Elevated Striatal Dopamine Transporters During Acute Cocaine Abstinence as Measured by [123I]β-CIT SPECT
Abstract
OBJECTIVE: The authors examined whether striatal dopamine transporters were altered in acutely (96 hours or less) abstinent cocaine-abusing subjects, as suggested by postmortem studies. METHOD: [123I]β-CIT and single photon emission computed tomography were used to assess striatal dopamine transporter levels in 28 cocaine-abusing subjects and 24 comparison subjects matched as a group for age and gender. RESULTS: Results showed a significant (approximately 20%) elevation in striatal V3″ values in acutely abstinent cocaine-abusing subjects relative to comparison subjects. An inverse correlation between dopamine transporter level and Hamilton Depression Rating Scale score was also observed. CONCLUSIONS: These findings indicate more modest elevations in striatal dopamine transporters in cocaine-abusing subjects than noted in previous postmortem reports and suggest a possible relationship between cocaine-related depression and dopamine transporter binding.
Cocaine's binding to the dopamine transporter is the mechanism most widely implicated in its addictive potential (1, 2). Less clearly understood, however, is the degree to which altered dopamine transporter regulation is a factor in the pathophysiology of cocaine abuse. A view of dopamine transporter regulation in human cocaine-abusing subjects may improve our understanding of clinical aspects of cocaine dependence, including drug-induced craving, dysphoria, and relapse.
A considerable, albeit inconsistent, body of research has examined the effects of cocaine administration on dopamine transporter regulation. Homogenate binding studies have documented increased, decreased, and unchanged brain densities (Bmax) of dopamine transporters in laboratory animals exposed to cocaine (3, 4). In contrast, several (5, 6), but not all (7), postmortem studies of human cocaine-related deaths have noted dramatic increases (50%–500%) in dopamine transporter binding sites as measured by cocaine analogues. On the basis of its structural similarity to cocaine, we have used the iodinated radioligand [123I]β-carbo~methoxy-3β-(4-iodophenyl)tropane ([123I]β-CIT; also known as [123I]RTI-55) to image striatal dopamine transporters in cocaine-abusing subjects with single photon emission computed tomography (SPECT).
METHOD
Twenty-eight cocaine-dependent (by DSM-III-R criteria) and 24 healthy comparison subjects, matched as a group for age (mean=32 years, SD=6, versus mean=33, SD=9, respectively) (t=0.20, df=51, p=0.84, two-tailed unpaired t test) and gender (10 women and 18 men versus nine women and 15 men) (χ2=0.02, df=1, p=0.89), were studied. Cocaine-dependent subjects were frequent (mean=25 days/month, SD=8) and heavy (mean=30 g/month, SD=23) intravenous or freebase (N=27) users of the drug and were free of other primary psychiatric or substance use disorders. Subjects had physical and neurological examinations, ECG, and routine blood and urine laboratory tests to rule out concurrent medical illness and pregnancy, as well as exclude or verify illicit drug use. All provided voluntary written informed consent for study procedures.
Cocaine-abusing subjects were studied during a period of acute (96 hours or less) drug abstinence and remained hospitalized on a locked inpatient research unit for the entire study. Subject assessments included the Cocaine Craving Scale (8), the Hamilton Depression Rating Scale (9), and the Hamilton Anxiety Rating Scale (10). Healthy comparison subjects were studied as outpatients.
Subjects received an injection of [123I]β-CIT (dose mean=355 MBq, SD=44, or mean=9.6 mCi, SD=1.2; radiochemical purity mean=97.4%, SD=1.9%; specific activity >185 GBq/mmol or >5,000 Ci/mmol) followed approximately 24 hours later by SPECT scanning on the brain-dedicated CERASPECT camera (Digital Scintigraphics, Waltham, Mass.) (11). Studies in healthy human subjects have demonstrated that [123I]β-CIT reaches equilibrium binding in the brain by 24 hours (12), so that a simple unitless ratio of regional radioactivities (V3″=Bmax/Kd/V2=specific/nondisplaceable binding=[striatum–occipital]/occipital) can be used to estimate dopamine transporter number (i.e., Bmax), by assuming comparable dopamine transporter affinities (1/Kd) and nondisplaceable distribution volumes (V2) in both study populations. Equilibrium assumptions were directly tested by obtaining three SPECT scans in the first 12 cocaine-abusing subjects and comparison subjects at 24, 27, and 30 hours. Before imaging, four Na99mTcO4-filled fiducial markers (6–10 µCi) were glued bilaterally along the canthomeatal line to permit identification of this plane during image analysis.
Images were reconstructed from photopeak counts (mean=159 keV, SD=16) by filtered (Butterworth; cutoff=1 cm, power factor=10) back-projection, displayed as a 64×64×32 matrix (voxel size=3.3×3.3×3.3 mm) and reoriented to the canthomeatal plane. The four slices with the highest striatal uptake were summed and attenuation corrected (µ=0.15 cm–1) to yield a final transaxial slice 13.3 mm thick. Standardized region of interest templates for left and right caudate/putamen and occipital lobe (13) were visually applied to obtain measures of regional radioactive density, decay corrected to the time of injection.
Statistical differences between groups were assessed by two-tailed unpaired t tests; comparisons of [123I]β-CIT binding and clinical ratings employed Pearson's product-moment correlation. Statistical significance was assumed at the p<0.05 level.
RESULTS
Results revealed modest (approximately 20%), albeit statistically robust (p=0.008), increases in V3″ in cocaine-abusing subjects as compared to comparison subjects (mean=9.5, SD=2.1, versus mean=8.1, SD=1.5) (figure 1). Elevations in V3″ did not derive from differences in nondisplaceable (i.e., occipital) binding between groups (cocaine versus comparison: mean=0.16, SD=0.05×10–2, versus mean=0.15, SD=0.05×10–2% ID · ml–1) (t=–1.07, df=51, p=0.29). Clearance rates for specific (mean=0.17%/hour, SD=0.6, versus mean=0.00%/hour, SD=0.6) (t=0.58, df=23, p=0.58) and nondisplaceable (mean=–1.91%/hour, SD=2.1, versus mean=–0.53%/hour, SD=2.3) (t=–1.73, df=23, p=0.11) binding were comparable for the addicted and nonaddicted groups and were consistent with equilibrium assumptions (12). Among severity of use and behavioral ratings, only levels of depression (Hamilton depression scale score) were significantly correlated (r=–0.50, df=26, p=0.02) with [123I]β-CIT binding in cocaine-abusing subjects.
DISCUSSION
The present study provides the first in vivo evidence of increased cocaine binding sites (i.e., striatal dopamine transporters) in cocaine-abusing subjects as measured by [123I]β-CIT SPECT. Our findings of increased V3″ in the addicted group are most likely explained by an increased density of dopamine transporters, as suggested by two previous postmortem reports. Little et al. (5), reporting on seven cocaine-related deaths, found [3H]~WIN 35,428 binding to be 50%–200% higher than in seven comparison subjects as measured by quantitative autoradiography. Staley et al. (6) independently confirmed these findings in six cocaine overdose victims, demonstrating selective increases (approximately 500%) in Bmax for the high- but not the low-affinity binding site. In contrast to these postmortem studies, we observed more modest (approximately 20%) and overlapping degrees of dopamine transporter elevation in our cocaine-abusing subjects (5, 6), results that may explain negative findings in other postmortem (7, 14, 15) and positron emission tomography (PET) (16) studies.
The pathophysiological significance, if any, of increased dopamine transporters in cocaine-abusing subjects is currently unknown. Specifically, it remains to be established whether increases in cocaine binding sites reflect a premorbid, perhaps predisposing, trait in susceptible individuals, or whether dopamine transporter elevations are a secondary, potentially homeostatic, response to chronic dopamine reuptake blockade by cocaine. Future SPECT studies of individuals at high risk for cocaine addiction or previously addicted individuals during periods of sustained drug abstinence may ultimately help to answer this question. In favor of the former possibility, we found no evidence of a relationship between a variety of severity of use measures and dopamine transporter binding. Conversely, our observation of an association between depressed mood and low dopamine transporter levels provides indirect support for an adaptive response. In the absence of a more detailed understanding of the molecular basis of elevations in [123I]β-CIT binding, such inferences remain highly speculative. However, our Hamilton depression scale finding is corroborated by previous PET findings of an inverse correlation between depressed mood and striatal D2 receptors (17). Such abnormalities in depressed cocaine-abusing subjects are consistent with preclinical research suggesting an important role for dopaminergic dysfunction in postcocaine anhedonia (18). Thus, prospective replication of this preliminary finding by future investigations will be critical and may ultimately improve our understanding of the neurobiological basis of cocaine-related dysphoria and relapse.
Received June 30, 1997; revision received Nov. 24, 1997; accepted Dec. 22, 1997. From the Departments of Psychiatry and Diagnostic Radiology, and VA Medical Center, Yale University School of Medicine, New Haven, Conn.; the Department of Psychiatry, Emory University School of Medicine, Atlanta; the Butler Hospital, Department of Psychiatry and Human Behavior, Brown University School of Medicine, Providence, R.I.; and the Dallas VA Medical Center. Address reprint requests to Dr. Malison, Clinical Neuroscience Research Unit, Abraham Ribicoff Research Facilities, Connecticut Mental Health Center, 34 Park St., New Haven, CT 06519; [email protected] (e-mail). Supported by National Institute on Drug Abuse grants DA-09330 (Dr. Price), DA-04060, DA-09250, DA-0112 (Dr. Kosten), DA-00167 (Dr. Malison), DA-00216 (Dr. McCance), and DA-09294 (Dr. Innis); NIH grant RR-00125 from the General Medical Clinical Research Center; the State of Connecticut Department of Mental Health and Addiction Services; the Stanley Foundation (Dr. Price); and the National Alliance for Research on Schizophrenia and Depression (Dr. Malison). The authors thank E.O. Smith, G. Wisniewski, S. Zoghbi, M.D. Stratton, Y. Zea-Ponce, M.K. Madrak, L. Pantages-Torok, and R. Weiss for their assistance and John Neumeyer, Ph.D., for supplying the tributylstannyl precursor used in the preparation of [123I]β-CIT.
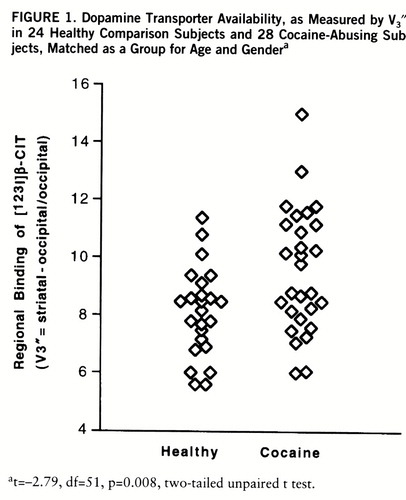
FIGURE 1. Dopamine Transporter Availability, as Measured by V3″, in 24 Healthy Comparison Subjects and 28 Cocaine-Abusing Subjects, Matched as a Group for Age and Gendera
at=–2.79, df=51, p=0.008, two-tailed unpaired t test.
1 Koob G, Bloom F: Cellular and molecular mechanisms of drug dependence. Science 1988; 242:715–723Crossref, Medline, Google Scholar
2 Kuhar MJ, Ritz MC, Boja JW: The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 1991; 14:299–302Crossref, Medline, Google Scholar
3 Kuhar MJ, Pilotte NS: Neurochemical changes in cocaine withdrawal. Trends Pharmacol Sci 1996; 17:260–264Crossref, Medline, Google Scholar
4 Mash DC, Staley JK: The dopamine transporter in human brain: characterization and effect of cocaine exposure, in Neurotransmitter Transporters: Structure, Function, and Regulation. Edited by Reith MEA. Totowa, NJ, Humana Press, 1997, pp 315–343Google Scholar
5 Little KY, Kirkman JA, Carroll FI, Clark TB, Duncan GE: Cocaine use increases [3H]WIN 35428 binding sites in human striatum. Brain Res 1993; 628:17–25Crossref, Medline, Google Scholar
6 Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC: High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. J Pharmacol Exp Ther 1994; 271:1678–1685Medline, Google Scholar
7 Wilson JM, Levey AI, Bergeron C, Kalasinsky K, Ang L, Peretti F, Adams VI, Smialek J, Anderson WR, Shannak K, Deck J, Niz~nik HB, Kish SJ: Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann Neurol 1996; 40:428–439Crossref, Medline, Google Scholar
8 Gawin F, Kleber H: Abstinence symptomatology and psychiatric diagnosis in chronic cocaine abusers. Arch Gen Psychiatry 1986; 43:107–113Crossref, Medline, Google Scholar
9 Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
10 Hamilton M: The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
11 Holman B, Carvalho P, Zimmerman R, Johnson K, Tumeh S, Smith A, Genna S: Brain perfusion SPECT using an annular single crystal camera: initial clinical experience. J Nucl Med 1990; 31:1456–1461Medline, Google Scholar
12 Laruelle M, Wallace E, Seibyl J, Baldwin R, Zea-Ponce Y, Zoghbi S, Neumeyer J, Charney D, Hoffer P, Innis R: Graphical, kinetic, and equilibrium analyses of in vivo [123I]β-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab 1994; 14:982–994Crossref, Medline, Google Scholar
13 Innis R, Seibyl J, Scanley B, Laruelle M, Abi-Dargham A, Wallace E, Baldwin R, Zea-Ponce Y, Zoghbi S, Wang S, Gao Y, Neu~meyer J, Charney D, Hoffer P, Marek K: Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci USA 1993; 90:11965–11969Crossref, Medline, Google Scholar
14 Hurd Y, Herkenham M: Molecular alterations in the neo~striatum of human cocaine addicts. Synapse 1993; 13:357–369Crossref, Medline, Google Scholar
15 Hitri A, Casanova MF, Kleinman JE, Wyatt RJ: Fewer dopamine transporter receptors in the prefrontal cortex of cocaine users. Am J Psychiatry 1994; 151:1074–1076Link, Google Scholar
16 Volkow ND, Wang G-J, Fowler JS, Logan J, Hitzemann R, Gatley SJ, MacGregor RR, Wolf AP: Cocaine uptake is decreased in the brain of detoxified cocaine abusers. Neuropsychopharmacology 1996; 14:159–168Crossref, Medline, Google Scholar
17 Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue C-Y, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, Hitzemann R, Henn F: Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 1990; 147:719–724Link, Google Scholar
18 Markou A, Koob G: Postcocaine anhedonia: an animal model of cocaine withdrawal. Neuropsychopharmacology 1991; 4:17–26Medline, Google Scholar



