Features of Structural Brain Abnormality Detected in First-Episode Psychosis
Abstract
OBJECTIVE: Structural magnetic resonance imaging (MRI) studies that focus on first-episode psychosis avoid some common confounds, such as chronicity of illness, treatment effects, and long-term substance abuse. However, such studies may select subjects with poor short-term treatment response or outcome. In this study, the authors focus on structural brain abnormalities in never or minimally treated patients who underwent MRI scanning early in their first episode of psychosis. METHOD: The authors examined 37 patients (13 medication naive, 24 previously treated) who were experiencing their first episode of psychosis; the mean duration of symptoms was short (31 weeks). These patients were comparable in age, gender, handedness, ethnicity, and parental socioeconomic status to a group of 25 healthy comparison subjects. A three-dimensional, inversion recovery prepared, fast spoiled gradient/recall in the steady state scan of the whole brain that used 1.5-mm contiguous sections was performed to acquire a T1-weighted data set. Human ratings of volumetric measurement of brain structures were performed with stereological techniques on three-dimensional reconstructed MRIs. RESULTS: The patient group had significant deficits in cortical gray matter, temporal lobe gray matter, and whole brain volume as well as significant enlargement of the lateral and third ventricles. Structural deviations were found in both treatment-naive and minimally treated subjects. No relationships were found between any brain matter volumes and positive or negative symptoms. CONCLUSIONS: Structural brain abnormalities were distributed throughout the cortex with particular decrement evident in gray matter. This feature is consistent with altered cell structure and disturbed neuronal connectivity, which accounts for the functional abnormality of psychosis.
Naturalistic studies indicate that the clinical progression of schizophrenia occurs in the early phase of the illness, predominantly in the first 5 years after onset (1, 2). Evaluation of brain structure during the first episode of psychosis is a powerful strategy for investigating fundamental questions about the disorder. Investigating patients early in the course of their illness identifies abnormalities that are relevant to the disease process itself. The first-episode design avoids confounding by the chronicity of the illness, longstanding substance abuse, and the effects of treatment (3). Furthermore, given that a substantial proportion of these patients will not experience another psychotic episode within 5 years (1), this type of study provides more generalizable results about the nature of the disorder than studies of chronic patients.
It is evident that the heterogeneity observed in the course and outcome of schizophrenia extends also to the pattern of structural brain abnormalities found in magnetic resonance imaging (MRI) studies. Although it is accepted that structural brain abnormalities play an important role in the pathology of schizophrenia, their relevance to the neurophysiology of the disease process is uncertain. The number of structural MRI studies in first-episode psychosis is still relatively small. Most have shown a similar pattern of cortical abnormalities to samples of chronic patients, although deficits may be less extensive (4). As is the case with studies of chronic patients, the most replicated finding is lateral ventricle enlargement (4–10), with fewer negative studies (11, 12). Third ventricle enlargement has more positive (7, 9, 12) than negative (6) reports. Of five studies of frontal lobe volume, three reported reduced volume (8, 13, 14), whereas two found no abnormality (6, 15). The temporal lobe has been examined most often in first-episode studies, with positive findings being shown in the medial areas and superior temporal gyrus (5, 10, 12–14, 16–19). However, studies of the whole temporal lobe show more negative (6, 8, 15) than positive (14) findings. Parietal and occipital lobe volumes have been reported as being normal (8, 15). The majority of first-episode studies fail to show any whole brain volume changes (6, 8, 10, 12, 17, 18), although such changes have been reported recently (14). Studies of subcortical structures also show abnormalities (e.g., references 20, 21). Deficits in gray matter (4) and reduced gray matter volume with normal white matter (9) have been shown in studies of total cortical volume that have been published to date. Inconsistencies remain as to whether there exists a pattern of diffuse abnormalities or selective deficits (22) in the brains of patients with schizophrenia and whether these abnormalities change over time (23). It is clear from the existing literature that the cortex in patients with schizophrenia is characterized by subtle pathology that is as yet not understood. Neither neurodegenerative processes or cell loss adequately define this neuropathology. Distributed gray matter malformation may be of greater relevance, although this has not been comprehensively assessed by high-resolution MRI in first-episode patients.
We hypothesized that structural brain abnormalities would be found when a high-resolution scanning technique and a rigorous stereologically unbiased methodology of volume estimation were used to examine a group of patients experiencing their first episode of psychosis and who had never or had just initiated medication treatment. We wished to determine whether such abnormalities are associated with distributed cortical pathology and occur in multiple sites independent of the effects of medication, chronic illness, age, gender, ethnicity, or socioeconomic factors. We hypothesized that gray matter deficit would be a prominent feature of abnormal brain structure in this group.
Method
Thirty-seven patients (26 men and 11 women) between 16 and 40 years of age were recruited as inpatients or outpatients from United Kingdom catchment areas comprising East, Southeast, and Southwest London and West Kent. Patients met DSM-IV criteria for schizophrenia (N=15), schizophreniform disorder (N=18), or schizoaffective disorder (N=4) as determined by the Structured Clinical Interview for DSM-IV (SCID) (24). Diagnostic interviews were performed by qualified research psychiatrists who had been trained in the administration of the SCID. All patients were experiencing their first episode of psychosis and had received no more than 12 weeks of previous neuroleptic medication exposure. Twenty-five comparison subjects (17 men and eight women) were recruited from the general population in the same catchment areas and were screened with the nonpatient version of the SCID (25). Patients and comparison subjects were similar in age (within 3 years), gender, ethnicity, and parental socioeconomic status. Both ethnicity and parental socioeconomic status were assessed by using standard classifications (26). Handedness was determined by using the Edinburgh Inventory (27). Subjects who had a DSM-IV diagnosis of substance abuse or dependence, mental retardation, neurological disorder, or a medical condition that could have affected brain structure or function were excluded from the study. In addition, comparison subjects were excluded if there was a personal history of any axis I or II disorder or a family history of psychosis. The duration of psychosis was calculated from the time of onset of first psychotic symptoms (based on patient interview, corroborative history from family members, and medical records when available) to the time of MRI scanning. Symptom measures were performed by using the Positive and Negative Syndrome Scale (28), which yielded good interrater reliability (kappa=0.87, SD=0.07). Ethical approval was obtained from the Bethlem and Maudsley ethics committee for research. After a complete description of the proposed study was given to each participant, written informed consent was obtained.
Image Acquisition
MRI scanning was performed on a 1.5-T Signa system (GE Medical Systems, Milwaukee) at the Maudsley Hospital in London. Initially, a series of sagittal and axial scout views were acquired to correct for head tilt and to localize imaging coordinates. Next, a three-dimensional, inversion recovery prepared, fast spoiled gradient/recall in the steady state scan of the whole brain was performed to acquire a T1-weighted data set. These T1-weighted images were obtained in the axial plane (parallel to the z axis of the magnet) with 1.5-mm contiguous sections (TR=11.3 msec, TI=300 msec, TE=2.2 msec, and the flip angle=20° with one data average and a 256 × 256 × 128 pixel matrix).
Measurements and Regions of Interest
The regions of interest used the landmarks outlined by DeLisi et al. (23) for whole brain volume, cortical gray matter, lateral ventricles, and temporal lobes. The third ventricular volume was that bounded by the anterior commissure, the fornix, the stria medullaris, the pineal body, the superior and inferior colliculi, the midbrain and mamillary body, the thalamus, and hypothalamus.
Brain images were reoriented parallel to the anterior-posterior commissure line and the interhemispheric fissure before measurement. Volumetric measurements were obtained by stereological assessment by using the MEASURE program (29). This methodology has been employed in previous studies (30, 31), and its validation has been previously described (29). A human rater used three orthogonal views (axial, coronal, and sagittal) and a three-dimensional grid of rotated L-shaped points superimposed on the images. The optimal grid size was based on the Cavalieri principle and calculated by using the Gundersen formula (32). Using the grid, the rater “painted” the brain matter in all three views for each region of interest. Pixels marked in one plane are automatically marked in the other two. The MEASURE program calculates volume by counting the total number of “painted” pixels within the region of interest. Grid points on the boundary of a structure are marked as within the structure only if the pixel within the acute angle of the L-shaped point is inside the structure. The use of three-dimensional reconstructed images allows for viewing from any orientation, thereby improving boundary recognition compared to single-plane techniques (33). The manual “painting” of structures reduces the confounds of MRI artifacts and partial volume effects. Two of the authors (A.S., J.L.), blind to diagnosis, performed all ratings and demonstrated high intrarater and interrater reliability. Intraclass correlations ranged from 0.90 to 0.98 for all measures.
Statistical Analysis
Data analysis was performed by using SPSS (Chicago). Chi-square analyses were used to look for differences in socioeconomic status, handedness, gender distribution, and ethnicity. Analyses of variance (ANOVA) were used to examine differences in age, years of education, and height. Between-group comparisons of regional volumes were performed by using the general linear model in SPSS. Analysis of covariance (ANCOVA) used total brain volume or regional volume as dependent variables and height, age, parental socioeconomic status, sex, and diagnosis as independent variables. No direct measure of head size was obtained. Age was used to control for possible age-related changes in the subjects (34). Height was used as the independent variable to control for body and head size. Height has been shown to be a predictor of general head size (35) and has been previously used in MRI studies of schizophrenia (8, 15). We compared previously treated and medication-naive patients in terms of regional brain volume. Pearson correlations were used to test for relationships between brain volume measures and age at onset, duration of psychosis, and duration of untreated psychosis. All analyses used two-tailed estimation of significance. The significance level applied to the data was set at p<0.05.
Results
The mean duration of the first psychotic episode was 31.3 weeks (SD=25.0). The mean duration of untreated psychosis was 28.1 weeks (SD=26.0). Thirteen patients (35.1%) had never received antipsychotic treatment at the time of scanning. The remaining patients had been treated for a mean duration of 4.6 weeks (SD=3.0). The mean age at onset of the psychotic episode was 23.8 years (SD=5.5, mode=18).
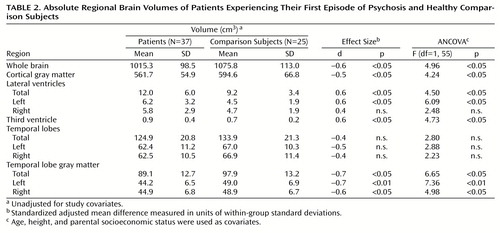
Table 1 shows comparative demographic variables for the patients experiencing their first psychotic episode and the comparison subjects. There were no differences between the groups for height, age, parental socioeconomic status, handedness, gender, or ethnicity. The patients experiencing their first psychotic episode had significantly fewer years of education than did the comparison subjects.
Table 2 shows absolute regional volumes in the groups. Figure 1 and Figure 1 show adjusted mean brain volumes in both groups. Significant differences were found after covarying for height. These differences remained after additional covariation with age and parental socioeconomic status. The patients with first-episode psychosis had significantly lower whole brain volume, cortical gray matter volume, and temporal lobe gray matter volume and significantly enlarged lateral and third ventricular volumes. The left temporal lobe gray matter reduction was particularly marked (ANCOVA F=7.36, df=1, 55, p<0.009). There were no differences in total or left or right temporal lobe volumes and no effect of medication status on any volume difference (ANCOVA F<2, df=1, 61, p>0.20 for all). Male subjects (both patients and comparison subjects) had larger brain and CSF volumes than female subjects, but there was no significant sex-by-diagnosis interaction for any volume measurement. Of the 12 regions of interest examined, eight were found to be significantly different at the p<0.05 level.
No correlations were found between any symptom measure and region of interest volumes (r<0.20, df=35, p>0.10 for all). Specifically, no relationship was found between positive symptoms, negative symptoms, or general psychopathology and temporal lobe volumes (r<0.20, df=35, p>0.10 for all). There were no relationships between duration of psychosis (treated or untreated) or age at onset and regional brain volumes.
Discussion
This study shows that significant deficits in cortical gray matter, temporal lobe gray matter, and reduced whole brain volume are present early in the course of first-episode psychosis in association with significant enlargement of the lateral and third ventricles. Structural deviations were found in both treatment-naive and minimally treated subjects. These findings represent marked, dispersed abnormalities in patients with no or minimal medication exposure (duration of treatment: mean=4.6 weeks, SD=3.0). This study group is of interest given the inclusion of the 13 medication-naive subjects (35% of the patient group) and the high-resolution volumetric analysis of a range of brain regions. As a comprehensive examination of brain structure close to the onset of psychosis in a clinically representative cohort, this study contributes to a relatively sparse literature on structural MRI in this patient group. The detection of dispersed structural deficits in subjects with these clinical characteristics confirms the view that such findings cannot be attributed to the effects of medication or disease chronicity.
Our findings are in keeping with earlier studies of patients in their first episode of schizophrenia showing, as would be expected, larger lateral (4–10) and third ventricles (7, 9, 12). Failure to detect enlargement of a structure as small as the third ventricle may be accounted for by the large slice thickness used in the single negative study (6). Enlargement of the third ventricle in the absence of lateral ventriculomegaly was found in a study of 20 first-episode schizophrenia patients and subjects at high risk of developing the illness relative to comparison subjects (12). Although a trend toward lateral ventricular enlargement was evident, that study was limited by the sample size. Our findings of both whole brain volume reduction and temporal lobe gray matter deficit are of interest in the context of existing studies. A recent study of schizophrenic subjects also showed reduced whole brain volume in addition to frontal and temporal lobe decrements at baseline (14). Our finding of significant cortical gray matter deficit both underlines this finding in patients with first-episode psychosis and strengthens it by the use of thinner contiguous slices than in earlier work (4, 9) and by the inclusion of medication-naive subjects and a larger cohort size (9).
Analysis of neuropsychological function in this group of first-episode patients, presented elsewhere (36), showed significant impairments on tasks of executive function, verbal learning, delayed recall from nonverbal memory, and psychomotor speed against a background of generalized underperformance on a wide range of neuropsychological functions. These findings are consistent with pathological involvement of language and verbal learning-related areas, which are thought to include the left temporal lobe as highlighted in recent work in first-episode patients (17). Cortical gray matter abnormality was conspicuous both in the temporal lobes and throughout the cortex in our study group. The presence of generalized neuropsychological differences between patients and comparison subjects and overall smaller gray matter volume suggest nonlocalized alteration of cell structure and disturbed neuronal connectivity. Such a model is characterized by putative pathology in a distributed neural network (37), which accounts for the functional abnormalities associated with psychosis. Although no causal associations have been proven between structural findings and functional deficits in schizophrenia, numerous lines of evidence are pertinent. Neuropathological studies have reported cytoarchitectural abnormalities and synaptic alterations in patients with schizophrenia, which may account for our finding of gray matter deficits (38). Diminished gray matter volume associated with reduced neuron size and increased neuronal density may reflect reduced synaptic number (39). This may arise through a process of excessive synaptic pruning (40).
The duration of psychotic symptoms and mean duration of antipsychotic treatment were short, yet the patients showed significant gray matter and CSF differences relative to comparison subjects. No associations were found between duration of illness or onset age and any regional volume. We found no relationships between symptoms and brain structure. In particular, there was no symptom association with temporal lobe deficit. This has a number of possible explanations. Our total temporal lobe measures may have lacked the specificity to detect a relationship between symptoms and superior temporal gyrus volume (which may be more selectively involved in the psychotic state). Alternatively, psychotic symptoms generated by a distributed network-wide deficiency associated with reduced gray matter are likely to show a more complex structure-function relationship with regional brain volume.
This study has a number of strengths compared to other first-episode work. The patients included both inpatients and those treated in the community, whereas other studies (e.g., references 4, 6–9, 11, 15, 19) looking at this group have focused on hospitalized or tertiary referral subjects. Consequently, we feel that this sample is representative of the first-episode schizophrenia spectrum population. We have demonstrated that patients presenting in a community setting have brain structural deficits. Although there were no significant group differences in age or parental socioeconomic status, the absence of differences does not exclude confounding by these factors. Therefore, these variables were also used as covariates. Some factors constrain the interpretation of these results. Not all potential subjects from each catchment area were recruited to this study, but an ethnically and sociodemographically representative sample was examined relative to a very similar comparison group. The first-episode design is limited by the fact that the stability of diagnoses is unproven and requires longitudinal follow-up for confirmation. This is currently in progress.
In conclusion, this study has demonstrated quantitative gray matter and CSF abnormalities in patients with first-episode psychosis. These abnormalities are present close to the onset of illness and are not attributable to the effects of chronicity of illness or medication. The presence of dispersed gray matter abnormalities underlines their relevance to the neurobiology of schizophrenia and adds credence to a model of the syndrome characterized by a network-wide alteration of neuronal structure and connectivity.
 |
 |
Received Nov. 30, 1999; revisions received April 3 and June 27, 2000; accepted June 30, 2000. From the Section of Cognitive Psychopharmacology, Institute of Psychiatry. Address reprint requests to Dr. Fannon, Department of Psychiatry, Institute of Psychiatry, Denmark Hill, London SE5 8AF, U.K.; [email protected] (e-mail).Support for this research was provided by Grosvenor House Group Estates and PsychMed Ltd.The authors thank Katy Piper, Jessica Sheringham, Steven Williams, Andrew Simmons, and the staff of the Institute of Psychiatry/Maudsley Hospital Neuroimaging Research Group for their collaboration.
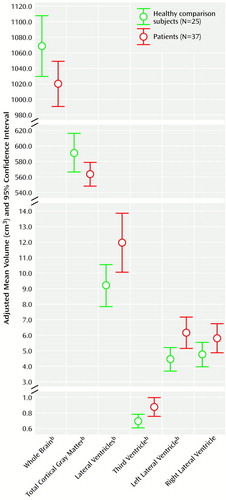
Figure 1. Adjusted Whole Brain, Cortical Gray Matter, and Ventricle Volumes of Patients Experiencing Their First Episode of Psychosis and Healthy Comparison Subjectsa
aVolumes were adjusted for height, age, and parental socioeconomic status.
bSignificant difference between groups (see Table 2 for ANCOVA results).
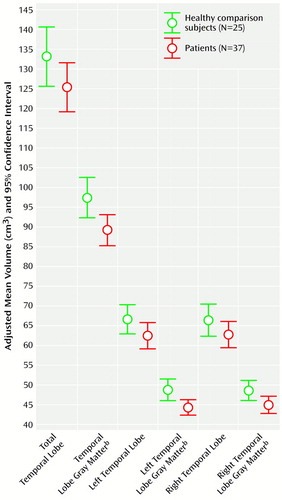
Figure 2. Adjusted Temporal Lobe and Temporal Lobe Gray Matter Volumes of Patients Experiencing Their First Episode of Psychosis and Healthy Comparison Subjectsa
aVolumes were adjusted for height, age, and parental socioeconomic status.
bSignificant difference between groups (see Table 2 for ANCOVA results).
1. Mason P, Harrison G, Glazebrook C, Medley I, Croudace T: The course of schizophrenia over 13 years: a report from the International Study on Schizophrenia (ISoS) coordinated by the World Health Organization. Br J Psychiatry 1996; 169:580–586Crossref, Medline, Google Scholar
2. McGlashan TH: A selective review of recent North American long term follow-up studies of schizophrenia. Schizophr Bull 1988; 14:515–542Crossref, Medline, Google Scholar
3. Hafner H, Hambrecht M, Loffler W, Munk-Jorgensen P, Riecher-Rossler A: Is schizophrenia a disorder of all ages? a comparison of first episodes and early course across the life cycle. Psychol Med 1998, 28:351–365Google Scholar
4. Zipursky RB, Lambe EK, Kapur SK, Mikulis DJ: Cerebral gray matter volume deficits in first episode psychosis. Arch Gen Psychiatry 1998; 55:540–546Crossref, Medline, Google Scholar
5. Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA: Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res 1990; 35:1–13Crossref, Medline, Google Scholar
6. DeLisi LE, Hoff AL, Schwartz JE, Shields GW, Halthore SN, Gupta SM, Henn FA, Anand AK: Brain morphology in first-episode schizophrenic-like psychotic patients: a quantitative magnetic resonance imaging. Biol Psychiatry 1991; 29:159–175Crossref, Medline, Google Scholar
7. Degreef G, Ashtari M, Bogerts B, Bilder RM, Jody DN, Alvir JM, Lieberman JA: Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Arch Gen Psychiatry 1992; 49:531–537Crossref, Medline, Google Scholar
8. Nopoulos P, Torres I, Flaum M, Andreasen NC, Ehrhardt JC, Yuh WTC: Brain morphology in first-episode schizophrenia. Am J Psychiatry 1995; 152:1721–1723Google Scholar
9. Lim KO, Tew W, Kushner M, Chow K, Matsumoto B, DeLisi LE: Cortical gray matter volume deficit in patients with first-episode schizophrenia. Am J Psychiatry 1996; 153:1548–1553Google Scholar
10. Whitworth AB, Honeder M, Kremser C, Kemmler G, Felber S, Hausmann A, Wanko C, Wechdorn H, Aichner F, Stuppaeck CH, Fleischhacker WW: Hippocampal volume reduction in male schizophrenic patients. Schizophr Res 1998; 31:73–81Crossref, Medline, Google Scholar
11. DeLisi LE, Strizke P, Riordan H, Holan V, Boccio A, Kushner M, McClelland J, Van Eyl O, Anand A: The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry 1992; 31:241–254; correction, 31:1172Google Scholar
12. Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC: Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet 1999; 353:30–33Crossref, Medline, Google Scholar
13. Ohnuma T, Kimura M, Takahashi T, Iwamoto N, Arai H: A magnetic resonance imaging study in first-episode disorganized-type patients with schizophrenia. Psychiatry Clin Neurosci 1997; 51:9–15Crossref, Medline, Google Scholar
14. Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC: A follow up magnetic resonance imaging study of schizophrenia: relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry 1998; 55:145–152Crossref, Medline, Google Scholar
15. Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JMJ, Snyder PJ, Lieberman JA: Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry 1994; 151:1437–1447Google Scholar
16. Fukuzako H, Kodama S, Fukuzako T, Yamada K, Hokazono Y, Ueyama K, Hashiguchi T, Takenouchi K, Takigawa M, Takeuchi K, Manchanda S: Shortening of the hippocampal formation in first-episode schizophrenic patients. Psychiatry Clin Neurosci 1995; 49:157–161Crossref, Medline, Google Scholar
17. Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley RW: Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 1998; 155:1384–1391Google Scholar
18. Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, Desmond P, Bridle N, Tierney P, Murrie V, Singh B, Copolov D: Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry 1999; 56:133–141Crossref, Medline, Google Scholar
19. Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun-Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW: Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry 1999; 156:1091–1093Google Scholar
20. DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R: Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res 1997; 74:129–140Crossref, Medline, Google Scholar
21. Corson PW, Nopoulos P, Andreasen NC, Heckel D, Arndt S: Caudate size in first-episode neuroleptic-naive schizophrenic patients measured using an artificial neural network. Biol Psychiatry 1999; 46:712–720Crossref, Medline, Google Scholar
22. Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A: Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry 1992; 49:194–205Crossref, Google Scholar
23. DeLisi LE, Tew W, Xie S, Hoff AL, Sakuma M, Kushner M, Lee G, Shedlack K, Smith AM, Grimson R: A prospective follow-up study of brain morphology and cognition in first episode schizophrenic patients: preliminary findings. Biol Psychiatry 1995; 38:349–360Crossref, Medline, Google Scholar
24. Spitzer RL, Williams JBW, Gibbon M: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
25. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID-I/NP), version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
26. Office of Population Censuses and Surveys: Classification of Occupations and Classification of Ethnicity. London, Her Majesty’s Stationery Office, 1991Google Scholar
27. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
28. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
29. Barta PE, Dhingra L, Royall R, Schwartz E: Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. J Neurosci Methods 1997; 75:111–118Crossref, Medline, Google Scholar
30. Frangou S, Sharma T, Sigmudsson T, Barta P, Pearlson G, Murray RM: The Maudsley Family Study 4: normal planum temporale asymmetry in familial schizophrenia: a volumetric MRI study. Br J Psychiatry 1997; 170:328–333Crossref, Medline, Google Scholar
31. Buchanan RW, Vladar K, Barta PE, Pearlson GD: Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry 1998; 155:1049–1055Google Scholar
32. Gundersen HJ, Jensen EB: The efficacy of systematic sampling in stereology and its prediction. J Microsc 1987; 147:227–263Crossref, Google Scholar
33. Mayhew TM: A review of recent advances in stereology for quantifying neural structure. J Neurocytol 1982; 21:313–328Crossref, Google Scholar
34. Cowell C, Turetsky BT, Gur RC, Grossman RI, Shtasel DL, Gur RE: Sex differences in aging of the human frontal and temporal lobe. J Neurosci 1994; 14:4748–4755Google Scholar
35. Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V II, O’Leary DS, Ehrhardt JC, Yuh WTC: Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA 1994; 272:1763–1769Google Scholar
36. Riley EM, McGovern D, Mockler D, Doku VCK, O’Ceallaigh S, Fannon DG, Tennakoon L, Santamaria M, Soni W, Morris RG, Sharma T: Neuropsychological functioning in first-episode psychosis—evidence of specific deficits. Schizophr Res 2000; 43:47–55Crossref, Medline, Google Scholar
37. Bullmore ET, Woodruff PW, Wright IC, Rabe-Hesketh S, Howard RJ, Shuriquie N, Murray RM: Does dysplasia cause anatomical dysconnectivity in schizophrenia? Schizophr Res 1998; 30:127–135Google Scholar
38. Harrison P: The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain 1999; 122:593–624Crossref, Medline, Google Scholar
39. Selemon LD, Goldman-Rakic PS: The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45:17–25Crossref, Medline, Google Scholar
40. Keshavan M, Anderson S, Pettegrew J: Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? the Feinberg hypothesis revisited. J Psychiatry Res 1994; 28:239–265Crossref, Medline, Google Scholar



