Association of Missense Mutation in FOLH1 With Decreased NAAG Levels and Impaired Working Memory Circuitry and Cognition
Abstract
Objective:
Altering the metabotropic glutamate receptor 3 (mGluR3) by pharmacology or genetics is associated with differences in learning and memory in animals and humans. GRM3 (the gene coding for mGluR3) is also genome-wide associated with risk for schizophrenia. The neurotransmitter N-acetyl-aspartyl-glutamate (NAAG) is the selective endogenous agonist of mGluR3, and increasing NAAG may improve cognition. Glutamate carboxypeptidase II (GCPII), coded by the gene folate hydrolase 1 (FOLH1), regulates the amount of NAAG in the synapse. The goal of this study was to determine the relationship between FOLH1, NAAG levels, measures of human cognition, and neural activity associated with cognition.
Methods:
The effects of genetic variation in FOLH1 on mRNA expression in human brain and NAAG levels using 7-T magnetic resonance spectroscopy (MRS) were measured. NAAG levels and FOLH1 genetic variation were correlated with measures of cognition in subjects with psychosis and unaffected subjects. Additionally, FOLH1 genetic variation was correlated with neural activity during working memory, as measured by functional MRI (fMRI).
Results:
A missense mutation in FOLH1 (rs202676 G allele) was associated with increased FOLH1 mRNA in the dorsolateral prefrontal cortex of brains from unaffected subjects and schizophrenia patients. This FOLH1 variant was associated with decreased NAAG levels in unaffected subjects and patients with psychosis. NAAG levels were positively correlated with visual memory performance. Carriers of the FOLH1 variant associated with lower NAAG levels had lower IQ scores. Carriers of this FOLH1 variant had less efficient cortical activity during working memory.
Conclusions:
These data show that higher NAAG levels are associated with better cognition, suggesting that increasing NAAG levels through FOLH1/GCPII inhibition may improve cognition. Additionally, NAAG levels measured by MRS and cortical efficiency during working memory measured by fMRI have the potential to be neuroimaging biomarkers for future clinical trials.
There is an abundance of evidence that the metabolic glutamate receptor 3 (mGluR3) plays a role in cognition (1–7), as well as in the pathophysiology of schizophrenia (8, 9). Selectively targeting the mGluR3 pathway through its endogenous agonist, N-acetyl-aspartyl-glutamate (NAAG) (10), may be a novel strategy for improving cognition in illnesses in which deficits in cognition are prominent (e.g., schizophrenia). NAAG acts as a peptide neurotransmitter that inhibits glutamate neurotransmission as a selective agonist of mGluR3 (10) and antagonist of N-methyl-d-aspartate (NMDA) receptors (11). Agonists of mGluR2/3 have improved cognitive performance and other behaviors in animal models (12, 13). However, in a phase 2 trial, an mGluR2/3 agonist was not efficacious in treating overall symptoms of schizophrenia (14). Failure of an mGluR2/3 agonist may be due, in part, to its lack of specificity for mGluR3 (over mGluR2) or differences in downstream signaling from this allosteric modulator or because the effects of targeting mGluR3 may be relatively specific to cognition.
NAAG levels in the synapse are primarily regulated by the enzyme glutamate carboxypeptidase II (GCPII), coded by the gene folate hydrolase 1 (FOLH1), which inactivates NAAG by cleaving it into N-acetylaspartate (NAA) and glutamate (15, 16). Inhibitors of GCPII/FOLH1 have been shown to increase extracellular NAAG levels and improve learning and memory in animal models of schizophrenia, ethanol intoxication, Alzheimer’s disease, and multiple sclerosis with cognitive impairment (17–21). It has not yet been shown how altering GCPII/FOLH1 in human brain affects NAAG levels. Although GCPII/FOLH1 inhibitors are in development, there is no GCPII/FOLH1 blocker currently available for human use.
Imaging genetics has been used to identify relationships between alterations in potential drug targets and pathways and changes in brain function during relevant tasks. In a previous study, we found that healthy participants with a GRM3 genotype that was associated with risk for schizophrenia and impaired cognition also had increased cortical activity at high performance during the N-back working memory task compared with noncarriers (3), which is a pattern of inefficient activation found in patients with schizophrenia (22) and their healthy siblings (23). Here, we exploited a putative functional genetic variation in FOLH1 to establish a relationship between FOLH1/GCPII, NAAG levels in human brain, and measures of cognition and cortical physiology. We hypothesized that genetic variation associated with less FOLH1 expression would be associated with higher NAAG levels in human brain, as measured by high-field magnetic resonance spectroscopy (MRS). Additionally, we hypothesized that higher NAAG levels, and the genetic variant associated with lower FOLH1 and higher NAAG, would be associated with better cognitive performance and more efficient cortical activity during working memory, as assayed with functional MRI (fMRI).
Methods
Postmortem Human Brain Samples
Postmortem human brain samples were obtained by autopsy, as part of the Lieber Institute for Brain Development (LIBD) brain repository, as previously described (24, 25). All samples were obtained with audiotaped informed consent from the legal next of kin to study brain tissue, as approved by the institutional review boards of the National Institutes of Health (NIH) and the University of Maryland, in lieu of written consent. Clinical characterization, diagnoses, and macro- and microscopic neuropathological examinations were performed on all samples using a standardized paradigm, and subjects with evidence of macro- or microscopic neuropathology were excluded. Details of tissue acquisition, handling, processing, dissection, clinical characterization, diagnoses, neuropathological examinations, RNA extraction, and quality control measures have been described previously (24, 25). Briefly, brains were hemisected and cut into 1.0-cm to 1.5-cm thick coronal slabs, flash frozen, and stored at −80°C. Dorsolateral prefrontal cortex (DLPFC) gray matter was dissected using a dental drill. The DLPFC (Brodmann’s areas 9 and 46) was dissected from the middle frontal gyrus of the coronal slab, immediately anterior to the genu of the corpus callosum. Dissected tissue was pulverized and stored at −80°C. Pulverized cerebellum was used to measure the pH.
RNA Sequencing
We identified expression quantitative trait loci (eQTL) in the DLPFC of 412 subjects older than age 13 (subjects with schizophrenia, N=175; unaffected control subjects, N=237) using RNA sequencing and genotype data. Genotyping of brain samples was conducted using standard methods, as previously described (25). RNA sequencing methodologies have also been described previously (25). Details of RNA sequencing and selection of the FOLH1 missense mutation, rs202676, are described in the Methods section of the online supplement. The eQTL modeling tested for additive genetic effects of rs202676 on FOLH1 expression while adjusting for sex, diagnosis, ancestry (multidimensional scaling components), and expression heterogeneity (principal components), as previously described (25). Multiple testing across the genome was controlled using the false discovery rate (FDR) within each feature summarization. These results can be viewed and visualized using the LIBD DLPFC eQTL browser (http://eqtl.brainseq.org) (25). FOLH1 rs202676 allele frequencies in the postmortem data set are presented in Table S1 in the online supplement.
Clinical Subjects
Neurologically and psychiatrically healthy volunteers (N=65) and patients with recent-onset psychosis (N=57) were recruited from the Johns Hopkins Schizophrenia Center as previously described (26). After receiving a complete description of the study, all clinical subjects ≥18 years old provided written informed consent. Parental consent and assent were obtained for all clinical subjects <18 years old. The clinical cohort was 59% male and comprised subjects ages 15–34 years (mean age, 23.9 years [SD=4.42]). For patients, onset of psychosis was within 24 months of the start of the study, and therefore these patients were characterized early in their illness. Further details about the clinical cohort have been previously published (27). Details on the genotyping of the clinical subjects are presented in the Methods section of the online supplement. FOLH1 rs202676 allele frequencies in the clinical subjects are presented in Table S2 in the online supplement. We analyzed the effects of FOLH1 rs202676 on neural NAAG levels in the Caucasian cohort (N=40) and the African American cohort (N=70) separately because of differences in allele frequencies.
Magnetic Resonance Spectroscopy
MRS imaging data were collected on a Philips Achieva 7-T scanner (Philips Healthcare, Amsterdam) at the Kennedy Krieger Institute, using a 32-receive-channel and eight-channel transmit Nova Medical head coil, as previously described (27). In each subject, spectra were collected from the left centrum semiovale (CSO), left DLPFC, left orbitofrontal cortex, anterior cingulate cortex (ACC), and thalamus. Details of the spectroscopy imaging acquisition and analyses are provided in the Methods section of the online supplement and have been described previously (27). Statistical analyses were performed using GraphPad Prism, version 7.00 for Windows (GraphPad Software, La Jolla, Calif.). Linear regression was used to test for the effect of rs202676 genotype (copies of minor allele G) on NAAG concentrations in each brain region. A Bonferroni-corrected p value of 0.05 (i.e., p=0.01 uncorrected) was considered statistically significant to control for multiple comparisons (five brain regions).
Neuropsychological Assessments
Healthy volunteers and patients with early psychosis completed neuropsychological assessments as previously described (28–31). Briefly, the study subjects completed a battery of neuropsychological tests to assess cognitive function in six domains: processing speed, attention/working memory, ideational fluency, executive function, verbal memory, and visual memory (32). Tests in this battery (29) were administered and scored according to standard instructions by a trained clinical research assistant who was blinded to the imaging data of the subjects. Factor scores were calculated for each of the six domains after controlling for age, sex, race, and premorbid intelligence based on a normative sample (33, 34) using the Hopkins Adult Reading Test (35). Statistical analyses were performed using GraphPad Prism, version 7.00 for Windows. Linear regression was used to test the relationship between NAAG levels in the CSO and measures of cognition for the six factor scores, the composite score, and IQ score. CSO was selected as the region for this analysis because it was found to have the highest correlation between the FOLH1 genotype and NAAG levels. In an exploratory analysis, based on our findings on visual memory, we then tested the effects of DLPFC NAAG levels on these measures of cognition. A linear regression was also used to test the relationship between FOLH1 rs202676 (number of G alleles) and the same measures of cognition. For both the linear regressions on NAAG levels and the FOLH1 rs202676 genotype, a Bonferroni-corrected p value of 0.05 (p=0.0063, uncorrected) was considered statistically significant to control for multiple comparisons (eight assessments: six cognitive factor scores, a composite score, and the IQ score).
Functional Neuroimaging Subjects
Healthy adults (N=183) participated in an fMRI study in the Clinical Brain Disorders Branch Sibling Study at the National Institute of Mental Health (NIMH) (36). The study was approved by the institutional review board at the NIMH Intramural Research Program. All study subjects were assessed using the Structured Clinical Interview for DSM-IV. All subjects were physically and psychiatrically healthy; specific exclusion criteria are reported elsewhere (37). Only Caucasian individuals of self-identified European descent were included in this data set to minimize population stratification artifacts. FOLH1 rs202676 was extracted by whole-genome typing of the imaging subjects, which was conducted using standard methods, as previously described (25). The allele frequency of the NIH fMRI cohort (all were Caucasian) was similar to the 1,000 Genomes Project data set (A=0.78, G=0.22) for the European cohort (Hardy-Weinberg equilibrium, p=0.91). Participants were 18–55 years old. Additional demographic data on the functional neuroimaging cohort are presented in Table S3 in the online supplement. A separate replication data set was collected for 23 healthy subjects who underwent scanning at Johns Hopkins School of Medicine, which is described in the Methods section of the online supplement.
N-Back Working Memory Task
As part of a battery of fMRI paradigms, participants performed a working memory (N-back) task administered using a block design, with the 2-back working memory condition alternating with a no-back control condition, as previously described (38). During the 0-back control task block, the subjects simply responded with the current digit presented (1–4 in a diamond-shaped box). This alternated with the 2-back block, in which the participants serially responded with numbers presented two places earlier in the sequence (n=2). Consistent with previous studies (23), before scanning, participants were trained until their accuracy or task performance was constant (plateaued peak). In order to isolate working memory circuitries, all subjects in this analysis (N=183) and the replication data set (N=23) achieved at least 80% accuracy on the 2-back working memory condition.
Functional Imaging Acquisition and Analysis
fMRI scanning was conducted at NIH, as part of the Clinical Brain Disorders Branch Sibling Study, as previously described (36). In each run, 120 functional scans were acquired for each subject to measure the T2*-weighted blood-oxygen-level dependent signal (gradient-recall echo planar imaging, TR=2,000 ms, TE=28 ms, flip angle=90°, field of view=240 mm, 24 slices, slice thickness=6 mm, matrix size=64×64, voxel size=3.75×3.75×6 mm). Five additional scans were acquired at the beginning to allow for steady-state magnetization and discarded from subsequent analyses. The fMRI data were preprocessed and analyzed using SPM12 implemented in MATLAB (MathWorks, Natick, Mass.). Images were realigned and normalized to the echo planar imaging template in Montreal Neurological Institute (MNI) space to a final voxel size (3×3×3 mm) and spatially smoothed using a 8.0-mm full width at half maximum Gaussian kernel.
Statistical analyses were performed following a two-level procedure. At the first level, a separate general linear model was defined for each participant, including a regressor for 0-back blocks and a regressor for 2-back blocks, both time-locked to block onset with a duration of 30 seconds (block duration). The block design was modeled using a canonical hemodynamic response function. The data were high-pass filtered (120-second cutoff), and serial correlations were accounted for by an autoregressive model of the first order. Because we were specifically interested in neural responses during working memory, for each subject, contrast images were calculated for the 2-back blocks versus 0-back blocks (2-back > 0-back). These individual contrast images were entered into a second-level random-effects whole-brain analysis using a multiple regression model to determine the influence of FOLH1 rs202676 on working memory neural activity. Specifically, we regressed the FOLH1 rs202676 genotype (G carriers and AA) on neural activity, which included a mask for working memory activation (2 > 0-back contrast). Sex, age, and IQ were included in the model of covariates of noninterest. A cluster-based whole-brain family-wise error-corrected p threshold of <0.05 was applied to the resulting group-level map using parameters from Monte Carlo simulations implemented in the AlphaSim program in AFNI (39). The simulations (1,000 iterations, 8-mm full width at half maximum, 3×3×3 mm3 voxels) yielded a combined threshold of t=3.14 (p<0.001, uncorrected) and a cluster extent of 19 contiguous voxels (513 mm3), as equivalent to a corrected threshold p value <0.05. Details on analyses of a separate fMRI replication data set collected at Johns Hopkins School of Medicine are presented in the Methods section in the online supplement.
Results
Missense Mutation Associated With Increased FOLH1 Expression in Human Brain
In postmortem brain samples of both patients with schizophrenia (N=175) and unaffected control subjects (N=237), carriers of the missense G allele of FOLH1 rs202676 had a 10.8% higher FOLH1 gene-level expression in the DLPFC per allele copy compared with carriers of AA homozygotes (Figure 1A, p=6.87e-19, pFDR=3.374e-16). This gene-level eQTL was also nominally significant in the independent CommonMind Consortium DLPFC data set (PMID: 27668389) (p=0.007) and showed eQTL association (t >2) in 10 out of 13 brain regions in the Genotype-Tissue Expression (GTEx) consortium (PMID: 29022597) (Figure 1B). Moreover, feature-level analysis focusing on the exon-exon splice junction (chr11:49204795–49207220) corresponding to the major protein-coding isoform (ENST00000256999.6, which encodes the 750 amino acid GCPII protein) revealed even stronger association in the LIBD discovery data set (p=1.65e-23, pFDR=3.66e-20, 24% increase per allele copy; see also Figure S1A in the online supplement), as well as in the CommonMind Consortium (PMID: 27668389) (p=8.60e-11, 27.0% increase per allele copy). Reanalysis of RNA sequencing data from the GTEx project (PMID: 29022597) for this exon-exon splice junction revealed similar allele direction expression across 12 of 13 brain regions (T >2; all regions except the amygdala, T=1.24), with the strongest effects in the caudate (T=5.32). Lastly, we found an additional association between rs202676 and the decreased expression of a splice junction in a transcript encoding a shorter 74-amino-acid protein (ENST00000525826, p=9.26e-21, pFDR=1.61e-17; see also Figure S1B in the online supplement) that was further replicated in the CommonMind Consortium (p=1.77e-9), suggesting that this single-nucleotide polymorphism (SNP) may influence the transcriptional splicing of the gene. These two junction-level associations were also significant across many of the brain regions in the GTEx data set (see Figures S1C and S1D in the online supplement). Additionally, these genotype associations were consistent in both races under study: when the Caucasian and African American cohorts were analyzed separately, the association between rs202676 and FOLH1 gene expression retained significance at the gene level (Caucasian: p=1.19e-07, pFDR=2.38e-05; African American: p=5.67e-10, pFDR=3.33e-07; see also Figure S2A–S2C in the online supplement) and junction level (see Figure S2D–S2I in the online supplement). These various results establish that the rs202676 genotype is associated with FOLH1 expression and the G allele is the gain-of-function allele.
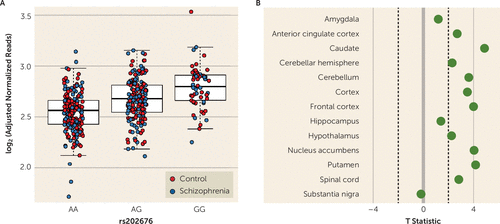
FIGURE 1. FOLH1 missense mutation association with higher FOLH1 expression in human braina
a Carriers of the G allele of the missense polymorphism rs202676 had higher FOLH1 mRNA expression in the dorsolateral prefrontal cortex (panel A) and other brain regions (panel B) compared with noncarriers. Control subjects (N=237), schizophrenia patients (N=175).
Missense Mutation Associated With Lower NAAG Levels Measured by MRS
We next investigated the association of rs202676 genotype with levels of NAAG, NAA, and glutamate and the ratio of NAAG to NAA in the brain of living human subjects using 7-T MRS. We limited our analyses to those metabolites in the NAAG metabolic pathway (i.e., NAAG catabolized to NAA and glutamate) because of the specific role of FOLH1/GCPII. Given the association of G alleles with increased FOLH1 expression, we would expect the same allele to be associated with increased FOLH1 activity, as measured by decreased NAAG. In fact, in patients with recent-onset psychosis (N=20) and in healthy volunteers (N=20, groups combined to enhance power), the FOLH1 rs202676 G allele was associated with lower NAAG levels in several brain regions in the Caucasian cohort, most robustly in the CSO (p=0.0084, r2=0.17, slope=−0.20) (Figure 2). Correlations in the other regions were not significant after correcting for multiple comparisons but showed a similar relationship in the ACC (p=0.04) and DLPFC (p=0.061) and showed no association in the orbitofrontal cortex (p=0.68) or thalamus (p=0.17). The FOLH1 rs202676 genotype did not significantly predict levels of NAA or glutamate in any brain region, but the G allele was associated with a lower NAAG:NAA ratio in the CSO (p=0.017, r2=0.14) and ACC (p=0.049, r2=0.099), although it did not reach significance after correcting for multiple comparisons. FOLH1 rs202676 was not significantly associated with levels of NAAG, NAA, and glutamate or the ratio of NAAG to NAA in any brain region in the African American cohort.
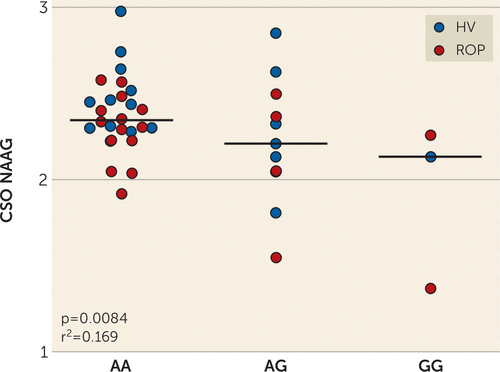
FIGURE 2. FOLH1 missense mutation association with lower N-acetyl-aspartyl-glutamate (NAAG) measured by magnetic resonance spectroscopya
a In 20 healthy volunteers (HV) and 20 patients with recent-onset psychosis (ROP), carriers of the G allele of the FOLH1 missense polymorphism rs202676 had lower NAAG concentrations in the centrum semiovale (CSO).
NAAG Levels Associated With Visual Memory Performance
The CSO region was selected to test the effects of NAAG on cognitive domains because it showed the highest correlation with FOLH1 genotype (Figure 2). Our data also showed that, of the regions we measured, this region had the highest levels and best estimates of NAAG (i.e., lowest Cramér-Rao lower bounds: mean, 5% in the CSO compared with 12%−18% in other regions) (27). CSO NAAG levels were most strongly associated with visual memory performance; subjects who had higher levels of CSO NAAG had a greater factor score for visual memory (Figure 3) (N=119, p=0.0033, r2=0.072, slope=13.65 [SD=4.544]). Healthy volunteers and patients with psychosis were combined in order to establish enough power to detect a significant association, but an analysis of each group separately revealed similar associations (healthy volunteer group: N=63, p=0.145, slope=8.295; recent-onset psychosis group: N=56, p=0.0511, slope=12.68). After correcting for multiple comparisons, CSO NAAG levels did not predict the other factor scores; however, higher CSO NAAG levels were associated with higher IQ, although it fell short of significance (p=0.043, see also Table S4 in the online supplement). FOLH1 missense mutation (rs202676) was associated with IQ score calibrated for age, sex, race, and premorbid intelligence; subjects who carried the G allele (associated with lower NAAG levels) had lower IQ scores (Figure 4) (N=122, p=3.7e-4, r2=0.10, slope=−5.543 [SD=1.513]). When the diagnostic groups were analyzed separately, they showed a similar relationship (healthy volunteer group: N=65, p=0.0032, r2=0.13, slope=−7.003; recent-onset psychosis group: N=57, p=0.043, r2=0.073, slope=−4.143).
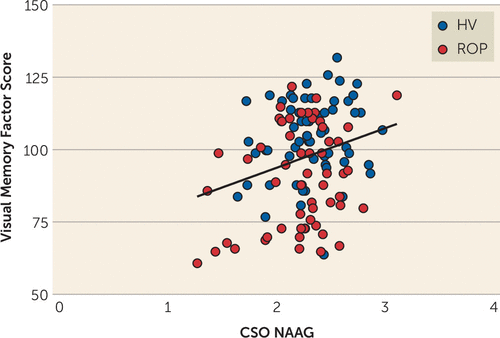
FIGURE 3. N-acetyl-aspartyl-glutamate (NAAG) association with visual memory performancea
a NAAG levels in the centrum semiovale (CSO) were positively correlated with visual memory performance in healthy volunteers (HV) and patients with recent-onset psychosis (ROP).
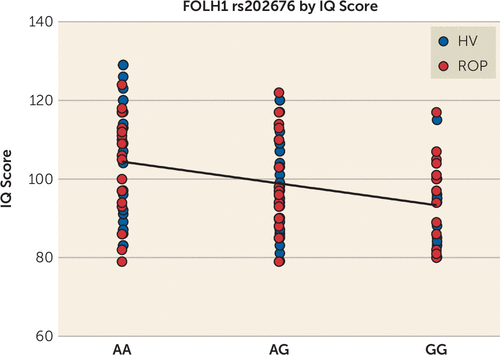
FIGURE 4. FOLH1 missense mutation (rs202676 G allele) association with lower IQ scores in healthy volunteers (HV) and patients with recent-onset psychosis (ROP)
NAA levels in the CSO were not significantly associated with visual memory factor scores or any other cognitive factor scores or IQ (see Table S5 in the online supplement). The NAAG:NAA ratio in the CSO was associated with higher visual memory factor scores, although statistical significance did not survive correction for multiple comparisons (p=0.0212). The NAAG:NAA ratio in the CSO did not significantly correlate with other cognitive factor scores or IQ (see Table S6 in the online supplement).
We acknowledge that the concept of regional functionality may be important. While fully ascertaining it would likely require investigations dedicated specifically to this question, given the significant visual memory finding and the known association between working memory and the prefrontal cortex, we performed a post hoc exploratory analysis testing the effects of DLPFC NAAG levels on measures of cognition. NAAG levels in the DLPFC were available for 109 subjects. The smaller sample size for the DLPFC was the result of losing subjects who had a Cramér-Rao lower bound greater than the 30% cutoff threshold. Levels of NAAG in the DLPFC were positively associated with visual memory factor scores (p=0.0089), although the association did not reach the threshold for statistical significance after correcting for multiple comparisons. Additionally, similar to CSO NAAG levels, DLPFC NAAG levels were not significantly correlated with other cognitive factor scores (see Table S7 in the online supplement).
Genetic Variation in FOLH1 Associated With Cortical Brain Activity During Working Memory
FOLH1 missense mutation was associated with less efficient (i.e., higher) cortical activity during working memory in three regions: the cuneus, the inferior parietal cortex, and the superior temporal gyrus. G allele carriers had significantly greater neural activity in these areas compared with AA carriers (Figure 5) (peak voxel for the cuneus: MNI coordinates: 9, −64, 49; k=43 voxels, T=4.11; peak voxel for the inferior parietal cortex: MNI coordinates: 33, −58, 58; k=97, T=4.06; peak voxel for the superior temporal gyrus: MNI coordinates: −36, −52, 49; k=35, T=3.89). The 2-back performance was not significantly different between genotype groups (see Table S3 in the online supplement). In a separate replication data set, G allele carriers had greater neural activity in the same brain regions, including the inferior parietal cortex, superior temporal gyrus, and cuneus (see Table S8 and Figure S4 in the online supplement).
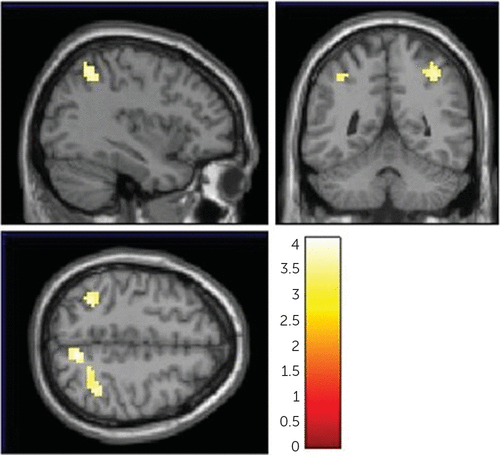
FIGURE 5. FOLH1 missense mutation association with cortical brain activity during the N-back working memory taska
a The images show less efficient cortical brain activity in healthy subjects who carried the FOLH1 missense mutation (rs202676 G allele) compared to non-carriers.
Discussion
There has been great interest in targeting the mGluR3 pathway for the treatment of cognitive dysfunction, and potentially other symptoms of schizophrenia. Targeting the GCPII/FOLH1 enzyme gives an alternative and perhaps more specific approach to modifying glutamate neurotransmission, specifically by modulating levels of its endogenous mGluR3 ligand, NAAG. We exploited genetic variation in FOLH1/GCPII to examine the relationship between FOLH1 expression, NAAG levels, cognition, and neural activity during working memory. One advantage to using genetic variation rather than comparing diagnostic groups is that it enabled us to avoid the inherent confounders between healthy subjects and those with severe mental illness. There are many reasons for an increase or decrease, or no difference, in these measurements between healthy and mentally ill cohorts, including but not limited to antipsychotic medication, smoking, drug abuse, and poor nutrition. Furthermore, as we found with this study, the effects of this missense mutation spans diagnostic groups (e.g., both unaffected/healthy subjects and patients with schizophrenia and psychosis), across multiple levels of biology, suggesting that altering mGluR3 by changing NAAG (via FOLH1/GCPII) may improve cognition in other disorders.
Our data show that among patients with schizophrenia and unaffected control subjects, carriers of the G allele of the FOLH1 missense polymorphism (rs202676) have higher mRNA expression. It should be noted that other genetic variants in linkage disequilibrium with this missense variant also showed strong associations with gene expression, and untangling the specific functional SNPs underlying these associations would be important follow-up molecular work. While we found higher levels of FOLH1 mRNA in carriers of this variant, it was not a direct measure of enzyme function, and therefore we cannot conclusively ascertain the effects of this variant on FOLH1/GCPII enzyme function, which should be explored in future studies.
The same mutation associated with higher FOLH1 expression was associated with lower NAAG levels in healthy subjects and in patients with psychosis. Collectively, these findings suggest that decreasing FOLH1/GCPII could increase NAAG levels. These data are the first, to our knowledge, to support such a relationship in human brain. Previous studies comparing NAAG levels in healthy subjects and schizophrenia patients used limited samples, with lower field-strength MRS, and results have been inconsistent. While some studies have found decreased NAAG levels in patients with schizophrenia (22, 40), other studies have found higher levels (21, 41) or no differences in NAAG levels (42), but these studies differed by the brain regions examined and age of the cohort (22). Additionally, previous research has found both increases and decreases in GCPII expression and NAAG levels between brains of unaffected control subjects and patients with schizophrenia, bipolar disorder, and major depression (43, 44). In a previous study, our group found that patients with psychosis had decreased NAAG levels compared with healthy control subjects, as measured by 7-T MRS (27). NAA and NAAG have very similar spectra and thus are hard to separate in in vivo MRS at the widely available field strengths of 1.5 T or 3 T. The present study made use of the increased spectral resolution available at the high magnet field strength of 7 T to provide more accurate estimates of NAAG, separate from the larger signal of NAA. It has been shown previously that NAAG expression is two to four times higher in white matter than in gray matter (45), and thus the strongest correlation in CSO may, in part, reflect the least uncertainty of the MRS determination in this white matter rich region. Differences in the effect of FOLH1 rs202676 genotype on NAAG levels between the Caucasian and African American cohorts could be due, in part, to differences in allele frequencies and the genetic background on which they are inherited, because CSO NAAG levels are similar between racial cohorts. In the 1000 Genomes Project, the frequency of the G allele was 22.6% in the Caucasian population and 60.2% in the African population (https://www.ncbi.nlm.nih.gov/projects/SNP). However, in the RNA-sequencing data, FOLH1 mRNA expression was similar in both the Caucasian and African American cohorts, suggesting that the potential feedback of increased expression associated with the missense variant is present in both racial groups and likely relates more to the individual sequence variant than the ancestral background in which it lies. The differences in the clinical data may reflect the influence of other variants on FOLH1 expression or on expression of the pseudogene (FOLH1B) that differs between racial groups.
Genetic variation in FOLH1 was not associated with levels of glutamate or NAA. Levels of glutamate are largely regulated by glutamate transporters. Levels of NAA are regulated by N-acetylaspartate synthase (i.e., NAA synthase) and aspartoacylase (which hydrolyses NAA into aspartate and acetate) (46), as well as FOLH1/GCPII, and therefore changes in NAA levels may not be predictive of changes in FOLH1/GCPII. NAAG levels are more than threefold lower than NAA levels and are regulated primarily by FOLH1/GCPII and, to a much lesser extent, because of its low abundance, NAAG synthetase (47); therefore, it is not surprising that genetic variation in FOLH1 influences NAAG levels. In addition, because brain levels of NAA are substantially higher than NAAG levels, modest changes in GCPII hydrolysis of NAAG would have a very small effect on basal NAA levels at best. Future studies should investigate the effects of genetic variation on FOLH1 expression and enzyme activity in both gray and white matter regions.
Animal studies have shown that blocking GCPII results in increases in brain NAAG levels (17–19, 48, 49), which is associated with improved cognition (18, 19) and decreases in schizophrenia-like behaviors (20, 50). However, the effects of differential NAAG levels on cognition have not previously been well studied in patients with schizophrenia or other disorders characterized by cognitive impairment. We found that subjects who had higher levels of NAAG in the CSO performed better on measures of visual memory. The effects of NAAG may be selective to visual memory performance or may correlate with other cognitive domains that we did not measure or measured in a way that did not capture the effect. It is interesting to note that the N-back task is a visual (working) memory task, similar to the tests used in the visual memory domain in the clinical cohort. Future studies will need to explore the effects of CSO NAAG levels on other cognitive tests, including other cognitive tasks during fMRI, in order to more conclusively determine the level of specificity, or not, of our findings in the visual memory domain. NAAG levels in CSO may correlate with visual memory because this measurement reflects general NAAG levels in cortical white matter and thus widespread integrity of the connections needed throughout the cortex for visual memory, whereas measurement of NAAG in other regions may correlate with other cognitive domains. Similarly, other investigators have reported a positive correlation between frontal lobe NAAG and NAAG/NAA ratio and measures of episodic memory in patients with schizophrenia (51), although they also reported higher NAAG levels in the ACC in patients with schizophrenia (N=20) compared with healthy subjects (N=20) (51). One study found that multiple sclerosis patients with cognitive impairment had lower hippocampal NAAG levels than multiple sclerosis patients without cognitive impairment (18) and a positive correlation between hippocampal NAAG/creatine and cognitive performance in multiple domains (18).
Here, we focused on the region providing the best NAAG measures (i.e., the CSO), because we did not have a specific hypothesis about a particular region, given that we were testing the effects on IQ and a composite score for cognition, as well as individual domains. Future studies will need to determine the effects of regional NAAG levels on cognition and symptoms of schizophrenia. NAAG levels in the DLPFC were positively associated with visual memory scores, although this finding may be less significant than associations with CSO NAAG, in part as a result of the greater variance in the NAAG estimates in the DLPFC. Recent data on monkeys have shown that mGluR3 receptors have an expansive role in primate DLPFC circuits governing cognition (4). In primates, mGluR3 receptors are expressed on both astrocytes and postsynaptically in spine synapses in the DLPFC (4). Increasing NAAG levels strengthens connectivity and greatly enhances persisting firing of DLPFC Delay cells needed for higher cognition (4). These data may be the mechanism behind our data showing that higher NAAG levels result in better cognitive performance in humans.
In order to determine the neural basis underlying the association between NAAG and working memory, we correlated FOLH1 genetics with cortical efficiency during working memory, using fMRI. The N-back working memory task (52) has been widely applied to study working memory dysfunction in schizophrenia (22, 23) and strongly engages a working memory neural network that includes the DLPFC and parietal cortex (53, 54) in a capacity-constrained response function (inverted U) (52). We found that carriers of this FOLH1 missense mutation had higher cortical activity during working memory. Because performance was similar between subjects (>80% accuracy) and did not differ between genotype groups, the working memory activity associated with the G allele was interpreted as inefficient (suboptimal) (23), because greater resources (activity) were required to reach the same level of performance. Increased cortical activity during working memory is a pattern of brain activation found in patients with schizophrenia (22) and their healthy siblings (23). These genetic-related inefficient activity patterns overlap with inefficient working memory responses from several other studies that found parietal, cuneus, and superior temporal hyperactivation during working memory in schizophrenia patients compared with healthy subjects (55–57). We have also shown that healthy subjects who carry SNPs associated with risk for schizophrenia have greater cortical activity during working memory, for genes including GRM3 (3), COMT (37), and CACNA1C (58). When investigating the correlation between genotype and working memory fMRI signals, it is common to find significance in a subset of the regions (58–60), rather than the entire network, as is the case here. While the block-design fMRI N-back task is advantageous for robust signals, it is difficult to make concrete functionality conclusions beyond those related to general working memory processes. Additionally, one study found that across a series of memory tasks, there was a linear increase in brain activity (measured by fMRI) with increasing age in areas that were normally decreased during task performance (e.g., the medial frontal and parietal regions), whereas activity in regions with task-related activation (e.g., the dorsolateral prefrontal cortex) decreased with age (61), suggesting a gradual, age-related reduction in the ability to suspend non-task-related or default-mode activity and engage areas involved in carrying out memory tasks. Cognitive dysfunction related to increased age may be similar in this way to what we have shown in patients with schizophrenia (10, 33, 34), shown by a shift in the balance between DLPFC activity and parietal activity, which could account for increased vulnerability to distraction from irrelevant information (61). This pattern of brain activity is consistent with weaker DLPFC connectivity (4) and a need for a more posterior cortical strategy (e.g., hyperactivity of the parietal cortex) to maintain a high working memory performance. In the present study, we found that healthy and psychotic subjects who are carriers of the FOLH1 variant that is associated with lower NAAG levels had a lower IQ score, which suggests that low NAAG levels could result in widespread cognitive dysfunction.
In addition to the cognitive effects reported here, this missense mutation has been associated with less severe negative symptoms (62) and greater reduction in negative symptoms following folic acid and vitamin B12 supplementation (63). Therefore, genetic variation in FOLH1 and related expression and NAAG concentration differences may be relevant not only to cognition in schizophrenia (and related potential therapeutics) but also to negative symptoms in schizophrenia (64). Future studies should test the relationship between NAAG levels and genetic variation in FOLH1 on negative symptoms in schizophrenia.
Consistent with the implications of these findings, previous research has suggested that increasing NAAG levels can improve cognition. One study showed that treatment with growth hormone-releasing hormone (GHRH) increases brain levels of NAAG and GABA, but not glutamate levels, in the DLPFC in older adults with mild cognitive impairment and cognitively intact older adults (65). The study also found an improvement in the cognitive domains of executive function and verbal memory, which may be related to the increased NAAG and/or GABA levels (65) or another aspect of GHRH actions. Inhibitors of GCPII/FOLH1 have been shown to increase neural NAAG levels and improve learning and memory in animal models (18, 19) but are not currently available for clinical use. We focused on NAAG because it is a substrate for mGluR3 (unlike NAA), and NAAG levels are primarily regulated by FOLH1/GCPII. We found that CSO NAA levels are not associated with cognitive performance, but the ratio of NAAG to NAA is associated with visual memory performance.
Before introducing a GCPII antagonist into a patient population, it is imperative to fully ascertain all the nuances of all the variables (e.g., cognitive domain, metabolite measurements, sample size), considering not only our findings but also synthesizing relevant findings from previous as well as future studies. It is important to develop a better understanding of how modulating NAAG levels (e.g., with a GCPII inhibitor) (20, 50, 66–68) will affect cognitive performance and functional neural activity in humans. Our study provides insight regarding the neurobiological impact of NAAG modulation, including which circuits are most likely to be affected and could serve as biomarkers in clinical trials of GCPII inhibitors. Measuring NAAG levels using MRS, cognitive function, and neural activation using fMRI in a battery of tasks in a large cohort of the same patients will be prudent. Additionally, future studies should test the relationship between differential FOLH1/GCPII and NAAG results and measures of cognition and/or other symptoms and behaviors in patients with schizophrenia, as well as other causes of cognitive impairment and/or decline.
1 : Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2004; 174:39–44Crossref, Medline, Google Scholar
2 : Pharmacogenetic associations of the type-3 metabotropic glutamate receptor (GRM3) gene with working memory and clinical symptom response to antipsychotics in first-episode schizophrenia. Psychopharmacology (Berl) 2015; 232:145–154Crossref, Medline, Google Scholar
3 : Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci USA 2004; 101:12604–12609Crossref, Medline, Google Scholar
4 : mGluR2 versus mGluR3 metabotropic glutamate receptors in primate dorsolateral prefrontal cortex: postsynaptic mGluR3 strengthen working memory networks. Cereb Cortex 2018; 28:974–987Crossref, Medline, Google Scholar
5 : mGluR2/3 mechanisms in primate dorsolateral prefrontal cortex: evidence for both presynaptic and postsynaptic actions. Mol Psychiatry 2017; 22:1615–1625Crossref, Medline, Google Scholar
6 : Evaluation of relationship between GRM3 polymorphisms and cognitive function in schizophrenia of Han Chinese. Psychiatry Res 2015; 229:1043–1046Crossref, Medline, Google Scholar
7 : mGluR3 knockout mice show a working memory defect and an enhanced response to MK-801 in the T- and Y-maze cognitive tests. Behav Brain Res 2014; 266:94–103Crossref, Medline, Google Scholar
8 Schizophrenia Working Group of the Psychiatric Genomics Consortium: Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511:421–427Crossref, Medline, Google Scholar
9 : Meta-analysis supports GWAS-implicated link between GRM3 and schizophrenia risk. Transl Psychiatry 2017; 7:
10 : N-acetylaspartylglutamate is an agonist at mGluR3 in vivo and in vitro. J Neurochem 2011; 119:891–895Crossref, Medline, Google Scholar
11 : Differential effects of N-acetyl-aspartyl-glutamate on synaptic and extrasynaptic NMDA receptors are subunit- and pH-dependent in the CA1 region of the mouse hippocampus. Neurobiol Dis 2015; 82:580–592Crossref, Medline, Google Scholar
12 : Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 1998; 281:1349–1352Crossref, Medline, Google Scholar
13 : The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther 1999; 291:161–170Medline, Google Scholar
14 : A double-blind, placebo-controlled comparator study of LY2140023 monohydrate in patients with schizophrenia. BMC Psychiatry 2014; 14:351Crossref, Medline, Google Scholar
15 : Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate: subcellular and regional distribution, ontogeny, and the effect of lesions on N-acetylated-alpha-linked acidic dipeptidase activity. J Neurochem 1988; 50:1200–1209Crossref, Medline, Google Scholar
16 : Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate: identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. J Biol Chem 1987; 262:14498–14506Medline, Google Scholar
17 : Effects of NAAG peptidase inhibitor 2-PMPA in model chronic pain - relation to brain concentration. Neuropharmacology 2006; 51:1163–1171Crossref, Medline, Google Scholar
18 : Inhibition of glutamate carboxypeptidase II (GCPII) activity as a treatment for cognitive impairment in multiple sclerosis. Proc Natl Acad Sci USA 2012; 109:20101–20106Crossref, Medline, Google Scholar
19 : Dose-dependent inhibition of GCPII to prevent and treat cognitive impairment in the EAE model of multiple sclerosis. Brain Res 2016; 1635:105–112Crossref, Medline, Google Scholar
20 : NAAG peptidase inhibitors block cognitive deficit induced by MK-801 and motor activation induced by d-amphetamine in animal models of schizophrenia. Transl Psychiatry 2012; 2:
21 : A role for N-acetylaspartylglutamate (NAAG) and mGluR3 in cognition. Neurobiol Learn Mem 2019; 158:9–13Crossref, Medline, Google Scholar
22 : Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 10:1078–1092Crossref, Medline, Google Scholar
23 : Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry 2003; 160:2209–2215Link, Google Scholar
24 : Critical factors in gene expression in postmortem human brain: focus on studies in schizophrenia. Biol Psychiatry 2006; 60:650–658Crossref, Medline, Google Scholar
25 : Developmental and genetic regulation of the human cortex transcriptome illuminate schizophrenia pathogenesis. Nat Neurosci 2018; 21:1117–1125Crossref, Medline, Google Scholar
26 : Olfactory functioning in first-episode psychosis. Schizophr Bull 2018; 44:672–680Crossref, Medline, Google Scholar
27 : Assessing brain metabolism with 7-T proton magnetic resonance spectroscopy in patients with first-episode psychosis. JAMA Psychiatry 2019; 76:314–323Crossref, Medline, Google Scholar
28 : Reduced superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with early psychosis in association with clinical features. Schizophr Res 2017; 183:64–69Crossref, Medline, Google Scholar
29 : Decoupling of N-acetyl-aspartate and glutamate within the dorsolateral prefrontal cortex in schizophrenia. Curr Mol Med 2015; 15:176–183Crossref, Medline, Google Scholar
30 : Neuropsychological functioning in bipolar disorder and schizophrenia. Biol Psychiatry 2007; 62:179–186Crossref, Medline, Google Scholar
31 : Confirmatory factor analysis reveals a latent cognitive structure common to bipolar disorder, schizophrenia, and normal controls. Bipolar Disord 2013; 15:422–433Crossref, Medline, Google Scholar
32 : Hierarchical structure of the cognitive processes in schizophrenia: the fundamental role of processing speed. Schizophr Res 2012; 135:72–78Crossref, Medline, Google Scholar
33 : The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2010; 16:6–16Crossref, Medline, Google Scholar
34 : Accounting for estimated IQ in neuropsychological test performance with regression-based techniques. J Int Neuropsychol Soc 2009; 15:1012–1022Crossref, Medline, Google Scholar
35 : Development, psychometric properties, and validity of the Hopkins Adult Reading Test (HART). Clin Neuropsychol 2009; 23:926–943Crossref, Medline, Google Scholar
36 : Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry 2000; 157:1309–1316Link, Google Scholar
37 : Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 2001; 98:6917–6922Crossref, Medline, Google Scholar
38 : Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry 2003; 160:709–719Link, Google Scholar
39 : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
40 : N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem 2000; 75:443–452Crossref, Medline, Google Scholar
41 : Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: a mechanism for antipsychotic drug action? Am J Psychiatry 2009; 166:812–820Link, Google Scholar
42 : Glutamate carboxypeptidase II gene expression in the human frontal and temporal lobe in schizophrenia. Neuropsychopharmacology 2004; 29:117–125Crossref, Medline, Google Scholar
43 : Dysregulation of glutamate carboxypeptidase II in psychiatric disease. Schizophr Res 2008; 99:324–332Crossref, Medline, Google Scholar
44 : Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry 1995; 52:829–836Crossref, Medline, Google Scholar
45 : Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed 1997; 10:73–78Crossref, Medline, Google Scholar
46 : Regulation of NAA-synthesis in the human brain in vivo: Canavan’s disease, Alzheimer’s disease and schizophrenia. Adv Exp Med Biol 2006; 576:263–273Crossref, Medline, Google Scholar
47 : Molecular characterization of N-acetylaspartylglutamate synthetase. J Biol Chem 2010; 285:29156–29164Crossref, Medline, Google Scholar
48 : GCP II (NAALADase) inhibition suppresses mossy fiber-CA3 synaptic neurotransmission by a presynaptic mechanism. J Neurophysiol 2004; 91:182–193Crossref, Medline, Google Scholar
49 : NAAG peptidase inhibition in the periaqueductal gray and rostral ventromedial medulla reduces flinching in the formalin model of inflammation. Mol Pain 2012; 8:67Crossref, Medline, Google Scholar
50 : Phencyclidine and dizocilpine induced behaviors reduced by N-acetylaspartylglutamate peptidase inhibition via metabotropic glutamate receptors. Biol Psychiatry 2008; 63:86–91Crossref, Medline, Google Scholar
51 : N-acetylaspartylglutamate (NAAG) and N-acetylaspartate (NAA) in patients with schizophrenia. Schizophr Bull 2013; 39:197–205Crossref, Medline, Google Scholar
52 : Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex 1999; 9:20–26Crossref, Medline, Google Scholar
53 : N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 2005; 25:46–59Crossref, Medline, Google Scholar
54 : Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage 2000; 12:268–275Crossref, Medline, Google Scholar
55 : A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry 2006; 60:11–21Crossref, Medline, Google Scholar
56 : Cerebral inefficient activation in schizophrenia patients and their unaffected parents during the N-back working memory task: a family fMRI study. PLoS One 2015; 10:
57 : Mechanisms of working memory impairment in schizophrenia. Biol Psychiatry 2016; 80:617–626Crossref, Medline, Google Scholar
58 : Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry 2010; 67:939–945Crossref, Medline, Google Scholar
59 : Characteristics of the cation cotransporter NKCC1 in human brain: alternate transcripts, expression in development, and potential relationships to brain function and schizophrenia. J Neurosci 2014; 34:4929–4940Crossref, Medline, Google Scholar
60 : Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch Gen Psychiatry 2010; 67:991–1001Crossref, Medline, Google Scholar
61 : Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci 2006; 18:227–241Crossref, Medline, Google Scholar
62 : Genetic variation throughout the folate metabolic pathway influences negative symptom severity in schizophrenia. Schizophr Bull 2013; 39:330–338Crossref, Medline, Google Scholar
63 : Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry 2013; 70:481–489Crossref, Medline, Google Scholar
64 : Folic acid/methylfolate for the treatment of psychopathology in schizophrenia: a systematic review and meta-analysis. Psychopharmacology (Berl) 2018; 235:2303–2314Crossref, Medline, Google Scholar
65 : Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging. JAMA Neurol 2013; 70:883–890Crossref, Medline, Google Scholar
66 : NAAG peptidase inhibition reduces locomotor activity and some stereotypes in the PCP model of schizophrenia via group II mGluR. J Neurochem 2004; 89:876–885Crossref, Medline, Google Scholar
67 : mGluR3 and not mGluR2 receptors mediate the efficacy of NAAG peptidase inhibitor in validated model of schizophrenia. Schizophr Res 2012; 136:160–161Crossref, Medline, Google Scholar
68 : Effects of N-acetylaspartylglutamate (NAAG) peptidase inhibition on release of glutamate and dopamine in prefrontal cortex and nucleus accumbens in phencyclidine model of schizophrenia. J Biol Chem 2012; 287:21773–21782Crossref, Medline, Google Scholar
69 : Critical factors in gene expression in postmortem human brain: Focus on studies in schizophrenia. Biol Psychiatry 2006; 60:650–658Crossref, Medline, Google Scholar
70 : NAAG peptidase inhibitors block cognitive deficit induced by MK-801 and motor activation induced by d-amphetamine in animal models of schizophrenia. Transl Psychiatry 2012; 2:



