Serotonin Transporter Distribution and Density in the Cerebral Cortex of Alcoholic and Nonalcoholic Comparison Subjects: A Whole-Hemisphere Autoradiography Study
Abstract
OBJECTIVE: Lesions of the medial prefrontal cortex and dysfunctions in serotonin turnover are two well-established factors associated with impulsive and sociopathic behaviors, but no firm neuroanatomical data have linked these pathophysiological findings. The aims of this study were to identify putative areas in the human brain that are rich in serotonin transporter sites, particularly within the medial prefrontal cortex, and to determine whether serotonin transporter density in this area is altered among alcoholic subjects. METHOD: Serotonin transporter density was measured among 17 alcoholic and 10 nonalcoholic comparison subjects by postmortem whole-hemisphere autoradiography with [3H]citalopram. RESULTS: In the human cerebral cortex, serotonin transporter binding sites were concentrated in the perigenual anterior cingulate cortex. Substantially sparser serotonin transporter density (up to 35%) was observed in the perigenual anterior cingulate cortex of alcoholic subjects in relation to nonalcoholic comparison subjects. After adjustment for age and postmortem delay, this finding remained statistically significant. CONCLUSIONS: A lower serotonin transporter density among the alcoholic subjects was observed, specifically in the so-called “affect” region, suggesting an association between ethanol addiction and dysfunctional serotonergic neurotransmission in this area.
Several studies (1, 2) have indicated that damage to the ventromedial frontal region leads to a defect in rational decision making and the processing of emotion. It has been observed that a particular area in the anterior cingulate cortex, the so-called “affect” division of the anterior executive region, which includes Brodmann’s areas 25 and 33 and rostral area 24 (areas 24a and 24b) (3), is involved in the regulation of emotions and social behavior (4). As cytoarchitectural and functional subdivisions, these areas form parts of the perigenual cortex, which mediates affect and motivation, the main contributions of the cingulate cortex (5). Patients with anterior cingulate cortex epilepsy, for example, often display psychopathic or sociopathic behaviors, while infarcts in or surgery to this area can result in aberrant social behavior. A similar disregard for the future consequences of one’s actions has also been observed to follow damage to the prefrontal cortex (6).
The behavior of some alcoholic subjects resembles that of patients with severe prefrontal injuries. In chronic alcoholism, in spite of apparently normal intellectual performance and at least occasional motivation to seek professional help, alcoholics often make decisions against their best interests and are typically unable to learn from their mistakes. Concerning metabolic findings, previous results indicate that circumscribed frontal dysfunction may occur in chronic alcoholics before clinically obvious neurological complications appear, which may account for some portion of alcohol-related neuropsychological and behavioral impairments (7). Studies suggest that brain metabolism in recovering alcoholics remains slower in the orbitofrontal cortex and anterior cingulate gyrus for several months after detoxification (8).
Low levels of CSF 5-hydroxyindoleacetic acid have been associated with alcoholism, especially with early-onset alcoholism, which is characterized by aggressiveness and severe impulsivity (9–11). Lower central serotonin transporter densities have been associated with alcoholism; a significantly smaller availability of brainstem serotonin transporter was reported in abstinent alcoholic subjects by means of single-photon emission tomography (12). This was significantly correlated with lifetime alcohol consumption and with relevant ratings of depression and anxiety during withdrawal. When comparing serotonin transporter densities between type 1 and type 2 alcoholics (13), the binding of [123I]βCIT to serotonin transporter in the midbrain of abstinent violent alcoholic offenders was lower than in healthy nonalcoholic comparison subjects or in abstinent nonviolent alcoholics (14). Serotonin reuptake inhibitors, such as citalopram and fluoxetine, have been reported to reduce alcohol consumption, which has not been explained by their antidepressant effect (15–17). In addition, several studies have implied fluoxetine’s efficacy in the treatment of impulsive-aggressive behavior (18–20). To our knowledge, there are no published human data on central serotonin transporter densities (a marker for serotonin presynaptic terminals) or serotonin receptors in this area. Cortes et al. (21) studied the distribution of serotonin reuptake sites in the postmortem brains of human nonalcoholic comparison subjects with [3H]imipramine and [3H]paroxetine and reported the highest densities of both ligands in the raphe nuclei of the midbrain, with only low densities in cortical areas. However, because of the limitations of the standard autoradiographic methods of that time, they were able to study only separate, relatively small areas, and a systematic whole-hemisphere screening of the entire cerebral cortex was not possible.
Although several studies have implied that lesions of the medial prefrontal cortex, as well as dysfunctions in serotonin turnover, are associated with the impulsive and sociopathic behaviors that are typically seen in alcoholics during periods of ethanol abuse, we know of no reports linking these two factors. The initial aim of this study was to identify putative areas within the medial prefrontal cortex of the human brain that are rich in serotonin transporter sites by means of whole-hemisphere autoradiography on horizontal (canthomeatal) sections (22). This novel technique offered a unique opportunity to study the distribution of serotonergic binding sites in the postmortem human brain and to determine if presynaptic serotonergic terminals are concentrated in the so-called “affect” region of the anterior cingulate cortex. A secondary goal was to determine if the serotonin transporter density in this area is altered among alcoholic subjects.
Method
Brain Sampling
Human brains were obtained during clinical necropsy from the Department of Forensic Medicine, University of Oulu, Finland; two nonalcoholic comparison brains were obtained from the Department of Forensic Medicine, University of Kuopio, Finland. The procedure was essentially the same in both locations. This portion of the study was approved by the Ethics Committees of the University of Oulu and the National Institute of Medicolegal Affairs, Helsinki, Finland. The brains were removed, cleaned of the dura, and divided at the midsagittal plane. The left hemisphere was placed with the midsagittal plane on a glass plate before freezing to –75°C. Medical records on the cause of death, previous diseases, and medications were collected. A postmortem analysis for drugs, including alcohol, and the normal necropsy protocol was also performed. None of the hemispheres exhibited damage or neuroanatomical abnormalities.
Diagnostics
Diagnoses were made by two physicians independently of each other. Medical record data were available for all 27 subjects. Mental disorders were coded according to DSM-III-R criteria, and alcoholic subjects were subclassified as type 1 or type 2 according to a work by Cloninger (13). The kappa coefficient of diagnostic agreement was 0.9; i.e., one type 2 alcoholic subject included in the study was diagnosed as a type 1 alcoholic by the second diagnostician. Otherwise, diagnoses were unanimous. Alcoholic subjects were selected when alcohol dependence or serious alcohol abuse was evident on the basis of autopsy, medical records, and anamnestic data. The most important criteria for defining the two groups of alcoholic subjects were early onset (before the age of 25) of alcohol abuse and documented, severe antisocial behavior among type 2 alcoholic subjects. Nonalcoholic comparison subjects were selected when autopsy or anamnestic data revealed no signs of harmful substance misuse. Subjects having psychotic disorders or any diseases (such as epilepsy) or taking medication that affected the CNS (such as neuroleptics or antidepressants) were excluded. Tobacco smoking history, based only on medical records, was considered unreliable and was not included in the final criteria. The causes of death were acute heart disease (all 10 nonalcoholic comparison subjects, four alcoholic subjects), suicide (N=4), homicide (N=3), pneumonia (N=2), alcohol toxification (N=2), acute pancreatitis (N=1), and subdural hematoma (N=1).
Study Subjects
All 27 subjects were white Caucasians. The study groups consisted of nine type 1 alcoholic subjects (seven men and two women; age: mean=52.7 years, SD=12.4; postmortem delay: mean=11.9 hours, SD=4.5); eight type 2 alcoholic subjects (all men; age: mean=34.6 years, SD=12.2; postmortem delay: mean=14.1 hours, SD=3.4); and 10 nonalcoholic comparison subjects (eight men and two women; age: mean=53.5 years, SD=10.7; postmortem delay: mean=14.8 hours, SD=9.2) who were free of psychiatric diagnosis. Individual demographic and diagnostic data is shown in Table 1. Because of methodological reasons, data were available for only 16 alcoholic subjects (nine type 1 and seven type 2 subjects) and eight nonalcoholic comparison subjects in the uppermost level studied of the perigenual anterior cingulate cortex.
Intervals between death and autopsy were not significantly different between the groups (p>0.05, Scheffé’s test for multiple comparisons, two-tailed). Eight of the nine type 1 alcoholic subjects had alcohol in their blood at the time of death, and one alcoholic subject had an abstinence period of 10 hours. One of the nonalcoholic comparison subjects had a small amount of alcohol in his blood at the time of death (0.04%). Two of the type 1 alcoholic subjects had traces of diazepam in their blood samples. Six type 2 alcoholic subjects had alcohol in their blood at the time of death, three had traces of benzodiazepines, and one tested positive for cannabinoids.
Cryosectioning
Cryosectioning and autoradiography were performed at the Department of Pharmacology and Toxicology, Kuopio University, Kuopio, Finland. (For detailed methods, see Hall et al. [22] and Tupala et al. [23, 24].)
Receptor Autoradiography
Extensive autoradiographic analyses were performed for one nonalcoholic comparison brain and one type 1 alcoholic brain in order to examine the ventral part of the frontal cortex and to determine the appropriate brain levels for quantitative serotonin transporter autoradiography (Figure 1). Subsequent brain sections were chosen from three different canthomeatal brain levels among the 27 subjects. The sizes of the corpus callosum and the head of caudate nucleus were used as anatomical criteria when matching the sections in the upper level of the perigenual anterior cingulate cortex (corresponding to level 9 in Figure 1). The width of the internal capsule and the sizes of putamen, the head of the caudate nucleus, and the globus pallidus were used as anatomical criteria to match selected brain sections in the lower level of the perigenual anterior cingulate cortex (corresponding to level 6 in Figure 1). Individual variations in brain size were also considered when selecting sections that were parallel to each other. Depending on brain size, level 9 was studied at a size ranging from 41.6 to 65.7 mm and level 6 at a range of 58.1 to 88.1 mm.
Each cryosection was coded for a blind analysis of the data. The incubation concentration for [3H]citalopram (specific activity=82 Ci/mmol, NEN Life Science Products, Inc., Boston) in phosphate buffer solution was 1.2 nM. The buffer solution contained 137 mM of sodium chloride, 2.7 mM of potassium chloride, 1.76 mM of potassium phosphate, and 10.14 mM of hydrosodium phosphate; the pH was 7.4. Cryosections were preincubated for 15 minutes in phosphate buffer solution at room temperature. Each section was surrounded with plasticine before addition of the incubation solution (14 ml of phosphate buffer solution). The incubation lasted 90 minutes at room temperature, followed by washing (3 × 10 minutes) in ice-cold phosphate buffer solution and a brief dip into ice-cold distilled water. Nonspecific binding was defined by incubating adjacent sections with 10 μM of fluoxetine (Research Biomedicals International, Natick, Mass.) with 0.1% ascorbic acid added. After washing, sections were dried under a gentle stream of cool air for 10 minutes and left for 5 days at room temperature. Sections were exposed for 4 weeks to β-sensitive film ([3H]-Hyperfilm, Amersham Pharmacia Biotech UK Ltd., Little Chalfont, Buckinghamshire, U.K.) in X-ray cassettes, together with tritium calibration standards (Amersham Microscales, Amersham Life Science, Buckinghamshire, U.K.). The autoradiograms were analyzed by means of computerized densitometry. Software packages included Photoshop 4.01 (Adobe, Mountain View, Calif.) and Scion Image for Windows, version Beta 3b (Frederick, Md.).
In the perigenual anterior cingulate cortex, the measured area included the most superficial half of the gray matter (a borderline was drawn through the midpoint, between the inner and outer edges of the gray matter), from the sulcus of the corpus callosum to the cingulate sulcus. The relative density of [3H]citalopram binding sites was determined by a log-10 transformation of film blackness (i.e., mean pixel values of the selected regions) into binding density (pmol/g) on the basis of measured values from tritium standards. The background was subtracted, and nonspecific binding was subtracted from the total binding of the anatomical region. Sections adjacent to the quantified levels were Nissl-stained to serve as anatomical correlates. A one-way analysis of variance (ANOVA) and Dunnett’s test for multiple-group comparisons were used as statistical methods; analysis of covariance (ANCOVA) was used for adjusting age and postmortem delay, followed by Bonferroni’s test for multiple comparisons. In the group of type 1 alcoholic subjects, the results were adjusted only for postmortem delay for two reasons. First, type 1 alcoholics and nonalcoholic comparison subjects were of the same age. Second, the dependence of age and the dependent covariate was not parallel in the group of type 1 alcoholic subjects. In the upper level of the perigenual anterior cingulate cortex (corresponding to level 9 in Figure 1), a square transformation was employed because of the inequality of standard deviations between the alcoholic and nonalcoholic comparison groups. Levene’s test was used to determine the inequality of standard deviations, and the limit of statistical significance was set at p=0.10.
Results
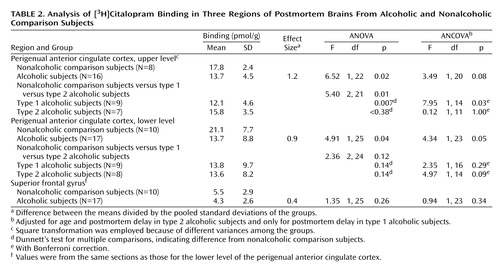
In Figure 1, an autoradiogram serial illustrates serotonin transporter distribution in the brain. The highest [3H]citalopram binding in the cerebral cortex was observed in the most superficial layers of the perigenual anterior cingulate cortex areas 24, 25, 32, and 33. The most dense labeling was in Brodmann’s area 33 in the callosal sulcus and rostral area 24, just anterior to the corpus callosum (detailed in Figure 2). High levels of binding were also observed in the raphe nuclei, entorhinal cortex, amygdala, insula, thalamus, and caudate (Figure 1). In this article, quantitative analyses were made on data from the frontal cortex.
[3H]Citalopram binding in each group is presented in Table 2 and Figure 3. Studies have reported the specific [3H]citalopram binding to be 40%–50% in frontal areas (25). In this study, the total specific [3H]citalopram binding ranged from 60% in the lower perigenual anterior cingulate cortex to 64% in the upper perigenual anterior cingulate cortex.
In the most dorsal level (corresponding to level 9 in Figure 1), representing the most rostral part of the perigenual anterior cingulate cortex (the upper level of the perigenual anterior cingulate cortex), ANOVA between nonalcoholic comparison subjects and type 1 and type 2 alcoholic subjects indicated a statistically significant difference among the groups (F=5.40, df=2, 21, p=0.01). The measured [3H]citalopram density in alcoholic subjects was 23% lower than in nonalcoholic comparison subjects (F=6.52, df=1, 22, p=0.02). In spite of the small number of subjects, however, the difference in [3H]citalopram density between type 1 alcoholic subjects and nonalcoholic comparison subjects was even clearer (–32%; p=0.007, Dunnett’s test for multiple comparisons). When adjusted for postmortem delay, this difference remained statistically significant (F=7.95, df=1, 14, p=0.03, ANCOVA with Bonferroni correction). At this level, type 2 alcoholic subjects had a 11% lower [3H]citalopram density in the perigenual anterior cingulate cortex than the nonalcoholic comparison subjects, but this finding was not statistically significant.
In the lower (ventral) level of the frontal cortex (corresponding to level 6 in Figure 1), the total group of alcoholic subjects had a 35% lower [3H]citalopram density in the perigenual anterior cingulate cortex than the nonalcoholic comparison subjects. This difference was statistically significant by ANOVA (F=4.91, df=1, 25, p=0.04); when age variation and different postmortem delays were adjusted, the p value was 0.05 (F=4.34, df=1, 23, ANCOVA). Analysis among type 1 and type 2 alcoholic subjects in relation to nonalcoholic comparison subjects revealed 35% and 36% lower bindings, respectively. However, these findings were not statistically significant after Bonferroni correction within these relatively small groups (N=9 and N=8, respectively). The serotonin transporter densities in the superior frontal gyrus (studied at a level corresponding to level 6 in Figure 1) were remarkably low in all groups, and the difference between the alcoholic and nonalcoholic comparison subjects was not statistically significant.
When observed within the nonalcoholic comparison group, the correlation between [3H]citalopram binding density and age was found to be negative (r=–0.38, p=0.28, N=10, two-tailed, the lower perigenual anterior cingulate cortex), whereas the correlation between [3H]citalopram density and postmortem delay was found to be positive (r=0.46, p=0.18, N=10). Within the alcoholic group, the respective correlations were also negative for age (r=–0.07, p=0.78, N=17, two-tailed, the lower perigenual anterior cingulate cortex) and positive for postmortem delay (r=0.29, p=0.26, N=17, two-tailed, the lower level).
Discussion
In this study, postmortem horizontal (canthomeatal plane) whole-hemisphere autoradiography was used to study the distribution of presynaptic serotonergic terminals by using sections from the human neocortex as a whole and [3H]citalopram, which is the most selective ligand currently available for serotonin transporter binding. A region of very high serotonin transporter density was identified in the perigenual anterior cingulate cortex. This accumulation is located exactly in the affect division of the anterior executive region, which is primarily involved in conditioned emotional learning (4). This region includes Brodmann’s areas 24, 25, 32, and 33 (perigenual anterior cingulate cortex), with extensive connections to the amygdala. Together, these areas—as part of the rostral limbic system—are considered to assess the motivational content of internal and external stimuli and regulate context-dependent behaviors.
The measured [3H]citalopram binding in the perigenual anterior cingulate cortex was markedly lower in the alcoholic subjects in relation to the nonalcoholic comparison subjects both in the upper (effect size=1.2) and in the lower (effect size=0.9) measured levels of the perigenual anterior cingulate cortex. No statistically significant difference was observed in the superior frontal gyrus (effect size=0.4). These results indicate that a lower serotonin transporter density in alcoholic subjects—whatever the mechanism mediating this effect—is specific to the affect region in the anterior cingulate cortex and is not explained by a nonspecific ethanol-induced down-regulation of serotonin transporter or by a general neuronal loss in the frontal cortex (26, 27). These results also indicate that lower serotonin transporter density in the perigenual anterior cingulate cortex is not explained by age at time of death (which would roughly reflect the duration of alcohol abuse).
Previous studies have identified lower serotonin transporter levels in various brain regions of depressed or nondepressed suicide victims (28), as well as in living depressed patients (29) and in the platelets of antidepressant-naive depressed patients (30). However, in the present study, the alcoholic subjects who had suffered from coexisting depression and committed suicide did not differ from the other alcoholic subjects regarding serotonin transporter densities.
Heinz et al. (12) observed a lower availability of brainstem serotonin transporter in abstinent alcoholic subjects. They suggested that a lower availability of raphe serotonin transporter most likely reflects an actual reduction in transporter density because of a cumulative toxic effect of ethanol consumption. On the other hand, they discussed that a lower availability of raphe serotonin transporter may predispose an individual to depression and anxiety under certain conditions, such as chronic ethanol intoxication or withdrawal-associated detoxification stress. In the present study, we did not present quantitative results for the raphe nucleus because it is a midline structure and is therefore problematic to measure. No significant difference was seen between serotonin transporter densities in any brain areas of type 1 and type 2 alcoholic subjects. The lower serotonin transporter density in the frontal cortex of the alcoholics may be mainly regarded as a state marker because of the lack of an abstinence period. On the other hand, the chronic and toxic effects of alcohol abuse may mask the possibility that abstinent type 2 alcoholics might have a genetic trait for lower serotonin transporter density than abstinent type 1 alcoholics, which could also be assumed on the basis of CSF studies (31). In addition to alcoholism, it is possible that altered serotonin transporter densities in the perigenual anterior cingulate cortex may be associated also with depression, schizophrenia, anxiety, anorexia, bulimia, or personality disorders. It is also unlikely that alcoholics in this study were polydrug abusers, which would complicate the interpretation of these results.
In his somatic-marker hypothesis on processes that concern human reasoning and decision making, Damasio (32) proposed that the memory of linkage between certain classes of situations and certain body states must be permanently held and that the system required for such memories is located in the ventral and medial prefrontal cortices. Also, results from other studies have confirmed various predictions from this hypothesis (33). In addition to other regions of the frontal cortex, the orbitofrontal cortex has also been suggested to play an important role in choosing appropriate courses of action (1, 34, 35), and Brodmann’s areas 33 and 25 and rostral area 24 have been observed to be involved in the regulation of social and emotional behavior (4). These studies are in line with the present results, which demonstrate serotonergic deficits in alcoholics that are localized in the perigenual anterior cingulate cortex, an area of the brain that is important for judgment, planning, and decision making.
 |
 |
Received Nov. 27, 2000; revisions received March 19 and Sept. 6, 2001; accepted Sept. 25, 2001. From the Department of Forensic Psychiatry, Niuvanniemi Hospital, the Department of Clinical Physiology, Kuopio University Hospital, and the Departments of Pharmacology and Toxicology and Pharmaceutical Chemistry, University of Kuopio, Kuopio, Finland; the Psychiatry Section, Department of Clinical Neuroscience, Karolinska Institute and Hospital, Stockholm, Sweden; the Departments of Forensic Medicine and Psychiatry, University of Oulu, Oulu, Finland; and the Department of Psychiatry, Lapinlahti Hospital, University of Helsinki. Address reprint requests to Dr. Tiihonen, Department of Psychiatry, Lapinlahti Hospital, University of Helsinki, P.O. Box 320, FIN-00029 HUS, Helsinki, Finland; [email protected] (e-mail). Supported by annual EVO financing (special government subsidy) from Niuvanniemi Hospital and the Swedish Medical Research Council (grant number 11640). The authors thank Kari Karkola, M.D., for providing brain samples from the Kuopio University Hospital; Pirjo Halonen, M.Sc., for statistical support, Raija Törrönen for photography, Aija Räsänen for secretarial assistance, and Siv Eriksson for advice.
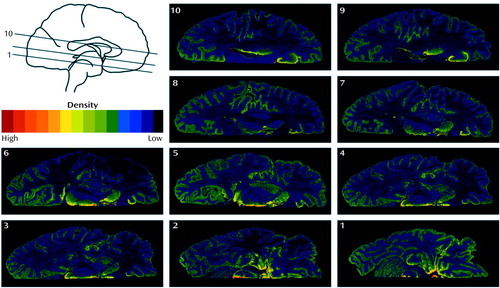
Figure 1. Autoradiograms Showing [3H]Citalopram Binding in 10 Serial Sections (With Approximately 5-mm Intervals) From the Ventral Frontal Cortex of the Postmortem Brain of a Nonalcoholic Comparison Subjecta
aThese images illustrate the serotonin-transporter-rich spot in the perigenual anterior cingulate cortex, just anterior to the corpus callosum. The posterior high-density region in the ventral brainstem in level 2 is considered to represent the dorsal raphe nucleus. High levels of binding were also observed in the entorhinal cortex (levels 1 and 2), amygdala (level 2), insula (level 8), thalamus (levels 5 and 6), and caudate (levels 5 and 6).
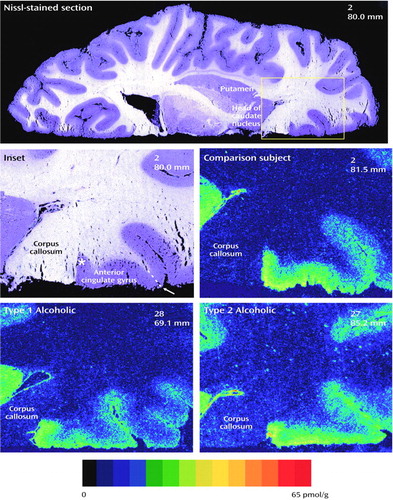
Figure 2. Autoradiograms Showing [3H]Citalopram Binding in the Perigenual Anterior Cingulate Cortex of Type 1 and Type 2 Alcoholic Subjects and a Nonalcoholic Comparison Subjecta
aA Nissl-stained section serves as an anatomical correlate to the comparison. Each brain was given a number for a blind analysis of data, and the brain sections are localized with brain numbers and levels in millimeters from the vertex (numbers in upper-right corner). The serotonin transporter accumulation is located just anterior to the corpus callosum, corresponding to Brodmann’s areas 33 (the depths of the callosal sulcus, indicated by an asterisk) and 24 (anterior cingulate gyrus). The small arrow indicates the anterior boundary of the region of interest.
Figure 3. [3H]Citalopram Binding in Three Regions of Postmortem Brains of Type 1 and Type 2 Alcoholic Subjects and Nonalcoholic Comparison Subjectsa
aStatistically significant difference (by ANOVA) was found in the upper level (F=5.40, df=2, 21, p=0.01) of the perigenual anterior cingulate cortex.
1. Damasio AR, Van Hoesen GW: Emotional disturbances associated with focal lesions of the limbic frontal lobe, in Neuropsychology of Human Emotion. Edited by Heilman KM, Satz P. New York, Guilford, 1983, pp 85-110Google Scholar
2. Damasio H, Grabowski T, Randall F, Galaburda AM, Damasio AR: The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 1994; 264:1102-1105Crossref, Medline, Google Scholar
3. Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR: Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol 1995; 359:490-506Crossref, Medline, Google Scholar
4. Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118:279-306Crossref, Medline, Google Scholar
5. Vogt BA, Vogt LJ, Nimchinsky EA, Hof PR: Primate cingulate cortex chemoarchitecture and its disruption in Alzheimer’s disease, in Handbook of Chemical Neuroanatomy, vol 13: The Primate Nervous System, Part I. Edited by Bloom FE, Björklund A, Hökfelt T. Amsterdam, Elsevier Science, 1997, pp 455-528Google Scholar
6. Bechara A, Damasio AR, Damasio H, Anderson SW: Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994; 50:7-15Crossref, Medline, Google Scholar
7. Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Féline A, Syrota A: Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med 1998; 28:1039-1048Crossref, Medline, Google Scholar
8. Volkow ND, Wang GJ, Overall JE, Hitzemann R, Fowler JS, Pappas N, Frecska E, Piscani K: Regional brain metabolic response to lorazepam in alcoholics during early and late alcohol detoxification. Alcohol Clin Exp Res 1997; 21:1278-1284Crossref, Medline, Google Scholar
9. Heinz A, Higley JD, Gorey JG, Saunders RC, Jones DW, Hommer D, Zajicek K, Suomi SJ, Lesch K-P, Weinberger DR, Linnoila M: In vivo association between alcohol intoxication, aggression, and serotonin transporter availability in nonhuman primates. Am J Psychiatry 1998; 155:1023-1026Link, Google Scholar
10. Virkkunen M, Rawlings R, Tokola R, Poland RE, Guidotti A, Nemeroff C, Bissette G, Kalogeras K, Karonen S-L, Linnoila M: CSF biochemistries, glucose metabolism, and diurnal activity rhythms in alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry 1994; 51:20-27Crossref, Medline, Google Scholar
11. Virkkunen M, Eggert M, Rawlings R, Linnoila M: A prospective follow-up study of alcoholic violent offenders and fire setters. Arch Gen Psychiatry 1996; 53:523-529Crossref, Medline, Google Scholar
12. Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, Gorey JG, Doty L, Geyer C, Lee KS, Coppola R, Weinberger DR, Linnoila M: Reduced central serotonin transporters in alcoholism. Am J Psychiatry 1998; 155:1544-1549Link, Google Scholar
13. Cloninger CR: The psychological regulation of social co-operation. Nat Med 1995; 1:623-624Crossref, Medline, Google Scholar
14. Tiihonen J, Kuikka JT, Bergström KA, Karhu J, Viinamäki H, Lehtonen J, Hallikainen T, Yang J, Hakola P: Single-photon emission tomography imaging of monoamine transporters in impulsive violent behaviour. Eur J Nucl Med 1997; 24:1253-1260Crossref, Medline, Google Scholar
15. Miller NS: Pharmacotherapy in alcoholism. J Addict Dis 1995; 14:23-46Crossref, Medline, Google Scholar
16. Naranjo CA, Poulos CX, Bremner KE, Lanctot KL: Fluoxetine attenuates alcohol intake and desire to drink. Int Clin Psychopharmacol 1994; 9:163-172Crossref, Medline, Google Scholar
17. LeMarquand D, Pihl RO, Benkelfat C: Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry 1994; 36:395-421Crossref, Medline, Google Scholar
18. Coccaro EF, Kavoussi RJ, Hauger RL: Serotonin function and antiaggressive response to fluoxetine: a pilot study. Biol Psychiatry 1997; 42:546-552Crossref, Medline, Google Scholar
19. Coccaro EF, Kavoussi RJ: Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry 1997; 54:1081-1088Crossref, Medline, Google Scholar
20. Salzman C, Wolfson AN, Schatzberg A, Looper J, Henke R, Albanese M, Schwartz J, Miyawaki E: Effect of fluoxetine on anger in symptomatic volunteers with borderline personality disorder. J Clin Psychopharmacol 1995; 15:23-29Crossref, Medline, Google Scholar
21. Cortes R, Soriano E, Pazos A, Probst A, Palacios JM: Autoradiography of antidepressant binding sites in the human brain: localization using [3H]imipramine and [3H]paroxetine. Neuroscience 1988; 27:473-496Crossref, Medline, Google Scholar
22. Hall H, Halldin C, Farde L, Sedvall G: Whole hemisphere autoradiography of the postmortem human brain. Nucl Med Biol 1998; 25:715-719Crossref, Medline, Google Scholar
23. Tupala E, Hall H, Särkioja T, Räsänen P, Tiihonen J: Dopamine-transporter density in nucleus accumbens of type-1 alcoholics (letter). Lancet 2000; 355:380Crossref, Medline, Google Scholar
24. Tupala E, Hall H, Bergström K, Särkioja T, Räsänen P, Mantere T, Callaway J, Hiltunen J, Tiihonen J: Dopamine D2/D3-receptor and transporter densities in nucleus accumbens and amygdala of type 1 and 2 alcoholics. Mol Psychiatry 2001; 6:261-267Crossref, Medline, Google Scholar
25. Arranz B, Marcusson J: [3H]Paroxetine and [3H]citalopram as markers of the human brain 5-HT uptake site: a nonalcoholic comparison study. J Neural Transm Gen Sect 1994; 97:27-40Crossref, Medline, Google Scholar
26. Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO: Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res 1997; 21:521-529Crossref, Medline, Google Scholar
27. Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO: A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry 1998; 55:905-912Crossref, Medline, Google Scholar
28. Owens MJ, Nemeroff CB: Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem 1994; 40:288-295Medline, Google Scholar
29. Malison RT, Price LH, Berman R, Van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS: Reduced brain serotonin transporter availability in major depression as measured by [123I]-2β-carbomethoxy-3β-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 1998; 44:1090-1098Crossref, Medline, Google Scholar
30. Nemeroff CB, Knight DL, Franks J, Craighead WE, Krishnan KR: Further studies on platelet serotonin transporter binding in depression. Am J Psychiatry 1994; 151:1623-1625Link, Google Scholar
31. Virkkunen M, Linnoila M: Serotonin in early onset, male alcoholics with violent behaviour. Ann Med 1990; 22:327-331Crossref, Medline, Google Scholar
32. Damasio AR: The somatic marker hypothesis and the possible functions of the prefrontal cortex. Phil Trans R Soc Lond B 1996; 351:1413-1420Crossref, Medline, Google Scholar
33. Bechara A, Damasio H, Damasio AR: Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 2000; 10:295-307Crossref, Medline, Google Scholar
34. Elliot R, Dolan RJ, Frith CD: Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex 2000; 10:308-317Crossref, Medline, Google Scholar
35. Blair RJR, Cipolotti L: Impaired social response reversal: a case of “acquired sociopathy.” Brain 2000; 123:1122-1141Crossref, Medline, Google Scholar



