Elevated Central Serotonin Transporter Binding Availability in Acutely Abstinent Cocaine-Dependent Patients
Abstract
OBJECTIVE: Recent work has underscored the role of serotonergic neurotransmission in chronic neural adaptations to cocaine dependence. The authors tested for evidence of serotonergic dysfunction during acute abstinence from cocaine, a period of high risk for relapse in cocaine dependence.METHOD: Binding availability of dopamine transporters and serotonin transporters was measured in 15 cocaine-dependent subjects during acute abstinence and in 37 healthy comparison subjects by using [123I]β-CIT and single photon emission computed tomography.RESULTS: Significant increases in diencephalic and brainstem serotonin transporter binding (16.7% and 31.6%, respectively) were observed in cocaine-dependent subjects. Brainstem serotonin transporter binding was significantly inversely correlated with age across diagnostic groups.CONCLUSIONS: These findings provide further evidence of serotonergic dysfunction during acute abstinence from chronic cocaine use. Age-related decline in brainstem serotonin transporter binding may underlie the poor response to selective serotonin reuptake inhibitor antidepressants seen in some elderly depressed patients.
Cessation of chronic cocaine abuse is associated with withdrawal symptoms that include dysphoric mood and cocaine craving, which may be important triggers for relapse to cocaine abuse (1). Cocaine blocks reuptake of dopamine, serotonin, and norepinephrine into presynaptic neurons by binding to the neuronal membrane transporters for these monoamines, thereby acutely increasing extracellular levels of all three monoamines (2). Neural adaptations to these effects of cocaine exposure, which persist following cessation of drug intake, may mediate cocaine withdrawal symptoms (3).
The central role of acute increases in extracellular dopamine in the reinforcing properties of cocaine and, conversely, the role of depleted brain dopamine in cocaine withdrawal have long been recognized (3, 4). However, pharmacotherapeutic strategies for treatment of cocaine dependence that exclusively target the dopamine system have been ineffective (5), and mice lacking the dopamine transporter self-administer cocaine, suggesting the importance of other neurotransmitter systems in this disorder (6).
Recent work has underscored the role of serotonergic neurotransmission in cocaine dependence. Rat microdialysis studies have shown that withdrawal of unlimited access to self-administered intravenous cocaine is associated with severe depletion of extracellular serotonin in the nucleus accumbens and potentiation of the dopamine-release-enhancing properties of serotonin (7). Both l-tryptophan and fluoxetine increase brain serotonin concentrations and decrease cocaine self-administration in animals, although not when high doses of cocaine are used (8). In mice, knockout of the serotonin transporter leads to enhancement of conditioned location preference for cocaine, a measure of the degree of cocaine-related reward experienced by these animals (9).
In humans, transient depletion of serotonin by means of administration of a solution of amino acids lacking the serotonin precursor tryptophan decreases the subjective effects of cocaine administration (10) and attenuates cue-induced cocaine craving (11). Fluoxetine pretreatment, which increases extracellular serotonin concentrations, significantly attenuates the subjective effects of cocaine administration in cocaine-dependent patients (12). However, double-blind, placebo-controlled trials of fluoxetine in the treatment of cocaine-dependent humans have failed to demonstrate efficacy (13).
Human postmortem studies have shown decreased serotonin transporter binding sites, quantified with the radioligand [123I]2-carbomethoxy-3-(4-iodophenyl)tropane ([123I]β-CIT), in cocaine users with concurrent opiate use (14). However, since opiates potently decrease brain [123I]β-CIT binding (15), it is unclear whether the decrease in serotonin transporter binding observed in this postmortem study group was due to the singular or combined effects of opiates or cocaine. In contrast, a recent postmortem study of non-opiate-using, cocaine-overdose victims demonstrated increased serotonin transporter binding in striatal and cortical regions, quantified with [123I]β-CIT in the presence of benztropine to occlude binding to the dopamine transporter (16).
In the present study, dopamine transporter and serotonin transporter binding were examined in acutely abstinent non-opiate-using, cocaine-dependent patients and healthy comparison subjects by using [123I]β-CIT and single photon emission computed tomography (SPECT). Previous work has shown that [123I]β-CIT binding in the striatum is displaced by the selective dopamine transporter inhibitor 1-[2-bis-(4-fluorophenyl) methoxy] ethyl]-4-[3-phenylpropyl] piperazine (GBR 12909) but not by the selective serotonin transporter inhibitor citalopram, whereas the reverse is true for binding of this ligand in the diencephalon and brainstem (17). This indicates that striatal [123I]β-CIT binding reflects dopamine transporter binding availability, whereas diencephalic and brainstem [123I]β-CIT binding reflects serotonin transporter binding availability. Brainstem [123I]β-CIT binding chiefly reflects binding to the serotonin transporter on neurons of the raphe nuclei, locus ceruleus, substantia nigra, ventral tegmental area, and superior and inferior colliculi (18). Improved methods of image analysis, including nonuniform attenuation correction of SPECT images and coregistration of SPECT scans with magnetic resonance images (MRIs), were used in the present study to permit accurate and reliable identification of diencephalic, brainstem, and cerebellar regions.
Given the recent postmortem findings in non-opiate-using, cocaine-overdose victims, the current study was performed to test the hypothesis that brainstem serotonin transporter binding availability is increased in cocaine-dependent patients. In addition, the relationship between symptoms of depression during acute withdrawal from cocaine and serotonin transporter binding availability was examined. Finally, the presence of age-related changes in serotonin transporter binding was investigated.
Method
Subjects
Healthy and cocaine-dependent subjects were assessed by means of the Structured Clinical Interview for DSM-IV Axis I Disorders (19), physical and neurologic examinations, and routine laboratory tests to rule out medical and neurologic illness. Fifteen subjects meeting the criteria for chronic cocaine dependence (five women and 10 men; mean age=33.7 years, SD=4.8, range=26–43) participated; five of these subjects also had comorbid attention deficit hyperactivity disorder (ADHD) (20). There were no other comorbid axis I disorders among the subjects. Patients who used opiates within the preceding 6 months or met the criteria for opiate dependence or physiologic alcohol dependence at any time were excluded. On average, cocaine-dependent subjects smoked 5.2 g (SD=3.2) of crack cocaine per week, consumed 4.5 alcoholic drinks per day (SD=5.9), usually in the context of cocaine use, and had used crack cocaine for 13.0 years (SD=6.0). All cocaine-dependent subjects had positive urine toxicology for the cocaine metabolite benzoylecgonine on admission to an unlocked inpatient unit, in which abstinence from alcohol and drug use before [123I]β-CIT injection and scanning were monitored by means of repeat urine toxicology and breathalyzer screens. Cocaine-dependent subjects were abstinent from drug and alcohol use a mean 3.7 days (SD=3.8) before injection of [123I]β-CIT. Thirty-seven healthy comparison subjects (17 women and 20 men; mean age=35.8 years, SD=9.0, range=22–56) participated and were free of any previous or current axis I disorders, including substance or alcohol abuse or dependence. Healthy comparison subjects consumed, on average, 0.4 alcoholic drinks per day (SD=0.6) (information about alcohol consumption was available from 34 healthy subjects). Both healthy and cocaine-dependent subjects were free of current or previous psychotropic medication exposure.
All subjects provided written informed consent for participation in this study, which was approved by the Yale Human Investigations Committee and the Human Studies Subcommittee, West Haven Campus, Department of Veterans Affairs Connecticut Healthcare System.
Symptom Assessment
Among the cocaine-dependent patients, symptoms of depression were measured by using the Hamilton Depression Rating Scale (21), and symptoms of ADHD were measured by using the Wender Utah Rating Scale (22). A measure of impulsivity and aggression, which are common features of ADHD, was derived by summing the following items of the Wender Utah scale: 7, 9, 13, 24, 27, 28, 35, 41, and 42.
SPECT Imaging
[123I]β-CIT was synthesized as has been described (17). Subjects were pretreated with stable iodine before injection of a mean 6.0 mCi (SD=0.2) of [123I]β-CIT. SPECT scanning was performed a mean 21.8 hours (SD=1.3) after injection, when [123I]β-CIT binding is at equilibrium and dopamine transporter and serotonin transporter binding potential can be estimated by computing the ratio of specific to nondisplaceable uptake (V3″) (23). SPECT scanning was performed with a triple-headed, Picker PRISM 3000 camera (Cleveland), equipped with a low-energy, high-resolution fan beam collimator. Resolution of this system, estimated with a line source in a scattering medium, is 12 mm full width at half maximum in all three axes. SPECT scanning began with the simultaneous acquisition of a transmission scan by using a line source containing 20 mCi 57Co and a 15-minute emission scan, followed by removal of the line source and acquisition of a 24-minute emission scan. Both emission scans were acquired in continuous mode with the following parameters: 128 ≥ 128 matrix, 3.56-mm slice thickness, 120° angular range, 3° angular step, 40 steps, 36 seconds per step, and 15.5-cm radius of rotation. An axial MRI scan was acquired on all subjects for coregistration with SPECT emission scans.
Image Analysis
SPECT emission scans were reconstructed from photo peak counts (20% energy window centered on 159 kiloelectron volts) by using a Butterworth filter (power factor=10, cutoff=0.24 cycles per second). Transmission scans were reconstructed by using a Bayesian algorithm and a smoothing Gibbs prior with locally developed software (24). The two emission images were then coregistered by means of SPM 96, and the same three-dimensional transformation was applied to the transmission image. Nonuniform attenuation correction of the second emission image was then performed by means of locally developed software (24). MR scans were then coregistered to the SPECT transmission scan by using a surface-matching algorithm in MEDx (Sensor Systems, Sterling, Va.) (25) and reoriented so that the axial plane was parallel to the intercommissural plane. The same three-dimensional transformation was then applied to the emission image. The coregistered, reoriented MRI was used to guide placement of standardized, two-dimensional, region-of-interest templates for the right and left striatum (eight slices), diencephalon (four slices), brainstem (eight slices), and right and left cerebellum (six slices). Three-dimensional volumes of interest were then created for the striatum (total of eight right and eight left striatal regions of interest, 9.5 cm3 each), diencephalon (four slices, 1.9 cm3), brainstem (eight slices, 3.7 cm3), and cerebellum (six right and left cerebellar regions of interest, 43.2 cm3 each). The diencephalic volume of interest included the thalamus and hypothalamus, while the brainstem volume of interest included the superior and inferior colliculi and nuclei of the midbrain (substantia nigra, ventral tegmental area, nucleus linearis, and dorsal and medial raphe nuclei) and pons (nucleus raphe pontis and nucleus raphe magnus) (25). The three-dimensional, volume-of-interest template was then transferred to the coregistered, reoriented SPECT emission scan to determine regional activity (counts per minute per pixel).
Scans from nine subjects measured by two raters (L.K.J. and J.K.S.) and remeasured by a single rater revealed inter- and intrarater reliabilities (intraclass correlation coefficients [27]), respectively, of 0.99 and 0.98 for striatal activity, 0.96 and 0.99 for diencephalic activity, 0.91 and 0.99 for brainstem activity, and 0.97 and 0.99 for cerebellar activity. All scans for this study were measured by a single rater who was blind to patient diagnosis. Given that the cerebellum is relatively devoid of the dopamine and serotonin transporters (28, 29), cerebellar activity was used as a measure of nondisplaceable uptake. Thus, dopamine transporter binding availability was estimated by using regional radioactivities measured from striatal and cerebellar volumes of interest in the following equation: [striatal – cerebellum]/cerebellum=V3″. Serotonin transporter binding availability was estimated by substituting, separately, diencephalic and brainstem for striatal volume of interest regional radioactivities in this equation.
Statistical Analyses
Differences between cocaine-dependent patients and healthy comparison subjects in demographic variables were assessed by means of chi-square and t tests for independent groups. Group differences in dopamine transporter and serotonin transporter binding availability were examined by means of repeated measures analysis of covariance (ANCOVA), with diagnosis as the independent variable and age as the covariate. Relationships between serotonin transporter and dopamine transporter binding availability and age were examined by using partial correlation coefficients to control for the effect of diagnosis. Relationships between dopamine transporter and serotonin transporter binding and symptoms of depression during acute cocaine withdrawal and symptoms of impulsivity and aggression were examined by using partial correlation coefficients controlling for the effect of age. Effects of ADHD on serotonin transporter and dopamine transporter availability were assessed in an exploratory analysis performed by using repeated measures ANCOVAs, with diagnosis (healthy, cocaine-dependent without ADHD, or cocaine-dependent with ADHD) as the between-subjects variable and age as the covariate.
Results
Cocaine-dependent and healthy subjects did not differ in age, sex, or number of cigarettes smoked per day. However, a significantly greater proportion of cocaine-dependent patients smoked cigarettes (11 cocaine-dependent patients and 15 healthy subjects) (χ2=4.6, df=1, p=0.03). Cocaine-dependent subjects consumed more alcohol than healthy subjects (t=4.0, df=47, p=0.0002). A significantly greater proportion of the healthy subjects were European American (χ2=14.7, df=1, p=0.0001). On average, healthy subjects had nearly 2 years more of education than cocaine-dependent subjects (35 healthy subjects: mean=14.6 years, SD=1.9; 15 cocaine-dependent subjects: mean=12.7 years, SD=1.5) (t=3.4, df=48, p=0.001).
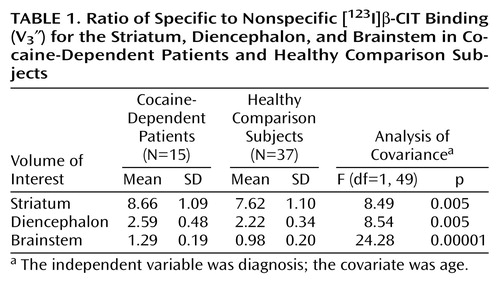
Mean values for V3″ for striatal, diencephalic, and brainstem volumes of interest for cocaine-dependent and healthy subjects are shown in Table 1. As shown, striatal V3″, reflecting dopamine transporter availability, increased by 13.6% in cocaine-dependent subjects, whereas diencephalic and brainstem V3″, reflecting serotonin transporter availability, increased by 16.7% and 31.6%, respectively, in cocaine-dependent subjects. Neither smoking status nor amount of alcohol consumed changed the results of this analysis when entered as covariates in an ANCOVA. Brainstem V3″ for cocaine-dependent patients and healthy comparison subjects is displayed in Figure 1.
Partial correlation coefficients, controlling for diagnosis, computed between age and V3″ for the striatum, diencephalon, and brainstem were as follows: striatum: r=–0.37, N=52, p=0.007; diencephalon: r=–0.18, N=52, p=0.16; and brainstem: r=–0.30, N=52, p=0.03. Partial correlation coefficients, controlling for age, computed between Hamilton depression scale scores and V3″ for the striatum, diencephalon, and brainstem within the cocaine-dependent group were not significant. Similarly, partial correlation coefficients, controlling for age, computed between scores on the Wender Utah Rating Scale subscale measure of impulsivity and aggression and V3″ for the striatum, diencephalon, and brainstem within the cocaine-dependent group were not significant. Partial correlation coefficients, controlling for age, computed between length of abstinence from cocaine before [123I]β-CIT injection, number of years of cocaine use, and recent weekly amounts of cocaine used and V3″ for the striatum, diencephalon, and brainstem within the cocaine-dependent group were not significant. The only exception was a significant negative correlation between recent weekly amounts of cocaine used and diencephalic V3″ (r=–0.62, N=15, p=0.01).
Exploratory ANCOVAs with diagnosis (healthy, cocaine-dependent without ADHD, and cocaine-dependent with ADHD) as the independent variable and age as the covariate revealed a significant main effect of diagnosis across all volumes of interest (striatum: F=4.16, df=1, 48, p=0.02; diencephalon: F=4.60, df=1, 48, p=0.01; and brainstem: F=11.95, df=1, 48, p=0.00006), with Bonferroni post hoc t tests indicating that V3″ was increased at all three regions for cocaine-dependent patients with and without ADHD relative to healthy comparison subjects. There were no differences between cocaine-dependent patients with and without ADHD in dopamine transporter or serotonin transporter availability.
Discussion
In the present group of acutely abstinent, cocaine-dependent patients, central dopamine transporter and serotonin transporter binding was significantly increased relative to that seen in healthy comparison subjects. To the best of our knowledge, this is the first demonstration of increased diencephalic and brainstem serotonin transporter binding availability in acutely abstinent, living, cocaine-dependent patients. Increases in serotonin transporter binding availability associated with cocaine dependence was greatest in the brainstem—nearly twice that observed in the diencephalon—likely reflecting the high concentration of serotonin transporter in this region (30). The significant negative correlation between recent weekly amounts of cocaine used and diencephalic serotonin transporter binding availability must be interpreted with caution, as other regions did not exhibit this relationship. In addition, this measure of cocaine use was based on self-report and thus may have been somewhat inaccurate.
The increase in striatal dopamine transporter binding availability observed in cocaine-dependent subjects was similar in magnitude to that observed in a separate group of cocaine-dependent patients in this laboratory (31) but smaller in magnitude to that observed in two postmortem studies of cocaine abusers (14, 32). This difference in magnitude of increase in dopamine transporter binding may reflect the shorter intervals between last cocaine use and measurement of dopamine transporter binding in postmortem studies than in in vivo imaging studies. However, the failure of other postmortem (33, 34) and in vivo (35) studies of cocaine-dependent subjects to show increases in dopamine transporter binding availability suggests that additional factors, such as the radioligand used to measure binding availability and cumulative cocaine exposure (36), may also influence the direction and magnitude of changes in the dopamine transporter binding measured.
The findings in the present study are consistent with previous work supporting a role for both dopaminergic and serotonergic systems in chronic neural adaptation to cocaine abuse (4, 6). Since dopamine transporters and serotonin transporters function to terminate synaptic neurotransmission by means of sodium-dependent reuptake of dopamine and serotonin, respectively (37), chronic inhibition of these monoamine transporters from repeated cocaine use may induce a compensatory up-regulation. Alternatively, cocaine has been shown to suppress serotonin synthesis, leading to decreased tissue levels of serotonin and its metabolite, 5-hydroxyindoleacetic acid (5-HIAA) (38). Thus, the increases in diencephalic and brainstem [123I]β-CIT binding observed in cocaine-dependent patients may instead reflect increases in the number of available binding sites on the serotonin transporter because of decreased competition from endogenous serotonin (39).
If [123I]β-CIT binding bears a direct relationship to removal of synaptic dopamine and serotonin by the dopamine transporter and serotonin transporter, then elevated dopamine transporter and serotonin transporter binding availability during acute abstinence from cocaine could be expected to lower synaptic concentrations of these monoamines. Consistent with this, in nonhuman primates, serotonin transporter binding availability, as measured by [123I]β-CIT and SPECT, has been found to be negatively correlated with cerebrospinal fluid levels of 5-HIAA (40). However, recent work in rats has shown that, under some conditions, changes in serotonin transporter binding availability do not parallel changes in the velocity of uptake of synaptic serotonin (41).
Dopamine and serotonin depletion both induce depressive relapse in remitted, depressed patients (42, 43). Striatal dopamine transporter binding availability, as measured by [123I]β-CIT and SPECT, has been found to be increased in a group of patients with major depression (44). However, central serotonin transporter binding availability has been found to be reduced in major depression (45) and negatively correlated with depressive symptoms during alcohol withdrawal (46), which suggests that reduced serotonergic neurotransmission leading to compensatory down-regulation of the serotonin transporter may be the primary serotonergic abnormality in depression. Our failure to observe a relationship between depressive symptoms during acute cocaine withdrawal and serotonin transporter or dopamine transporter binding availability likely reflects the very low rates of depressive symptoms reported by this group. This is consistent with previous observations that depressive symptoms experienced by patients withdrawing from chronic cocaine use in the inpatient setting are mild (47). A low central serotonin level has also been associated with impulsivity and aggression (48); however, levels of these symptoms among cocaine-dependent patients were not significantly correlated with serotonin transporter or dopamine transporter binding availability.
Analyses that controlled for group differences in cigarette smoking and alcohol consumption indicated that these variables did not contribute to the elevations in dopamine transporter and serotonin transporter binding availability observed in cocaine-dependent subjects. In fact, previous work in alcohol-dependent patients has shown that dopamine transporter binding is low during very early withdrawal (the first 4 days) and normalizes after 4 weeks of abstinence (49), whereas serotonin transporter binding appears to remain low for at least 3 weeks after the cessation of drinking (46).
The inverse relationship between age and dopamine transporter binding availability observed in this study was consistent with, but weaker than, that previously observed in groups ranging in age from 20 to the mid-80s (50, 51), most likely because of our exclusion of subjects above the age of 60 years. The significant inverse relationship between brainstem serotonin transporter binding availability and age was weaker than that observed for striatal dopamine transporter binding availability. As noted above, studies of age-related changes in serotonin transporter binding availability have produced conflicting results. Two human postmortem studies using [3H]paroxetine and examining the frontal cortex (52) and the dorsal raphe nucleus (53) found no age-related changes in serotonin transporter binding, whereas a third using [3H]imipramine found increased serotonin transporter binding with age in the frontal and parietal cortices and in the hypothalamus (54). One in vivo study of healthy and alcoholic subjects using [123I]β-CIT and SPECT found no age-related changes in serotonin transporter binding (46, 55, 56), whereas two [123I]β-CIT SPECT studies have demonstrated age-related declines in serotonin transporter binding in healthy subjects. Cortical serotonin transporter binding, measured by using [3H]paroxetine and [3H]imipramine, has been found to increase with age in one rat strain (Sprague-Dawley) (41), show no age-related changes in another rat strain (Fischer) (57, 58), and decline with age in a mammalian species (the house musk shrew) (58). Of importance, one study of the Fischer rat showed early maturational increases in hippocampal serotonin transporter binding, followed by significant declines during senescence (57). These disparate findings suggest that the observation of age-related changes in central serotonin transporter binding availability may be sensitive to a number of factors, including the radioligand used, as well as the brain region, species, and age range examined. More work will be needed to achieve a complete understanding of the relationship between age and serotonin transporter binding availability. However, the age-related decline in brainstem serotonin transporter binding availability observed in the present study and in two others (55, 56) may mediate the poor response to selective serotonin reuptake inhibitors seen in some groups of elderly depressed patients (59).
Limitations of this study include the lack of a direct measure of impulsivity and aggression, poor spatial resolution of SPECT, and the use of a radiotracer that binds to both the dopamine transporter and serotonin transporter. Although earlier work, previously noted, has demonstrated specific displacement of [123I]β-CIT in the striatum by pharmaceuticals that bind to the dopamine transporter and in the brainstem by pharmaceuticals that bind to the serotonin transporter (17), the contribution to brainstem [123I]β-CIT binding from the dopamine transporter, particularly in the region of the substantia nigra, cannot be completely ruled out. Thus, these results will require replication with a serotonin transporter selective radiotracer and a higher-resolution imaging modality, such as positron emission tomography.
 |
Presented in part at the College of Problems in Drug Dependence, Acapulco, Mexico, June 12–17, 1999. Received Sept. 13, 1999; revision received Dec. 14, 1999; accepted Jan. 27, 2000. From the Department of Psychiatry, Yale University School of Medicine, New Haven, Conn.; and the West Haven Campus, VA Connecticut Healthcare System. Address correspondence to Dr. Jacobsen, Psychiatry 116A, 950 Campbell Ave., West Haven, CT 06516; [email protected] (e-mail). Supported in part by NIH grant RR-00125 from the National Center for Research Resources and by NIH grants DA-00167, DA-04060, DA-09250, DA-10764, and DA-00397 from the National Institute on Drug Abuse. The authors thank the staffs of the Yale Neurospect Center, the Yale MRI Center, and the Yale Medications Development Research Center for their assistance with this study; Louis A. Amici for radiotracer synthesis; and Sylvia Hu, Ph.D., for statistical consultation.
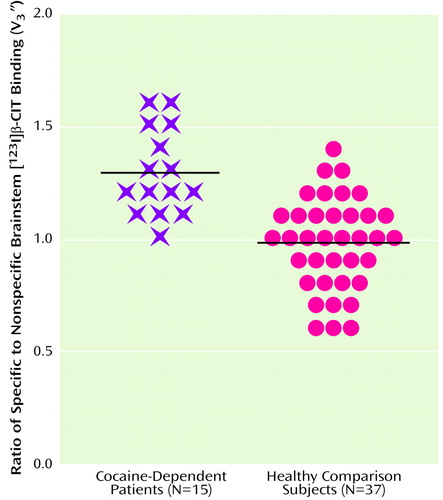
Figure 1. Ratios of Specific to Nonspecific [123I]β-CIT Binding (V3″) for the Brainstem in Cocaine-Dependent Patients and Healthy Comparison Subjects
1. Gawin FH, Kleber HD: Abstinence symptomatology and psychiatric diagnosis in chronic cocaine abusers. Arch Gen Psychiatry 1986; 43:107–113Crossref, Medline, Google Scholar
2. Ritz MC, Cone EJ, Kuhar MJ: Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure activity study. Life Sci 1990; 46:635–645Crossref, Medline, Google Scholar
3. Koob GF, Bloom FE: Cellular and molecular basis of drug dependence. Science 1988; 242:715–723Crossref, Medline, Google Scholar
4. Dackis CA, Gold MS: New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev 1985; 9:469–477Crossref, Medline, Google Scholar
5. Mendelson JH, Mello NK: Management of cocaine abuse and dependence. N Engl J Med 1996; 334:965–972Crossref, Medline, Google Scholar
6. Baumann MH, Rothman RB: Serotonergic dysfunction during cocaine withdrawal: implications for cocaine-induced depression, in Neurochemistry of Drug Abuse. New York, CRC Press, 1998, pp 463–484Google Scholar
7. Parsons LH, Koob GF, Weiss F: Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. J Pharmacol Exp Ther 1995; 274:1182–1191Google Scholar
8. Walsh SL, Cunningham KA: Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl) 1997; 130:41–58Crossref, Medline, Google Scholar
9. Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR: Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA 1998; 95:7699–7704Google Scholar
10. Aronson SC, Black JE, McDougle CJ, Scanley BE, Jatlow P, Kosten TR, Heninger GR, Price LH: Serotonergic mechanisms of cocaine effects in humans. Psychopharmacology (Berl) 1995; 119:179–185Crossref, Medline, Google Scholar
11. Satel SL, Krystal JH, Delgado PL, Kosten TR, Charney DS: Tryptophan depletion and attenuation of cue-induced craving for cocaine. Am J Psychiatry 1995; 152:778–783Link, Google Scholar
12. Walsh SL, Preston KL, Sullivan JT, Fromme R, Bigelow GE: Fluoxetine alters the effects of intravenous cocaine in humans. J Clin Psychopharmacol 1994; 14:396–407Medline, Google Scholar
13. Grabowski J, Rhoades H, Elk R, Schmitz J, Davis C, Creson D, Kirby K: Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled, double-blind trials. J Clin Psychopharmacol 1995; 15:163–174Crossref, Medline, Google Scholar
14. Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, DelProposto ZS, Hill E, Cassin BJ, Watson SJ, Cook EH: Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. Am J Psychiatry 1998; 155:207–213Link, Google Scholar
15. Bergstrom KA, Jolkkonen J, Kuikka JT, Akerman KK, Viinamaki H, Airaksinen O, Lansimies E, Tiihonen J: Fentanyl decreases beta-CIT binding to the dopamine transporter. Synapse 1998; 29:413–415Crossref, Medline, Google Scholar
16. Basile MJ, Izenwasser S, Staley JK, Mash DC: Serotonin transporter regulation in human cocaine abusers. Abstracts of the Society for Neuroscience 1999; 25:562Google Scholar
17. Laruelle M, Baldwin RM, Malison RT, Zea-Ponce Y, Zoghbi SS, Al-Tikriti M, Sybirska EH, Zimmermann RC, Wisniewski G, Neumeyer JL, Milius RA, Wang S, Smith EO, Roth RH, Charney DS, Hoffer PB, Innis RB: SPECT imaging of dopamine and serotonin transporter with [ 123I] β-CIT: pharmacological characterization of brain uptake in nonhuman primates. Synapse 1993; 13:295–309Crossref, Medline, Google Scholar
18. Cortes R, Soranoi E, Pazos A, Probst A, Palacios JM: Autoradiography of antidepressant binding sites in the human brain: localization using [3H]imipramine and [3H]paroxetine. Neuroscience 1988; 27:473–496Crossref, Medline, Google Scholar
19. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1997Google Scholar
20. Kaufman J, Birmaher B, Brent D, Rao U, Ryan N: The Schedule for Affective Disorders and Schizophrenia for School Aged Children: Present and Lifetime Version. Pittsburgh, University of Pittsburgh, Western Psychiatric Institute and Clinic, 1996Google Scholar
21. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
22. Ward MF, Wender PH, Reimherr FW: The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 1993; 150:885–890Link, Google Scholar
23. Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea-Ponce Y, Zoghbi SS, Neumeyer JL, Charney DS, Hoffer PB, Innis RB: Graphical, kinetic and equilibrium analyses of in vivo [123I] β-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab 1994; 14:982–994Crossref, Medline, Google Scholar
24. Rajeevan N, Zubal IG, Ramsby SQ, Zoghbi SS, Seibyl J, Innis RB, Zoghbi S: Significance of nonuniform attenuation correction in quantitative brain SPECT imaging. J Nucl Med 1998; 39:1719–1726Google Scholar
25. Pelizzari CA, Chen GTY, Spelbring DR, Weichselbaum RR, Chen CT: Accurate three-dimensional registration of CT, PET and/or MR images of the brain. J Comput Assist Tomogr 1989; 13:20–26Crossref, Medline, Google Scholar
26. Parent A: Carpenter’s Human Neuroanatomy. Baltimore, Williams & Wilkins, 1996Google Scholar
27. Fleiss JL: The Design and Analysis of Clinical Experiments. New York, John Wiley & Sons, 1986Google Scholar
28. Hall H, Halldin C, Guilloteau D, Chalon S, Emond P, Besnard J, Farde L, Sedvall G: Visualization of the dopamine transporter in the human brain postmortem with the new selective ligand [ 125I]PE 2I. Neuroimage 1999; 9:108–116Crossref, Medline, Google Scholar
29. B㢫str�, Bergstr�, Marcusson J: High affinity [ 3H]paroxetine binding to serotonin uptake sites in human brain tissue. Brain Res 1989; 486:261–268Crossref, Medline, Google Scholar
30. Dahlstrom A, Fuxe K: Evidence for the existence of monoamine-containing neurons in the central nervous system, I: demonstration of monoamines in the cell bodies of brainstem neurons. Acta Physiol Scand Suppl 1964; 232:1–55Google Scholar
31. Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB: Elevated striatal dopamine transporters in acute cocaine abstinence as measured by [123I] β-CIT SPECT. Am J Psychiatry 1998; 155:832–834Abstract, Google Scholar
32. Staley JK, Hearn L, Ruttenber J, Wetli CV, Mash DC: High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. J Pharmacol Exp Ther 1994; 271:1678–1685Google Scholar
33. Hurd YL, Herkenham M: Molecular alterations in the neostriatum of human cocaine addicts. Synapse 1993; 13:357–369Crossref, Medline, Google Scholar
34. Wilson JM, Levey AI, Bergeron C, Kalasinsky K, Ang L, Peretti F, Adams VI, Smialek J, Anderson WR, Shannak K, Deck J, Niznik HB, Kish SJ: Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol 1996; 40:428–439Crossref, Medline, Google Scholar
35. Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Gatley SJ, MacGregor RR, Wolf AP: Cocaine uptake is decreased in the brain of detoxified cocaine abusers. Neuropsychopharmacology 1996; 14:159–168Crossref, Medline, Google Scholar
36. Little KY, McLaughlin DP, Zhang L, McFinton PR, Dalack GW, Cook EH, Cassin BJ, Watson SJ: Brain dopamine transporter messenger RNA and binding sites in cocaine users: a postmortem study. Arch Gen Psychiatry 1998; 55:793–799Crossref, Medline, Google Scholar
37. Amara SG, Kuhar MJ: Neurotransmitter transporters: recent progress. Annu Rev Neurosci 1993; 16:73–93Crossref, Medline, Google Scholar
38. Baumann MH, Raley TJ, Partilla JS, Rothman RB: Biosynthesis of dopamine and serotonin in the rat brain after repeated cocaine injections: a microdissection mapping study. Synapse 1993; 14:40–50Crossref, Medline, Google Scholar
39. Jones DW, Gorey JG, Zajicek K, Das B, Urbina RA, Lee KS, Heinz A, Knable MB, Higley D, Weinberger DR, Linnoila M: Depletion-restoration studies reveal the impact of endogenous dopamine and serotonin on [123I] β-CIT SPECT imaging in primate brain (abstract). J Nucl Med 1998; 39:42PMedline, Google Scholar
40. Heinz A, Higley JD, Gorey JG, Saunders RC, Jones DW, Hommer D, Zajicek K, Suomi SJ, Lesch K-P, Weinberger DR, Linnoila M: In vivo association between alcohol intoxication, aggression, and serotonin transporter availability in nonhuman primates. Am J Psychiatry 1998; 155:1023–1026Google Scholar
41. Brunello N, Riva M, Volterra A, Racagni G: Age-related changes in 5HT uptake and [3H]imipramine binding sites in rat cerebral cortex. Eur J Pharmacol 1985; 110:393–394Crossref, Medline, Google Scholar
42. Delgado PM, Miller HL, Salomon RM, Licinio J, Krystal JH, Moreno FA, Heninger GR, Charney DS: Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol Psychiatry 1999; 46:212–220Crossref, Medline, Google Scholar
43. Berman RM, Narasimhan M, Miller HL, Anand A, Cappiello A, Oren DA, Heninger GR, Charney DS: Transient depressive relapse induced by catecholamine depletion: potential phenotypic vulnerability marker? Arch Gen Psychiatry 1999; 56:395–403Google Scholar
44. Laasonen-Balk T, Kuikka J, Viinamaki H, Husso-Saastamoinen M, Lehtonen J, Tiihonen J: Striatal dopamine transporter density in major depression. Psychopharmacology (Berl) 1999; 144:282–285Crossref, Medline, Google Scholar
45. Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS: Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 1998; 44:1090–1098Google Scholar
46. Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, Gorey JG, Doty L, Geyer C, Lee KS, Coppola R, Weinberger DR, Linnoila M: Reduced central serotonin transporters in alcoholism. Am J Psychiatry 1998; 155:1544–1549Google Scholar
47. Satel SL, Price LH, Palumbo JM, McDougle CJ, Krystal JH, Gawin F, Charney DS, Heninger GR, Kleber HD: Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Am J Psychiatry 1991; 148:1712–1716Google Scholar
48. Higley JD, Linnoila M: Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior: a nonhuman primate model investigating genetic and environmental influences on neurotransmission. Ann NY Acad Sci 1997; 836:39–56Crossref, Medline, Google Scholar
49. Laine TPJ, Ahonen A, Torniainen P, Heikkila J, Pyhtinen J, Rasanen P, Niemela O, Hillbom M: Dopamine transporters increase in human brain after alcohol withdrawal. Mol Psychiatry 1999; 4:189–191Crossref, Medline, Google Scholar
50. van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Wallace E, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Charney DS, Hoffer PB: Age-related decline in striatal dopamine transporter binding with iodine-123-beta-CITSPECT. J Nucl Med 1995; 36:1175–1181Google Scholar
51. Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley SJ, Hitzemann R, Smith G, Fields SD, Gur R: Dopamine transporters decrease with age. J Nucl Med 1996; 37:554–559Medline, Google Scholar
52. Arranz B, Eriksson A, Mellerup E, Plenge P, Marcusson J: Effect of aging in human cortical pre- and postsynaptic serotonin binding sites. Brain Res 1993; 620:163–166Crossref, Medline, Google Scholar
53. Stockmeier CA, Shapiro LA, Haycock JW, Thompson PA, Lowy MT: Quantitative subregional distribution of serotonin-1A receptors and serotonin transporters in the human dorsal raphe. Brain Res 1996; 727:1–12Crossref, Medline, Google Scholar
54. Severson JA, Marcusson JO, Osterburg HH, Finch CE, Winblad B: Elevated density of [3H]imipramine binding in aged human brain. J Neurochem 1985; 45:1382–1389Google Scholar
55. Pirker W, Asenbaum S, Hauk M, Kandlhofer S, Tauscher J, Willeit M, Neumeister A, Praschak-Reider N, Angelberger P, Brucke T: Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. J Nucl Med 2000; 41:36–44Medline, Google Scholar
56. van Dyck CH, Malison RT, Seibyl JP, Laruelle M, Klumpp H, Zoghbi SS, Baldwin RM, Innis RB: Age related decline in central serotonin transporter availability with [123I]β-CIT SPECT. Neurobiol Aging (in press)Google Scholar
57. Arora RC, Gulati A, Crayton JW: Aging and 3H-paroxetine binding in rat brain: effect of imipramine and tetrahydroacridine. Life Sci 1993; 52:1767–1775Google Scholar
58. Yamaguchi T, Yamagata A: Serotonergic ligand binding in aging brain of experimental animals. Biochem Res 1991; 16:469–473Google Scholar
59. Roose SP, Glassman AH, Attia E, Woodring S: Comparative efficacy of selective serotonin reuptake inhibitors and tricyclics in the treatment of melancholia. Am J Psychiatry 1994; 151:1735–1739Google Scholar



