relationship between prepulse inhibition deficits and thought disorder (17) and distractibility (16) extends to positive and negative symptoms. Because of sex differences and potential hormonal influences on prepulse inhibition modulation in female subjects (18, 19), we elected to reduce variance in this study and examined a group of male schizophrenic patients and normal male comparison subjects. In parallel, we are also continuing to study female schizophrenic patients.
METHOD
Subjects
Subjects consisted of 51 male schizophrenic patients and 26 male normal comparison subjects. Exclusion of 12 “nonreactive” subjects, defined as having a mean pulse-alone magnitude of less than 10 digital units in the first trial block (see later discussion), resulted in analysis of data from 39 schizophrenic and 24 normal comparison subjects. Schizophrenic patients were recruited from inpatient (N=21) and outpatient (N=6) programs at the University of California, San Diego, and the Alpine Convalescent Center (N=12), a long-term locked treatment facility for chronically ill psychiatric patients. All subjects had been screened carefully to ensure that they did not have an axis I diagnosis other than schizophrenia and had not experienced a neurologic insult, which could potentially affect brain functioning. After subjects were given a detailed description of their participation in the study, written consent was obtained. All subjects were diagnosed and assessed by a doctoral-level clinician through use of the Structured Clinical Interview for DSM-IV (SCID) (20). Symptoms were also assessed with the Scale for the Assessment of Positive Symptoms (SAPS) (21), the Scale for the Assessment of Negative Symptoms (SANS) (22), and the Brief Psychiatric Rating Scale (BPRS) (23). Patients were diagnosed with the following subtypes: paranoid (N=20), undifferentiated (N=8), disorganized (N=9), catatonic (N=1), and residual (N=1).
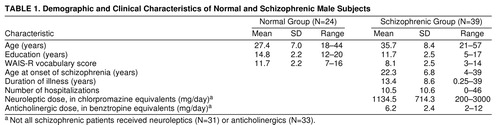
On average, subjects had been ill for 13.4 years (SD=8.6) and had 10.5 (SD=10.6) hospitalizations. Six patients were unmedicated. Patients receiving medications averaged 1134.5 mg (SD=714.3) of chlorpromazine equivalents. Other demographic and clinical information is in (table 1.
Comparison subjects underwent screening interviews to rule out head trauma, exposure to psychoactive medication, past or present axis I or II diagnoses, neurological illness, or drug abuse. Subjects were excluded if they had a positive result on toxicology screen (N=1) or if they met an MMPI-based algorithm for psychosis or substance abuse proneness (N=8) (24).
Schizophrenic patients were significantly older than the comparison subjects (t=4.05, df=61, p<0.001), had significantly fewer years of education (t=5.04, df=61, p<0.001), and had significantly lower WAIS-R vocabulary scores (t=5.54, df=55, p<0.001).
Startle Measures
An audiometer was used to exclude any subject who could not detect 45-dB tones at 500, 1000, or 6000 Hz. Each subject was seated comfortably. Two miniature silver/silver chloride electrodes were positioned below and to the right of the subject’s right eye, over the orbicularis oculi muscle; electrode resistances were less than 10 kOhm. A ground electrode was placed behind the right ear over the mastoid. EMG activity recorded by the electrodes was directed through an SR-LAB computerized startle response monitoring system (San Diego Instruments, Inc., San Diego) for digitization and analysis. The system recorded 250 1-msec epochs, starting with the onset of the startle stimulus. In addition, EMG activity was band-pass filtered (100 to 500 Hz). A 60-Hz notch filter was also used to eliminate 60-Hz interference. A square wave calibrator established sensitivity to be 4.7 µV/digital unit. Acoustic startle stimuli were presented binaurally through headphones. Sound levels were calibrated monthly by using a continuous tone and sound pressure level meter with a 6-cc coupler in an artificial ear.
Startle Session
A 5-minute acclimation period of 70-dB[A] SPL broadband noise that continued as the background noise was followed by two blocks of 36 trials, each block consisting of six of each of the six trial types presented in a pseudorandom order. All startle pulse stimuli were 40-msec 118-dB[A] SPL bursts of noise. In addition to the pulse-alone trials, there were four different prepulse trial types in which a 20-msec burst of noise preceded the startling stimulus by 60 msec. The four prepulse trial types included prepulses 2, 4, 8, or 16 dB above the 70-dB[A)] background level. In the sixth trial type, no stimulus was presented, but EMG was recorded. The mean intertrial interval was 15 seconds (range=8–22); the entire session lasted 23 minutes.
The software parameters by which voluntary and spontaneous eyeblinks were recognized, scored, and excluded have been described in a previous report (10). The onset latency, reported in milliseconds, was defined by a shift of 10 digital units from the baseline value, occurring 20–85 msec after the onset of the pulse stimulus, per the SR-LAB version of Graham’s program that we have used previously (10). Peak latency was defined as the point of maximal amplitude occurring within 85 msec of the pulse stimulus. Trials were identified for visual inspection when baseline values during the first 20 msec shifted by more than 10 units and were excluded if significant EMG activity occurred at the time of stimulus presentation by a blind investigator (interrater agreement=97.5%). Any subject for whom more than 10% of the 72 trials were excluded, or for whom more than two of the same trial type within the same block were excluded, was eliminated from subsequent analysis. In total, among normal comparison subjects, seven individuals (29%) had one or more trials excluded (range=one to two, mean=1.43) out of a 72-trial session). Among schizophrenic patients, nine individuals (23%) had trials excluded (range=one to seven, mean=2.77). The difference between the number of trials excluded across groups was not significant by t test. No subjects were excluded through use of these criteria.
Statistical Analysis
The paradigm was designed to provide several measures of startle. First, responsiveness (versus nonresponsiveness) was measured by examining whether subjects responded to the pulse-alone stimuli in the first trial block at a predetermined level of magnitude (10). Second, the startle response to the first startling stimulus was assessed as a measure of startle reactivity. Third, the level of the reflex magnitude, defined as the response magnitude during the pulse-alone trials of the first and second blocks, was measured. Fourth, latency characteristics of the startle responses were assessed on pulse-alone trials, including both the onset and peak latencies, and latency facilitation was assessed by comparing latencies on pulse-alone trials with latencies on the various prepulse trials. Fifth, prepulse inhibition was assessed by comparing the percent decrement in digital units between pulse-alone and the various prepulse trials. Sixth, habituation of startle, reported to be reduced in schizophrenic patients (10, 11, 25), was assessed as the decrement in response magnitude across the session.
Group differences in responsivity were analyzed by chi-square analysis. Reactivity was analyzed by two-way analysis of variance (ANOVA). Pulse-alone reflex magnitude was analyzed by two-way ANOVA with block as a repeated measure. Onset and peak latencies were analyzed by two-way ANOVA with repeated measures on trial type. Analyses of group differences in prepulse inhibition were conducted by using percentage scores, defined as the difference score divided by the corresponding pulse-alone value, multiplied by 100. Such an analysis tends to correct for the potential influence of individual differences in startle reactivity on measures of prepulse inhibition. Data were analyzed by using a two-way ANOVA with repeated measures on block and prepulse intensity. Spearman’s rank order correlations were used to assess the relationships between selected measures. All statistical tests were done with BMDP programs (26).
RESULTS
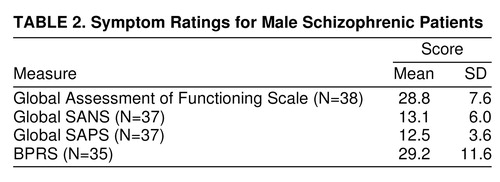
Some demographic characteristics of subjects are summarized in table 1. Data from the BPRS, SANS, and SAPS for schizophrenic patients are summarized in table 2.
Responsivity.
Subjects who averaged less than 10 digital units for pulse-alone magnitude in block 1 were defined as nonresponders. Twelve (23.5%) of the 51 schizophrenic patients and two (7.7%) of the 26 normal comparison subjects were defined as nonresponders. This difference was not significant by chi-square analysis.
Startle reactivity.
The initial reactivity to the first (unique) pulse-alone trial was not significantly different between schizophrenic and comparison subjects (F=1.00, df=1, 60, p>0.05).
Pulse-alone magnitude
Mean startle magnitude for pulse-alone trials was assessed by ANOVA. The effect of diagnostic group was not significant (F=2.45, df=1, 61, p>0.05), but schizophrenic patients appeared to have nonsignificantly lower pulse-alone magnitudes than comparison subjects in (table 3). There was a significant block effect (F=77.48, df=1, 61, p<0.001), with lower pulse magnitude in the second block of trials, reflecting habituation to the startling stimuli in both groups. The diagnosis-by-trial-block interaction was not significant.
Latency facilitation.
To assess the effect of the various prepulse conditions on onset and peak latency, means for each trial type were examined for block 1, before significant habituation had taken place, and, in addition, were collapsed across the session (figure 1.). In block 1, the main effect of diagnosis was significant (F=7.72, df=1, 55, p<0.01), with schizophrenic patients having significantly longer onset latencies than comparison subjects. All groups showed significant latency facilitation (F=13.92, df=4, 220, p<0.001), with the shortest onset latencies occurring with the most intense prepulse levels. The same patterns (longer latencies in schizophrenic patients but normal latency facilitation) were evident when the data were collapsed across the session. Peak latency was also assessed for block 1 and across the session. Schizophrenic patients had longer peak latencies in block 1 (F=8.99, df=1, 55, p<0.005) and across the session (F=4.75, df=1, 45, p<0.05). In addition, all groups showed significant latency facilitation in block 1 (F=39.7, df=4, 220, p<0.001) and across the session (F=21.56, df=4, 180, p<0.001). The diagnosis-by-prepulse-intensity interaction was not significant.
Prepulse inhibition.
Percent prepulse inhibition was assessed in schizophrenic patients and comparison subjects across the two blocks of the session in (table 3). As seen in previous studies (18), magnitude facilitation rather than inhibition occurred for all groups with the weakest prepulse (2 dB above background), so that our analyses were restricted to the three prepulse intensities at which prepulse inhibition was evident. Repeated measure ANOVA, with diagnosis as a between-subject factor and prepulse intensity and trial block as within-subject factors, revealed that the main effect of diagnosis was not significant (F=2.14, df=1, 58, p>0.05). There was a significant effect of prepulse intensity (F=54.79, df=2, 116, p<0.001), with louder prepulses producing more prepulse inhibition. There was a significant prepulse-intensity-by-diagnosis interaction (F=3.23, df=2, 116, p<0.05), with greater group differences observed with the more intense prepulse levels. Post hoc t tests revealed that male schizophrenic patients had significantly less prepulse inhibition in the 16-dB prepulse condition than did male comparison subjects (t=2.28, df=1, 61, p<0.05). In addition, the block-by-prepulse-intensity interaction was significant (F=3.25, df=2, 116, p<0.05) because of trends for increased prepulse inhibition for the 4- and 16-dB conditions in block 2 (versus block 1), whereas prepulse inhibition tended to decrease for the 8-dB prepulse condition in the second block.
Because the schizophrenic patients were significantly older and had significantly less education than did the comparison subjects, prepulse inhibition analyses were repeated and age and education were used as covariates. The main effect of prepulse intensity (F=54.79, df=2, 116, p<0.001), diagnosis-by-prepulse-intensity interaction (F=3.23, df=2, 116, p<0.05), and the block-by-prepulse-intensity interaction (F=3.25, df=2, 116, p<0.05) remained significant. A subset of schizophrenic patients (N=30, mean age=32.5 years) and comparison subjects (N=19, mean age=29.5) was matched for mean age. The main effect of prepulse intensity (F=40.45, df=2, 90, p<0.001), the diagnosis-by-prepulse-intensity interaction (F=3.36, df=2, 90, p<0.05), and the block-by-prepulse-intensity interaction (F=3.64, df=2, 90, p<0.05) remained significant. In addition, a subset of schizophrenic patients (N=26, mean years of education=13.0) and comparison subjects (N=15, mean years of education=13.5) was matched for mean educational level. The main effect of prepulse intensity (F=43.58, df=2, 74, p<0.001) and the diagnosis-by-prepulse-intensity interaction (F=3.24, df=2, 74, p<0.05) remained significant. The block-by-prepulse-intensity interaction failed to achieve significance in this subset.
Prepulse facilitation.
Both comparison subjects and schizophrenic patients showed startle magnitude facilitation with 2-dB prepulses, but main effects of diagnosis and of block were not significant, nor was the interaction.
Habituation.
To assess group differences in habituation, mean startle magnitude for the pulse-alone trials was assessed by ANOVA. As described earlier, both groups showed significant habituation, with lower pulse magnitude in the second trial block (F=77.48, df=1, 61, p<0.001). There were no differences in habituation between the two groups, as reflected by a nonsignificant diagnosis-by-trial-block interaction (F=0.34, df=1, 61, p>0.05). When percent habituation for the 12 pulse-alone trials was analyzed, the effect of group was also not significant.
Correlations of prepulse inhibition and symptoms.
Spearman rank order correlations for prepulse inhibition in the 16-dB condition (in which both prepulse inhibition and prepulse inhibition group differences were maximal) and the three symptom rating scales (BPRS, SANS, and SAPS) for schizophrenic subjects (N=37 because of missing SANS and SAPS scores for two patients) revealed that less prepulse inhibition correlated significantly with increased global SAPS (r=–0.489, N=37, p<0.01) and increased global SANS (r=–0.441, N=37, p<0.01) scores.
Spearman rank order correlations with a limited number of patient demographic variables, including age, age at onset, duration, number of hospitalizations, and anticholinergic and chlorpromazine equivalents, revealed that age was significantly correlated with mean prepulse inhibition on 16-dB trials (r=–0.460, p<0.01), with older subjects having less prepulse inhibition. No other correlations were statistically significant (N=39).
DISCUSSION
This study shows that schizophrenic patients have maximal prepulse inhibition deficits when stronger prepulses are used and that these prepulse inhibition deficits correlate with both positive and negative symptoms. Thus, when the prepulse is 16 dB (versus 2, 4, or 8 dB) above an experimentally delivered 70-dB background noise level, schizophrenic patients are maximally deficient in prepulse inhibition compared to the normal subjects. This finding reinforces the importance of precisely controlling background noise levels, prepulse intensity, and other parameters in studies of prepulse inhibition and psychopathological groups (see below). In the 16-dB prepulse condition, we also observed significant correlations between diminished prepulse inhibition and both positive and negative symptoms. Previous studies have shown strong associations between prepulse inhibition deficits and thought disorder (17) and distractibility (16). To our knowledge, this is the first study that has reported a significant correlation between prepulse inhibition and SAPS- and SANS-derived positive and negative symptoms.
The current pattern of schizophrenia-linked prepulse inhibition deficits at only the strongest prepulse inhibition levels at first appears discrepant with the finding by Grillon et al. (12) of prepulse inhibition deficits across a range of prepulse intensities. There are several differences in the parametric characteristics of the two experiments that may account for this difference. In the Grillon et al. study, a less intense startling stimulus was used (106 dB versus 118 dB), and prepulse intensities were more intense: prepulse stimuli were 5, 10, 15, and 20 dB above background. Grillon et al. also used a longer interstimulus interval (120 msec versus 60 msec) and an intertrial interval with a duration twice as long as that used in the present study. Cumulatively, the Grillon et al. study used prepulse trials that were expected to produce more prepulse inhibition due to the use of generally greater prepulse-to-background decibel levels and longer prepulse-to-startle interstimulus intervals and were thus more similar to the most intense prepulse condition used in the current study, where, in fact, we did find a significant group difference.
Until now, the major functional correlates of prepulse inhibition deficits in schizophrenic patients have been the association of decreased prepulse inhibition with thought disorder (17) and distractibility (16). It now appears that in male schizophrenic patients, both global positive and negative symptoms correlate with prepulse inhibition deficits. From a theoretical standpoint, positive symptoms have been associated with increased subcortical dopamine activity, whereas negative symptoms have been associated with frontal cortical hypodopaminergia in schizophrenic patients (27). Animal model studies provide evidence that prepulse inhibition is regulated by both subcortical and frontal cortical dopaminergic substrates: prepulse inhibition is reduced by manipulations that directly decrease frontal cortical dopamine activity (28) and by manipulations that directly increase dopamine activity in subcortical mesolimbic terminal fields (29, 30). Furthermore, reduced prepulse inhibition after frontal cortical dopamine depletion may result from increased subcortical dopamine activity (28), consistent with a reciprocal relationship between frontal cortical and mesolimbic dopamine activity (31). The significant symptom correlations with prepulse inhibition deficits in the present study may thus reflect the fact that the brain substrates that regulate prepulse inhibition include those associated with the genesis of both positive and negative symptoms in schizophrenia. It is important to note that prepulse inhibition deficits are not unique to schizophrenic patients and are observed in a family of “gating disordered” groups that are characterized by abnormalities within frontal cortical and/or subcortical dopaminergic circuitry (32–34).
While numerous investigators have found prepulse inhibition deficits and correlates of those deficits in schizophrenic and psychosis-prone patients (9–17), the paradigm itself is complex. In human and animal model studies, even minor-appearing alterations in background noise, prepulse type (e.g., tone versus noise), prepulse-to-background noise intensity differences, and startle stimulus characteristics may alter the observed group differences (35). It is important to note that we originally selected the uninstructed prepulse inhibition paradigm as a way to assess possible involuntary or automatic processing deficits in schizophrenic patients (9) because voluntary processing deficits are so frequently reported in schizophrenic patients and may be the result of distraction or simple “inattention.” Subsequently, these uninstructed prepulse inhibition deficits have been replicated in schizophrenic and related subject populations (9–17). Similar uninstructed processing deficits have been reported through use of a two-stimulus P50-evoked response paradigm (36, 37) and other two-stimulus paradigms (38). Dawson and Nuechterlein (39) used an innovative variant of the prepulse inhibition paradigm in which attending to the prepulse itself is controlled in order to assess attentional allocation to the prepulse. Still, this is a different paradigm that used a population of younger, treatment-responsive patients. These investigators found voluntary attentional modulation deficits in these young schizophrenic patients but no deficits in the uninstructed condition in which the parametric conditions were quite different from the paradigm presented here. In parallel, we have reported that older schizophrenic patients show a similar lack of attentional modulation of prepulse inhibition (40) compared with older normal subjects who exhibit fairly robust increased prepulse inhibition in the “attend” condition. In similar paradigms, though, we have failed to observe increased prepulse inhibition in normal subjects in various iterations of an attend condition (41). This rather complex pattern of results across paradigms is not particularly surprising and probably represents 1) parametric differences used in various experiments (as cited earlier); 2) differences in patient characteristics, such as duration of illness and symptom level (as reflected here); and 3) other factors including the increased use of patients treated with atypical (versus typical) antipsychotic medications, which may selectively normalize prepulse inhibition deficits in schizophrenic patients, per our findings in animal model data (42) and as reported by Nagamoto et al. (43) in a two-stimulus P50-suppression paradigm. It is clear that more work needs to be done in this interesting domain of the attentional modulation of prepulse inhibition.
The use of prepulse inhibition in clinical research is made particularly salient by a clear homologous animal model and a corresponding known neural circuit basis of prepulse inhibition (see Introduction). The power of this model of deficient prepulse inhibition in schizophrenia is evident from the fact that the ability of antipsychotics to restore prepulse inhibition in dopamine agonist-treated rats correlates significantly (r=0.99) with their clinical antipsychotic potency (30). The results across studies indicate that thought disorder, distractibility, and positive and negative symptoms all correlate with deficient prepulse inhibition in male schizophrenic patients. Several caveats should be noted. First, the important issue of gender effects in schizophrenia warrants further examination. Second, the level of correlation between prepulse inhibition deficits and SANS and SAPS ratings (versus measures of thought disorder used in previous studies [17]) is of interest but is probably not robust enough to pursue in great detail. On the basis of these results, a next logical and generative step would be to examine the relationship of prepulse inhibition (and other information processing measures) to cognitive and functional measures of outcome (17, 44). We are now conducting these studies in both male and female schizophrenic patients. In addition, studies of regional brain activation and the genetics of prepulse inhibition may offer unique insights into brain/behavior relationships in patients afflicted with schizophrenia.
Received April 14, 1998; revision received Oct. 29, 1998; accepted Nov. 10, 1998. From the Department of Psychiatry, University of California, San Diego. Address reprint requests to Dr. Braff, Department of Psychiatry, University of California, San Diego, 9500 Gilman Dr., La Jolla, CA 92093-0804; [email protected] (e-mail). Supported in part by NIMH grants MH-42228, MH-01223, and MH-01436 and by a grant from the VA (VISN 22, Mental Illness Research Education and Clinical Center). Dr. Geyer holds an equity position in San Diego Instruments.
 |
 |
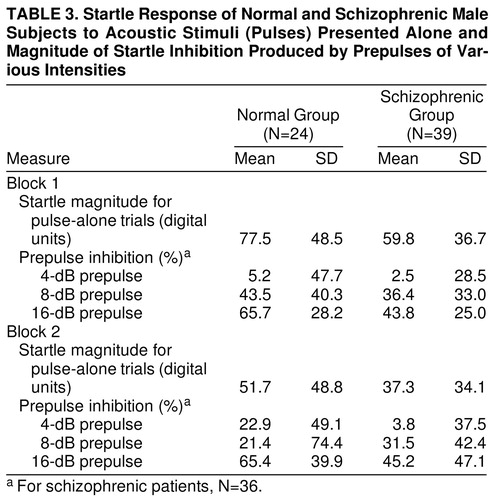 |
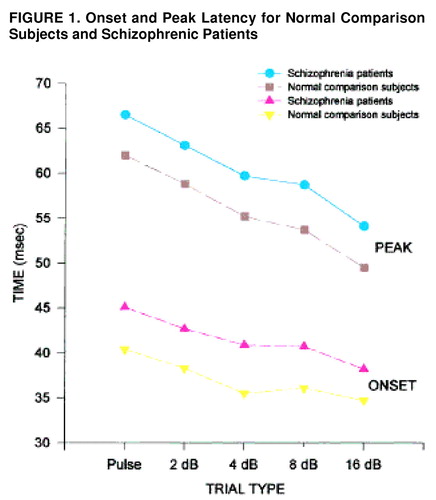
FIGURE 1. Onset and Peak Latency for Normal Comparison Subjects and Schizophrenic Patients
1. Braff DL: Information processing and attention in schizophrenia. Schizophr Bull 1993; 19:233–259Crossref, Medline, Google Scholar
2. Holzman PS: Recent studies of psychophysiology in schizophrenia. Schizophr Bull 1987; 13:49–75Crossref, Medline, Google Scholar
3.. Nuechterlein KH, Dawson ME: Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 1984; 10:160–203Crossref, Medline, Google Scholar
4. Braff DL, Geyer MA: Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry 1990; 47:181–188Crossref, Medline, Google Scholar
5. Braff DL: Psychophysiological and information processing approaches to schizophrenia, in Neurobiological Foundation of Mental Illness. Edited by Charney DS, Nestler E, Bunney BS. New York, Oxford University Press (in press)Google Scholar
6. Davis M, Gendelman D, Tischler M, Gendelman P: A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci 1982; 2:791–805Crossref, Medline, Google Scholar
7. Graham FK: The more or less startling effects of weak prestimuli. Psychophysiology 1975; 12:238–248Crossref, Medline, Google Scholar
8. Swerdlow NR, Caine SB, Braff DL, Geyer MA: The neural substrates of sensorimotor gating of the startle reflex: a review of recent findings and their implications. J Psychopharmacol 1992; 6:176–190Crossref, Medline, Google Scholar
9. Braff DL, Stone C, Callaway E, Geyer MA, Glick ID, Bali L: Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology 1978; 14:339–343Crossref, Google Scholar
10. Braff DL, Grillon C, Geyer MA: Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 1992; 49:206–215Crossref, Medline, Google Scholar
11. Bolino F, Di Michele V, Di Cicco L, Manna V, Daneluzzo E, Casacchia M: Sensorimotor gating and habituation evoked by electro-cutaneous stimulation in schizophrenia. Biol Psychiatry 1994; 36:670–679Crossref, Medline, Google Scholar
12. Grillon C, Ameli R, Charney DS, Krystal J, Braff DL: Startle gating defects occur across prepulse intensities in schizophrenic patients. Biol Psychiatry 1992; 32:939–949Crossref, Medline, Google Scholar
13. Cadenhead KS, Geyer MA, Braff DL: Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry 1993; 150:1862–1867Link, Google Scholar
14. Simons RF, Giardina BD: Reflex modification in psychosis-prone young adults. Psychophysiology 1992; 29:8–16Crossref, Medline, Google Scholar
15. Swerdlow NR, Filion D, Geyer MA, Braff DL: “Normal” personality correlates of sensorimotor, cognitive, and visuospatial gating. Biol Psychiatry 1995; 37:286–299Crossref, Medline, Google Scholar
16. Karper LP, Freeman GK, Grillon C, Morgan CA, Charney DS, Krystal JH: Preliminary evidence of an association between sensorimotor gating and distractibility in psychosis. J Neuropsychiatry Clin Neurosci 1996; 8:60–66Crossref, Medline, Google Scholar
17. Perry W, Braff DL: Information-processing deficits and thought disorder in schizophrenia. Am J Psychiatry 1994; 151:363–367Link, Google Scholar
18. Swerdlow NR, Monroe SM, Hartson HJ, Braff DL, Geyer MA, Auerbach PP: Men are more inhibited than women by weak prepulses. Biol Psychiatry 1993; 34:253–261Crossref, Medline, Google Scholar
19. Swerdlow NR, Hartman PL, Auerbach PP: Changes in sensorimotor inhibition across the menstrual cycle: implications for neuropsychiatric disorders. Biol Psychiatry 1997; 41:452–460Crossref, Medline, Google Scholar
20. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
21. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
22. Andreasen NC: Modified Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1984Google Scholar
23. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
24. Butler RW, Jenkins MA, Braff DL: The abnormality of normal comparison groups: the identification of psychosis proneness and substance abuse in putatively normal research subjects. Am J Psychiatry 1993; 150:1386–1391Link, Google Scholar
25. Geyer MA, Braff DL: Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology 1982; 19:1–6Crossref, Medline, Google Scholar
26. Dixon WJ: BMDP Biomedical Computer Programs. Los Angeles, University of California Press, 1988Google Scholar
27. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
28. Koch M, Bubser M: Deficient sensorimotor gating after 6-hydroxydopamine lesion of the rat medial prefrontal cortex is reversed by haloperidol. Eur J Neurosci 1994; 6:1837–1845Crossref, Medline, Google Scholar
29. Swerdlow NR, Masten V, Geyer MA, Braff DL: Schizophrenic-like abnormalities in sensory gating following accumbens dopamine infusions in rats. Psychopharmacology (Berl) 1990; 101:414–420Crossref, Medline, Google Scholar
30. Swerdlow NR, Braff DL, Taaid N, Geyer MA: Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry 1994; 51:139–154Crossref, Medline, Google Scholar
31. Pycock CV, Kervin RW, Carter CJ: Effects of lesions of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature 1980; 286:74–77Crossref, Medline, Google Scholar
32. Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL: A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry 1993; 33:298–301Crossref, Medline, Google Scholar
33. Castellanos FX, Fine EJ, Kaysen DL, Kozuch PL, Hamburger SD, Rapoport JL, Hallett M: Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biol Psychiatry 1996; 39:33–41Crossref, Medline, Google Scholar
34. Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR: Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiatry 1995; 58:192–200Crossref, Medline, Google Scholar
35. Davis M, Mansbach RS, Swerdlow NR, Campeau S, Braff DL, Geyer MA: Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology (Berl) 1990; 102:1–4Crossref, Medline, Google Scholar
36. Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, Drebing C, Nagamoto H, Bickford-Wimer P, Franks R: Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull 1987; 13:669–671Crossref, Medline, Google Scholar
37. Clementz BA, Geyer MA, Braff DL: P50 suppression among schizophrenia and normal comparison subjects: a methodological analysis. Biol Psychiatry 1997; 41:1035–1044Crossref, Medline, Google Scholar
38. Schall U, Schon A, Zerbin D, Eggers C, Oades RD. Event-related potentials during an auditory discrimination with prepulse inhibition in patients with schizophrenia, obsessive-compulsive disorder, and healthy subjects. Int J Neurosci 1996; 84:15–33Crossref, Medline, Google Scholar
39. Dawson MK, Nuechterlein KH: Psychophysiological dysfunctions in the developmental course of schizophrenic disorders. Schizophr Bull 1984; 10:204–232Crossref, Medline, Google Scholar
40. McDowd JM, Filion DL, Harris MJ, Braff DL: Sensory gating and inhibitory function in late-life schizophrenia. Schizophr Bull 1993; 19:733–746Crossref, Medline, Google Scholar
41. Braff DL, Perry W, Cadenhead KS, Swerdlow NR, Geyer MA: Attentional manipulations of prepulse inhibition of the startle reflex (abstract). Biol Psychiatry 1997; 41:65SCrossref, Medline, Google Scholar
42. Bakshi VP, Swerdlow NR, Geyer MA: Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J Pharmacol Exp Ther 1994; 271:787–794Medline, Google Scholar
43. Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R: Gating of the auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry 1996; 40:181–188Crossref, Medline, Google Scholar
44. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Google Scholar



