In Vivo Association Between Alcohol Intoxication, Aggression, and Serotonin Transporter Availability in Nonhuman Primates
Abstract
OBJECTIVE: Studies on brain serotonin metabolism in human and nonhuman primates have indicated that dysfunction of serotonin transmission may play a role in the biological vulnerability to dependence on alcohol. Among young men, low sensitivity to alcohol intoxication predicts subsequent alcohol abuse and dependence. METHOD: The authors used single photon emission computed tomography and the radioligand [I123]β-CIT ([I123]methyl 3β-(4-iodophenyl) tropane-2-carboxylate) to measure the availability of serotonin transporters in 11 male rhesus monkeys, and the monkeys were genotyped for a functional polymorphism of the serotonin transporter gene. The 11 monkeys had experienced parental separation after birth; their behavior and 5-hydroxyindoleacetic acid (5-HIAA) concentrations in CSF had been assessed regularly. RESULTS: In the 5-year-old monkeys, there was a significant negative correlation between β-CIT binding to serotonin transporters in the brainstem and 5-HIAA concentrations in CSF. Animals with greater β-CIT binding and low CSF 5-HIAA concentrations displayed greater aggressiveness and were less sensitive to alcohol-induced intoxication. The genetic constitution of the serotonin transporter promoter gene did not significantly contribute to the availability of brainstem serotonin transporters as measured by β-CIT binding. CONCLUSIONS: In adult nonhuman primates who underwent early developmental stress, variables indicating a low serotonin turnover rate were associated with behavior patterns similar to those predisposing to early-onset alcoholism among humans. (Am J Psychiatry 1998; 155:1023–1028)
Several lines of evidence suggest that serotonin dysfunction may play a role in the pathogenesis of alcoholism. Among rats selectively bred for alcohol preference, brain serotonin (5-HT) turnover is low and 5-HT1A receptor density is higher in the hippocampus, cerebral cortex, and nucleus accumbens than in non-alcohol-preferring animals (1–4). Among human and nonhuman primates, a low CNS serotonin turnover rate, as measured by the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) in CSF, has been implicated in the pathogenesis of alcohol abuse and impulsively aggressive behavior (5–10). Among young men, both so-called “antisocial personality traits” and less intoxication after alcohol consumption are predisposing variables for the later development of alcohol abuse and dependence (11, 12). Low CSF 5-HIAA concentrations have been found in early-onset alcoholics (13), who exhibit a more severe course of alcoholism, problems of social isolation, and impaired social functioning (14–16). Whether serotonin turnover rate affects the sensitivity to alcohol intoxication has not, however, been directly investigated.
In a study of the adopted offspring of alcoholic parents (17), investigators observed that environmental variables, such as parental neglect and late placement in adoptive families, were associated with heavy alcohol consumption during adulthood. Among nonhuman primates, environmental stressors during early development, such as maternal deprivation, were associated with low 5-HIAA concentrations in CSF, more alcohol consumption, greater aggression, and more anxiety-like behaviors during adolescence (9, 10, 18, 19). The central serotonin system is further implicated in the pathogenesis of anxiety-related traits and alcoholism by animal experiments and human studies on genetic variation in the expression of the serotonin transporter (20, 21).
We postulated that the availability of serotonin transporters interacts with serotonin turnover rate and with behavior traits predisposing to excessive alcohol consumption, especially among individuals who have been exposed to severe social stress during early development. In addressing these neurobehavioral hypotheses, the assessment of nonhuman primates offers the possibility of regulating and monitoring environmental stress, aggression, and alcohol consumption and of relating these data to biological and genetic markers. We measured serotonin reuptake sites in the brainstem by using the radioligand [I123]β-CIT and single photon emission computed tomography (SPECT) (22, 23) and analyzed the allelic variation of the serotonin transporter promoter region (21), the serotonin turnover rate (5-HIAA in CSF), social behavior, and intrinsic tolerance to alcohol in nonhuman primates who had been under close and structured behavioral observation since birth.
METHOD
Subjects and Methods of Behavioral Assessment
All protocols used in this study were reviewed and approved by the animal care and use committees of the intramural research programs of the National Institute on Alcohol Abuse and Alcoholism or the National Institute of Mental Health. The subjects were 11 male rhesus macaques (Macaca mulatta), aged 5 years. The rearing conditions of these monkeys have been described in detail elsewhere (9, 10). Briefly, 10 rhesus monkeys had been separated from their mothers after birth and were reared with their age-matched peers; one monkey was reared in a single cage with its mother. At 6 months of age, all animals underwent a series of four 4-day social isolation periods, each one followed by 3 days of home-cage reunion. CSF was obtained monthly during infancy and weekly during the social isolation period. One month after the social isolation, the subjects were placed in same-age social groups, where they remained until the present study. Further CSF samples were obtained at the ages of 2, 3, and 5 years.
For all animals, ratings of aggression were made by using a Likert scale (24) and the computer program Clemco (R. Hannes Beinert, Madison, Wis.), which served as a battery of clocks and counters. Aggression included the following behaviors: bites, aggressive chases, hitting, and slapping. The behavioral data were collected on three different occasions between 9:00 a.m. and 4:00 p.m. within the same month as the SPECT scans were acquired. The mean score of the three observation periods was used for correlations with neurobiological variables. Ratings of alcohol intoxication were available for 10 monkeys and were based on sedation, body sway, and ataxia after a single administration of a standardized quantity of alcohol (10 ml/kg body weight of an 8.4% volume-for-volume alcohol solution). Trained observers were reliable at r>0.85 according to Cohen's kappa (9, 10) and were unaware of the results of the biochemical, genetic, and imaging studies.
Measurement of 5-HIAA in CSF
All CSF samples were obtained on the same day between 1:00 and 2:00 p.m. Samples were obtained by quickly anesthetizing the subjects (in less than 10 minutes) while they were naturally interacting in their socially stable home-cage social groups when they were 2, 3, and 5 years of age. The 5-year sample was obtained just before the SPECT scan. The subjects were anesthetized with ketamine hydrochloride (15 mg/kg i.m.). CSF, 2–3 ml, was obtained from the cisterna magna of each subject by using a 22-gauge needle with a 5-cc syringe, and it was immediately frozen in liquid nitrogen. Samples from each subject were obtained within 29 minutes following induction of anesthesia. Previous studies (18, 25, 26) have shown that CSF monoamine metabolite concentrations are unaffected by the procedures if the samples are obtained within this window of time. Concentrations of 5-HIAA were quantified with high-performance liquid chromatography and electrochemical detection as previously described (8, 27). At the 5-year sampling, CSF samples for 10 animals were available 1 week before the behavioral testing.
Genetic Analyses
A genetic contribution to the functional capacity of the serotonin transporter has recently been implicated in humans (21). Compared to the “long” allele of the serotonin transporter promoter region, the “short” allele is associated with less serotonin uptake capacity. In rhesus monkeys, a similar polymorphic region within the promoter region of the serotonin transporter exists (rh5-HTTLPR), and its functional implications are currently being investigated. We assessed the genotype of the rh5-HTTLPR with polymerase chain reaction by using oligonucleotide primers (stpr5, 5′-GGCGTTGCCGCTCTGAATGC; int1, 5′-CAGGGGAGATCCTGGGAGGA). Polymerase chain reaction amplification was carried out in a final volume of 30 µl consisting of 50 ng genomic DNA, 2.5 mM deoxyribonucleotides (dGTP/7-deaza-2′-dGTp=1/1), 0.1 µg of sense and antisense primers, 10 mM Tris HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 1 U of Taq DNA polymerase. Annealing was carried out at 61°C for 30 seconds, extension at 72°C for 1 minute, and denaturation at 95°C for 30 seconds for 35 cycles.
[I123]β-CIT SPECT Procedure
On the day before SPECT scanning and for 3 subsequent days, the animals received 5 drops of Lugol's solution orally, in order to reduce uptake of radioactive iodine in the thyroid gland. Before each SPECT study, the animals were anesthetized with ketamine (10 mg/kg i.m.); there was usually an interval of at least 2 hours between the induction of anesthesia and the beginning of the SPECT scan. [I123]β-CIT binds in the brainstem with high affinity to serotonin transporters (22). It is specifically displaced in the brainstem by ligands binding to serotonin reuptake sites (22, 23). The synthesis of [I123]β-CIT and assessment of radiochemical purity have been described previously (28). The monkeys received 407–518 MBq (11–14 mCi) of [I123]β-CIT. Two 30-minute SPECT scans were acquired 2 and 3 hours after injection, when equilibrium at the serotonin transporter was approached in previous studies (22). SPECT data were acquired by using a CERASPECT gamma camera (Digital Scintigraphics, Waltham, Mass.). Reproducible head positioning was ensured by using a stereotactic headholder specifically designed for the SPECT scanner. Its use facilitated the positioning of all animals in the same orientation. Stereotactic atlases and magnetic resonance imaging (MRI) data sets obtained in the same scanning plane were used for the placement of regions of interest. SPECT images were acquired by using a high-resolution (7.5 mm full width at half maximum) collimator in 120-projection step-and-shoot mode. The photopeak (145–175 keV) and two windows used for scatter correction (127–143 keV and 175–191 keV) were acquired. Reconstruction by backprojection with a 10th-order Butterworth filter (1 cm cutoff) generated an isotropic volume (1.67-mm voxels) of 64 128×128 transverse slices.
Individual regions of interest were drawn on the basis of a standard atlas of rhesus macaque brain and comprised the basal ganglia, thalamus, hypothalamus, brainstem, and cerebellum (figure 1). Of these regions, the highest density of serotonin transporters is found in the brainstem, which contains the raphe system (29). Placement of the regions of interest was confirmed on coregistered MRI scans for a subset of the subjects. Regions of interest were placed on five consecutive slices, and the average count per minute per milliliter (cpm/ml) in the volume encompassed by each set of regions of interest was recorded and corrected for decay. Brainstem binding of [I123]β-CIT was analyzed by using the ratio of specific to nonspecific binding, V3″ (30).
Statistical Analyses
Statistical analyses were computed by using Statistica for Windows (Stat Soft, Tulsa, Okla.). The a priori postulated correlations between [I123]β-CIT binding, 5-HIAA in CSF, and behavioral data were assessed by means of Spearman's correlation coefficient.
RESULTS
In the 5-year-old monkeys, the availability of the serotonin transporter in the brainstem was found to be negatively correlated with current CSF 5-HIAA concentrations and severity of intoxication during the first exposure to alcohol (figure 2), and it was positively correlated with aggressiveness (rs=0.69, N=11, p=0.02). We found no other significant correlations between [I123]β-CIT binding in any other region of interest, behavioral data, and CSF 5-HIAA concentrations.
In this small study group, the genotype of the serotonin transporter promoter region did not predict the availability of brainstem serotonin transporters. Nine monkeys carried both the long and short versions of the promoter region of the serotonin transporter, while two monkeys were homozygotic for the long allele. The availability of brainstem serotonin transporters, the CSF 5-HIAA concentrations, and the behavioral ratings for these two primates were well within the ranges of variation displayed by the heterozygotic monkeys (figure 2).
The monkeys assessed in this study are part of a larger cohort that showed reduced CSF 5-HIAA concentrations after social separation stress (9, 10). Consistent with prior observations, the peer-reared monkeys displayed lower 5-HIAA concentrations during infancy (range: 494 to 605 pmol/ml) than the one mother-reared monkey (831 pmol/ml). After the separation stress at 6 months, 5-HIAA concentration showed a developmental decline in all monkeys (preseparation mean: 362 pmol/ml, postseparation mean: 331 pmol/ml). In accord with previous observations (18), an age-related decline in CSF 5-HIAA concentration was found (mean during infancy: 585 pmol/ml, mean at 5 years: 266 pmol/ml). Nevertheless, individual CSF 5-HIAA concentrations showed considerable correlation over the animals' lifetimes: the CSF 5-HIAA concentrations measured at 2 and 3 years were significantly correlated with 5-HIAA concentrations at 5 years (rs=0.65 and rs=0.77, respectively, N=10, p<0.05) and with [I123]β-CIT binding (rs=-0.63 and rs=–0.60, respectively, N=11, p<0.05).
DISCUSSION
In this study of 11 rhesus monkeys, greater availability of the serotonin transporter as measured by [I123]β-CIT binding was found to be significantly correlated with low levels of the serotonin metabolite 5-HIAA in CSF, less intoxication upon initial exposure to alcohol, and greater aggressiveness. Similar behavioral and biochemical patterns have been reported to predispose humans to the development of alcoholism (11, 12). The correlation between [I123]β-CIT binding to serotonin transporters in the brainstem and 5-HIAA in CSF may be due to a difference in the density of serotonin transporters or to a difference in the serotonin concentrations in the synapses, both of which can change the relative availability of serotonin uptake sites for the radioligand (22, 31, 32). Either way, the implication of the findings is the same: animals with low CSF 5-HIAA concentrations and high transporter availability should have low serotonin concentrations in the synapse. Our findings suggest that interindividual differences in CSF 5-HIAA concentrations may have their genesis in altered serotonin transport and that CSF 5-HIAA concentrations may be related to the availability of serotonin transporters in the brainstem. Previous ex vivo findings in alcohol-preferring vervet monkeys support the hypothesis that low levels of monoamine metabolites in the CSF are associated with a higher density of monoamine transporters (33).
Our findings are compatible with a role for dysregulated serotonin neurotransmission in the pathogenesis of excessive alcohol consumption. They indicate that CSF 5-HIAA concentrations are correlated with serotonin uptake in the brainstem, the site of the raphe nuclei and the central origin of cortical and subcortical serotonin projections (29, 34). A dysfunction of serotonin transmission in the raphe system may be one of the environmentally and genetically influenced biological variables predisposing to excessive alcohol consumption (35, 36). Among young rhesus monkeys, heritable influences account for more than 60% of the variance in 5-HIAA metabolite concentrations (37). Among adult human and nonhuman primates, the influence of heritable variables on central serotonin turnover rates is less than 50% and environmental variables account for more than one-half of the variance (9, 10, 38). In this study, all monkeys underwent social separation stress during infancy. A number of studies with nonhuman primates have shown that influences by adults, particularly maternal input, are critical to modulate the development of the central serotonin system (27, 37, 39). In the absence of influence by adults, the development of serotonin function is impaired. When CSF 5-HIAA was obtained from 51 neonatal peer-only-reared and mother-reared monkeys on postnatal days 14, 30, 60, 90, 120, and 150, the peer-reared subjects exhibited lower CSF 5-HIAA concentrations than did the mother-reared subjects (40). One study with a limited number of subjects suggested that the effect of early rearing experiences on CSF 5-HIAA concentration may disappear by adolescence (18). However, in a larger study in which peer-reared and mother-reared subjects were longitudinally studied from infancy into adulthood, the peer-reared subjects exhibited lower CSF 5-HIAA concentrations than the mother-reared subjects in both infancy and adulthood (9, 10). Similar findings have been observed by other investigators as well (19). Our observation of a significant association between serotonin transporter availability and aggressiveness in adult nonhuman primates that had experienced parental separation during early development is interesting. It may be relevant to humans, because findings from a recent twin study (41) also emphasized the importance of environmental factors in the pathogenesis of antisocial behavior among alcoholics.
In conclusion, our findings indicate that central serotonin dysfunction is associated with lower initial sensitivity to alcohol intoxication and with greater aggressiveness, two variables implicated in the pathogenesis of alcoholism. By characterizing the molecular neurobiology of addiction, the present in vivo imaging data may aid in the development of therapeutic strategies that target specific neurotransmission dysfunctions among alcoholics and other substance abusers.
Received Sept. 19, 1997; revision received Feb. 19, 1998; accepted April 2, 1998. From the Clinical Brain Disorders Branch, NIMH Neuroscience Center at St. Elizabeths Hospital, Washington, D.C.; the Laboratory of Clinical Studies, National Institute on Alcohol Abuse and Alcoholism, Bethesda, Md.; and the Department of Psychiatry, University of W<129>rzburg, Germany. Address reprint requests to Dr. Heinz, Department of Neurology, Ruhr-University of Bochum, St. Josef-Hospital, Gudrunstrasse 56, 44791 Bochum, Germany; [email protected] (e-mail). Dr. Linnoila died in February 1998. Supported in part by the Deutsche Forschungsgemeinschaft (Az: He 2597/1-1).
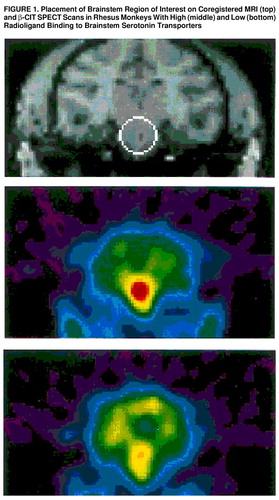
FIGURE 1. Placement of Brainstem Region of Interest on Coregistered MRI (top) and β-CIT SPECT Scans in Rhesus Monkeys With High (middle) and Low (bottom) Radioligand Binding to Brainstem Serotonin Transporters
FIGURE 2. Correlation of CSF 5-HIAA Concentrations and of Degree of Alcohol Intoxication to Binding of [I123]β-CIT to Brainstem Serotonin (5-HT) Transporters Among 11 Rhesus Monkeys Subjected to Early Developmental Stressa,b
aData missing for one monkey in each graph but not the same monkey in both graphs.
bSignificant Spearman correlations for both 5-HIAA (rs=–0.76, N=10, p=0.01) and degree of intoxication (rs=–0.65, N=10, p=0.04).
cRatings of alcohol intoxication were based on sedation, body sway, and ataxia after a single administration of a standardized quantity of alcohol (10 ml/kg body weight of an 8.4% volume-for-volume alcohol solution). Behavioral data were collected by using a standardized scoring system and the computer program Clemco (R. Hannes Beinert, Madison, Wis.), which served as a battery of clocks and counters. The trained observers were unaware of the results of the biochemical, genetic, and imaging studies.
1 Murphy JM, McBride WJ, Lumeng L, Li TK: Contents of monoamines in forebrain regions of alcohol-preferring (P) and non-preferring (NP) lines of rats. Pharmacol Biochem Behav 1987; 26:389–392Crossref, Medline, Google Scholar
2 McBride WJ, Murphy JM, Lumeng L, Li TK: Serotonin, dopamine and GABA involvement in alcohol drinking in selectively bred rats. Alcohol 1989; 7:199–205Crossref, Google Scholar
3 Wong DT, Threlkeld PG, Lumeng L, Li TK: Higher density of serotonin-1A receptors in the hippocampus and cerebral cortex of alcohol-preferring P rats. Life Sci 1990; 46:231–235Crossref, Medline, Google Scholar
4 Zhou FC, Bledsoe S, Lumeng L, Li TK: Serotonergic immunostained terminal fibers are decreased in selected brain areas of alcohol-preferring rats (abstract). Alcohol Clin Exp Res 1990; 14:355AGoogle Scholar
5 Linnoila M, Virkunnen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK: Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 1983; 33:2609–2614Crossref, Medline, Google Scholar
6 Kruesi MJ, Rapoport JL, Hamburger S, Hibbs E, Potter WZ, Lenane M, Brown GL: Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behavior disorders of children and adolescents. Arch Gen Psychiatry 1990; 47:419–426Crossref, Medline, Google Scholar
7 Virkunnen M, Kallio E, Rawlings R, Tokola R, Poland RE, Guidotti A, Nemeroff C, Bissette G, Kalogeras K, Karonen SL, Linnoila M: Personality profiles and state aggressiveness in Finnish alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry 1994; 51:28–33Crossref, Medline, Google Scholar
8 Higley JD, Mehlman PT, Taub DM, Higley SB, Suomi SJ, Vickers JD, Linnoila M: Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Arch Gen Psychiatry 1992; 49:436–441Crossref, Medline, Google Scholar
9 Higley JD, Suomi SJ, Linnoila M: A nonhuman primate model of type II excessive alcohol consumption? part 1: low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res 1996; 20:629–642Crossref, Medline, Google Scholar
10 Higley JD, Suomi SJ, Linnoila M: A nonhuman primate model of type II alcoholism? part 2: diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcohol Clin Exp Res 1996; 20:643–650Crossref, Medline, Google Scholar
11 Schuckit MA, Klein J, Twitchell G, Smith T: Personality test scores as predictors of alcoholism almost a decade later. Am J Psychiatry 1994; 151:1038–1042Link, Google Scholar
12 Schuckit M, Smith TL: An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry 1996; 53:202–210Crossref, Medline, Google Scholar
13 Fils-Aime ML, Eckhardt MJ, George DT, Brown GL, Mefford I, Linnoila M: Early-onset alcoholics have lower cerebrospinal fluid 5-hydroxyindoleacetic acid levels than late-onset alcoholics. Arch Gen Psychiatry 1996; 53:211–216Crossref, Medline, Google Scholar
14 Cloninger CR: A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Developments 1986; 4:167–226Google Scholar
15 Cloninger CR: Neurogenetic adaptive mechanisms in alcoholism. Science 1987; 236:410–416Crossref, Medline, Google Scholar
16 Irwin M, Schuckit M, Smith TL: Clinical importance of age at onset in type 1 and type 2 primary alcoholics. Arch Gen Psychiatry 1990; 47:320–324Crossref, Medline, Google Scholar
17 Cloninger CR, Bohman M, Sigvardsson S: Inheritance of alcohol abuse. Arch Gen Psychiatry 1981; 38:861–868Crossref, Medline, Google Scholar
18 Higley JD, Suomi SJ, Linnoila M: CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology (Berl) 1991; 103:551–556Crossref, Medline, Google Scholar
19 Clarke AS, Hedecker DR, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW: Rearing experience and biogenic amine activity in infant rhesus monkeys. Biol Psychiatry 1996; 40:338–352Crossref, Medline, Google Scholar
20 Goldman D: Why mice drink. Nature Genetics 1996; 13:137–138Crossref, Medline, Google Scholar
21 Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Mller CR, Hamer DH, Murphy DL: Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531Crossref, Medline, Google Scholar
22 Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, al-Tikriti MS, Sybirska EH, Zimmermann RC, Wiesniewski G, Neumeyer JL, Milius RA, Wang S, Smith EO, Roth RH, Charney DS, Hoffer PB, Innis RB: SPECT imaging of dopamine and serotonin transporters with [123I]β-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse 1993; 13:295–309Crossref, Medline, Google Scholar
23 Pirker W, Asenbaum S, Kasper S, Walter H, Angelberger P, Koch G, Pozzera A, Deecke L, Podreka I, Bruecke T: β-CIT SPECT demonstrates blockade of 5HT-uptake sites by citalopram in the human brain in vivo. J Neural Transm 1995; 100:247–256Crossref, Medline, Google Scholar
24 Doudet D, Hommer D, Higley JD, Andreason PJ, Moneman R, Suomi SJ, Linnoila M: Cerebral glucose metabolism, CSF 5-HIAA levels, and aggressive behavior in rhesus monkeys. Am J Psychiatry 1995; 152:1782–1787Link, Google Scholar
25 Bacopoulos NG, Redmond DE, Roth RH: Serotonin and dopamine metabolites in brain regions and cerebrospinal fluid of primate species: effects of ketamine and fluphenazine. J Neurochem 1979; 32:1215–1218Crossref, Medline, Google Scholar
26 Brammer GL, Raleigh MJ, McGuire MT, Rubinstein EH: Comparison of ketamine, physical restraint, halothane and pentobarbital: lack of influence on serotonergic measures in monkeys and rats. Neuropharmacology 26:1615–1621Google Scholar
27 Higley JD, Suomi SJ, Linnoila M: A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry 1992; 32:127–145Crossref, Medline, Google Scholar
28 Baldwin RM, Zea-Ponce Y, Zoghbi SS, Laruelle M, al-Tikriti MS, Sybirska EH, Malison RT, Neumeyer JL, Milius RA, Wang S, Stabin M, Smith EO, Charney DS, Hoffer PB, Innis RB: Evaluation of the monoamine uptake site ligand [123I]methyl 3 β-(4-iodophenyl)-tropane-2 β-carboxylate ([123I]-βCIT) in non-human primates: pharmacokinetics, biodistribution and SPECT brain imaging coregistered with MRI. Nuclear Medicine and Biology 1993; 20:597–606Crossref, Medline, Google Scholar
29 Jagust WJ, Eberling JL, Biegon A, Taylor SE, VanBrocklin HF, Jordan S, Hanrahan SM, Roberts JA, Brennan KM, Mathis CA: Iodine-123-5-iodo-6-nitroquipazine: SPECT radiotracer to image the serotonin transporter. J Nucl Med 1996; 37:1207–1214Medline, Google Scholar
30 Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea-Ponce Y, Zoghbi SS, Neumeyer JL, Charney DS, Hoffer PB, Innis RB: Graphical, kinetic, and equilibrium analyses of in vivo [123-I]β-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab 1994; 14:982–994Crossref, Medline, Google Scholar
31 Fisher RE, Morris ED, Alpert NM, Fischman AJ: In vivo imaging of neuromodulatory synaptic transmission using PET: a review of relevant neurophysiology. Human Brain Mapping 1995; 3:24–34Crossref, Google Scholar
32 Morris ED, Fisher RE, Alpert NM, Rauch SL, Fischman AJ: In vivo imaging of neuromoduation using positron emission tomography: Optimal ligand characteristics and task length for detection of activation. Hum Brain Mapping 1995; 3:35–55Crossref, Google Scholar
33 Mash DC, Staley JK, Doepel FM, Young SN, Palmour RM: Altered dopamine transporter densities in alcohol-preferring vervet monkeys. Neuroreport 1996; 7:457–462Crossref, Medline, Google Scholar
34 Baumgarten HG, Grozdanovic Z: Psychopharmacology of central serotonergic systems. Pharmacopsychiatry 1995; 28(suppl 2):73–79Google Scholar
35 Hesselbrock VM: The genetic epidemiology of alcoholism, in The Genetics of Alcoholism: Alcohol and Alcoholism, vol 1. Edited by Begleiter H, Kissin B. Oxford, Oxford University Press, 1995Google Scholar
36 Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, Eaves LJ: The structure of the genetic and environmental risk factors for six major psychiatric disorders in women. Arch Gen Psychiatry 1995; 52:374–383Crossref, Medline, Google Scholar
37 Higley JD, Thompson WW; Champoux M, Goldman D, Hasert MF, Kraemer GW, Scanlan JM, Suomi SJ, Linnoila M: Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoamine metabolites in rhesus monkeys (Macaca mulatta). Arch Gen Psychiatry 1993; 50:615–623Crossref, Medline, Google Scholar
38 Oxenstierna G, Edman G, Iselius L, Oreland L, Ross SB, Sedvall G: Concentrations of monoamine metabolites in the cerebrospinal fluid of twins and unrelated individuals—a genetic study. J Psychiatr Res 1986; 20:19–29Crossref, Medline, Google Scholar
39 Kraemer GW, Ebert MH, Schmidt DT, McKinney WT: A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology 1989; 2:175–189Crossref, Medline, Google Scholar
40 Shannon C, Champoux M, Higley JD, Dodson A, Higley SB, Suomi SJ, Linnoila M: Interindividual differences in neonatal serotonin functioning: stability of interindividual differences and behavioral correlates (abstract). Am J Primatology 1995; 36:155Google Scholar
41 Johnson EO, van den Bree MBM, Pickins RW: Subtypes of alcohol-dependent men: a typology based on relative genetic and environmental loading. Alcohol Clin Exp Res 1996; 20:1472–1480Crossref, Medline, Google Scholar



