Diagnostic Precursors to Bipolar Disorder in Offspring of Parents With Bipolar Disorder: A Longitudinal Study
Abstract
Objective:
The authors sought to identify diagnostic risk factors of manic, mixed, or hypomanic episodes in the offspring of parents with bipolar disorder (“high-risk offspring”).
Method:
High-risk offspring 6–18 years old (N=391) and demographically matched offspring (N=248) of community parents without bipolar disorder were assessed longitudinally with standardized diagnostic instruments by staff blind to parental diagnoses. Follow-up assessments were completed in 91% of the offspring (mean follow-up interval, 2.5 years; mean follow-up duration, 6.8 years).
Results:
Compared with community offspring, high-risk offspring had significantly higher rates of subthreshold mania or hypomania (13.3% compared with 1.2%), manic, mixed, or hypomanic episodes (9.2% compared with 0.8%), and major depressive episodes (32.0% compared with 14.9%). They also had higher rates of attention deficit hyperactivity disorder (30.7% compared with 18.1%), disruptive behavior disorders (27.4% compared with 15.3%), anxiety disorders (39.9% compared with 21.8%), and substance use disorders (19.9% compared with 10.1%), but not unipolar major depressive disorder (major depression with no bipolarity; 18.9% compared with 13.7%). Multivariate Cox regressions showed that in the high-risk offspring, subthreshold manic or hypomanic episodes (hazard ratio=2.29), major depressive episodes (hazard ratio=1.99), and disruptive behavior disorders (hazard ratio=2.12) were associated with subsequent manic, mixed, or hypomanic episodes. Only subthreshold manic or hypomanic episodes (hazard ratio=7.57) were associated when analyses were restricted to prospective data.
Conclusions:
Subthreshold manic or hypomanic episodes were a diagnostic risk factor for the development of manic, mixed, or hypomanic episodes in the offspring of parents with bipolar disorder and should be a target for clinical assessment and treatment research. Major depressive episodes and disruptive behavior disorders are also indications for close clinical monitoring of emergent bipolarity in high-risk offspring.
Most adults with bipolar disorder recall having mood symptoms before age 18, and there is growing evidence that those with childhood or adolescent onset have a worse prospective course of mood symptoms and higher rates of comorbid psychiatric illness as compared with those with adult onset (1–3). Furthermore, there is on average a lag of more than a decade between the onset of impairing mood symptoms and a bipolar diagnosis in bipolar adults with onset during youth (4). Improved identification of bipolar disorder in childhood and adolescence may increase the opportunity to apply evidence-based treatments in earlier phases of the illness, which in turn has the potential to improve long-term course and outcomes. Given that bipolar disorder runs in families (5) and has a strong genetic basis (6), the offspring of parents with bipolar disorder are an excellent population in which to examine diagnostic precursors of bipolar disorder.
Most studies of the child and adolescent offspring of bipolar parents show that the offspring have elevated rates of bipolar disorder, major depression, and anxiety disorders relative to comparison groups (7, 8). However, results are mixed regarding attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder, and conduct disorder (9–14). There are only a few high-risk studies with longitudinal data, but low numbers of individuals with bipolar disorder in the high-risk group limit the studies’ ability to identify predictors of developing bipolar disorder. Depression was found to precede manic or hypomanic episodes in nearly all high-risk offspring with bipolar disorder in two longitudinal studies, although the mean age at intake was 16.5 years in both studies (15, 16). Episodic symptoms such as crying, anxiety, hyperalertness, and decreased sleep in childhood preceded the onset of mania in a small sample of offspring of Amish parents with bipolar disorder, but power limitations precluded formal statistical analyses (17).
The Pittsburgh Bipolar Offspring Study (BIOS) is the largest study to date of offspring of parents with bipolar disorder. A unique feature of BIOS is that offspring were assessed for subthreshold episodes of manic symptoms using a priori operationalized criteria from the Course and Outcome of Bipolar Youth (COBY) study (18). Previous results from the intake assessment demonstrated that the offspring of parents with bipolar disorder had higher rates of anxiety disorders, bipolar I disorder, and bipolar spectrum disorders (bipolar I and II disorders or bipolar disorder not otherwise specified) relative to comparison offspring (12). A diagnosis of a bipolar spectrum disorder in the high-risk offspring was associated with younger parental age at the child’s birth, older offspring age, higher rates of anxiety and disruptive behavior disorders in the offspring, and both parents having a bipolar diagnosis (19).
Our primary aims in this study were 1) to confirm and extend the findings regarding diagnostic differences between the offspring of parents with bipolar disorder (subsequently referred to as “high-risk offspring”) and offspring of community comparison parents now that longitudinal data are included and the mean age of the sample is 18 years; and 2) to describe the developmental trajectory of mood episodes and identify diagnostic precursors of full-threshold bipolar disorder (defined as the presence of manic, mixed, or hypomanic episodes, which we refer to hereafter as “mania/hypomania”) in the offspring of parents with bipolar disorder. Based on the studies noted above, we hypothesized that subthreshold mania or hypomania, depression, anxiety, and disruptive behavior disorders would be associated with future mania/hypomania.
Method
The methods of BIOS have been described in detail elsewhere (12). The study was approved by the University of Pittsburgh Institutional Review Board.
Sample
The sample was recruited through the parent probands. Parents with bipolar disorder were recruited through advertisements (53%), other research studies (31%), and outpatient clinics (16%). Parents with bipolar disorder had to meet DSM-IV criteria for bipolar I or II disorder and live within 200 miles of Pittsburgh. Exclusion criteria were a lifetime diagnosis of schizophrenia, mental retardation, a mood disorder secondary to medical illness, substance use, or use of psychoactive medications. Comparison parents were recruited from the community using random digit dialing and were group-matched to the bipolar parents by age, sex, and neighborhood. Comparison parents could not have a parent or sibling with bipolar disorder, and the biological coparent could not have bipolar disorder. The study included all offspring of the parent probands who were in the age range of 6 to 18 years (including siblings and half-siblings), unless the child had mental retardation.
Procedures
Study procedures were initiated after informed consent was obtained from the parents and assent from the children (12). Parent probands and participating biological coparents (31%) were assessed for DSM-IV disorders by direct interview using the Structured Clinical Interview for DSM-IV (SCID) and the ADHD, oppositional defiant disorder, conduct disorder, and separation anxiety disorder sections of the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL). The psychiatric history of nonparticipating biological coparents was obtained from the parent proband using the Family History Research Diagnostic Criteria method (20) plus the ADHD, oppositional defiant disorder, conduct disorder, and separation anxiety items from the K-SADS-PL.
At intake, parents were interviewed about their offspring and the children were directly interviewed using the K-SADS-PL for non-mood disorders and the K-SADS Mania Rating Scale and the depression items from the K-SADS–Present Version. Symptoms that are criteria for more than one diagnosis (e.g., distractibility) were not rated as fulfilling criteria for a mood disorder unless they had their onset or significantly intensified during a period of abnormal mood. Subthreshold mania or hypomania was diagnosed using the bipolar disorder not otherwise specified criteria from the COBY study (18) and was defined as distinct periods of abnormally elevated, expansive, or irritable mood that met the following criteria: 1) at least two DSM-IV manic symptoms (three if the mood is irritable only) that were clearly associated with the onset of abnormal mood; 2) a clear change in functioning associated with the onset of these affective symptoms; 3) the presence of the elated and/or irritable mood and manic symptoms for a significant part of the day (a minimum of 4 hours, not necessarily consecutive); and 4) a minimum of 4 days (not necessarily consecutive) of meeting criteria 1–3 over the subject’s lifetime.
Socioeconomic status was determined using the Hollingshead scale (21).
Follow-up evaluations were performed approximately every 2 years using the same diagnostic instruments cited above for parents and offspring younger than age 19. Offspring age 19 and older were assessed using the SCID for non-mood disorders, and the K-SADS Mania Rating Scale and the depression items from the K-SADS–Present Version for mood disorders. If a participant was not able to complete an interview at the 2-year interval, attempts to schedule and complete the evaluation would continue unless the participant or the parent or guardian asked to withdraw from the study. Follow-up evaluations focused on assessment of the interval since the previous interview.
Assessments were performed by interviewers with bachelor’s or master’s degrees who had intensive training with the diagnostic instruments and were required to achieve 80% agreement with a certified rater. Interviewers who assessed offspring were blind to the parents’ diagnoses, as different interviewers were used to assess the parents. All information was presented to a child psychiatrist, who reviewed the data to confirm diagnoses. Psychiatrists were blind to parental diagnoses. As specified in the K-SADS instructions, all available data were used to assign summary symptom and diagnostic scores, and discrepant information was discussed at a case conference with the psychiatrist.
Diagnostic reliability was assessed using audiotapes of 44 actual BIOS assessments, which were rated by two to eight BIOS interviewers (mean=5.4). The kappa statistic for diagnostic reliability was 0.86 for bipolar spectrum disorders, 0.77 for bipolar I or II disorder versus bipolar disorder not otherwise specified versus no bipolar disorder, 0.64 for major depressive episode, 0.71 for any depressive episode, 0.86 for ADHD, 0.78 for anxiety disorders, 0.84 for oppositional defiant disorder and/or conduct disorder, and 1.0 for substance use disorders.
The onset age of specific disorders and mood episodes was set to the estimated age at which the participant met full DSM-IV criteria. For consistency with other longitudinal high-risk studies, the onset of full-threshold bipolar disorder in the offspring was set to when they first met DSM-IV criteria for a manic, mixed, or hypomanic episode. The onset age of bipolar spectrum disorder was set to the age of the first time the participant met criteria for subthreshold mania or hypomania or full-threshold bipolar disorder.
Statistical Analysis
Comparisons of demographic and clinical characteristics were evaluated between parent and biological coparent groups using t tests and chi-square tests. Comparisons between offspring groups used mixed logistic regression models to control for within-family correlation. Demographic variables that showed group differences below a p value of 0.2 were included as potential covariates in subsequent models comparing diagnostic differences between the high-risk and comparison offspring. Potential covariates were entered in a forward stepwise approach (with the criteria of p<0.2 for entry and p<0.1 for retention used for all stepwise models). Mixed regression models controlling for parental psychopathology were also used to compare diagnostic differences among the offspring of bipolar parents and three different comparison offspring groups: all comparison offspring, the offspring of comparison parent probands who had a lifetime diagnosis of an axis I disorder, and the offspring of healthy comparison parent probands. Potential covariates were entered in a forward stepwise approach and included the demographic variables from the previous analyses, as well as parental proband and coparent non-bipolar diagnoses. Offspring groups were compared on the cumulative risk of developing mania/hypomania and of developing any bipolar spectrum disorder (bipolar I or II disorder or bipolar disorder not otherwise specified) using Kaplan-Meier survival analyses.
Diagnostic precursors of mania/hypomania were evaluated in the high-risk offspring using Cox regression models that included family membership as a random effect. Diagnostic factors that were associated with hazard of developing mania/hypomania at a level of p<0.2 in univariate models were included in a stepwise multivariate Cox regression with the retention criterion of p<0.1. Since mania/hypomania would be diagnosed in an offspring with a prior substance use disorder only if there was clear evidence that the two disorders were independent, the temporal association between the two conditions may have been distorted. Therefore, we did not include substance use disorders in the multivariate models. Potential confounding factors were then evaluated by adding to them to the model one at a time, and they were retained if they significantly affected results (defined as a change >15% in the hazard ratio of diagnostic factors retained in the model). Potential confounding factors were as follows: offspring age at intake; offspring age at last assessment; year at intake; number of assessments; duration of follow-up; whether the first follow-up assessment occurred within 3 years of intake; whether the second follow-up assessment occurred within 5 years of intake; offspring sex; offspring race; socioeconomic status; parent proband sex; parent proband age at onset of their own bipolar disorder; and parent proband bipolar subtype (I or II). Sensitivity analyses repeating the Cox regressions using the same methodology were performed in the subset of high-risk offspring, with follow-up for those who did not have a history of mania/hypomania at intake. To minimize the influence of recall bias, mood episodes and diagnoses were considered to precede the onset of mania/hypomania in the sensitivity analyses only if they were diagnosed at an assessment prior to the assessment at which mania/hypomania was identified.
Results
Participants
The study included 391 offspring of 236 parents with bipolar disorder (bipolar I disorder, N=170; bipolar II disorder, N=66) and 248 offspring of 141 community comparison parents. Two community comparison parents developed bipolar disorder over follow-up and were reassigned to the bipolar disorder group. One additional bipolar disorder family was entered into the study after the initial analyses, and one child of a parent with bipolar disorder was determined to have mental retardation and was removed from the study.
At least one follow-up visit was completed for 90.9% of the offspring, and follow-up rates did not differ between offspring groups (Table 1). The initial follow-up assessment was completed “on schedule” (within 3 years of intake) for 78.6% of offspring, with no difference between offspring groups. The rate of second follow-up assessments that were on schedule (within 5 years of intake) was nonsignificantly lower for the high-risk offspring than for the comparison offspring (63.4% compared with 71.8%). There were no differences between the high-risk offspring and comparison offspring in the mean number of follow-up assessments (2.7 [SD=1.3] compared with 2.7 [SD=1.2]), duration of follow-up (6.8 years [SD=2.2] compared with 6.9 years [SD=2.0]), or interval between assessments (2.5 years [SD=1.1] compared with 2.4 years [SD=0.8]).
| Characteristic | High-Risk Offspring (N=391) | Comparison Offspring (N=248) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Female | 191 | 48.9 | 134 | 54.0 |
| White | 317 | 81.1 | 184 | 74.2 |
| Living with both biological parentsa | 163 | 41.7 | 154 | 62.1 |
| Had at least 1 follow-up assessment | 357 | 91.3 | 224 | 90.3 |
| First follow-up assessment completed within 3 years of intake | 307 | 78.5 | 195 | 78.6 |
| Second follow-up assessment completed within 5 years of intake | 248 | 63.4 | 178 | 71.8 |
| Mean | SD | Mean | SD | |
| Age at intake (years) | 11.9 | 3.7 | 11.8 | 3.6 |
| Age at last assessment (years) | 18.1 | 4.8 | 18.0 | 4.5 |
| Mother’s age at offspring’s birth (years)a | 26.7 | 5.8 | 28.3 | 5.6 |
| Father’s age at offspring’s birth (years) | 29.5 | 7.6 | 30.4 | 7.0 |
TABLE 1. Demographic Characteristics of Offspring of Parents With Bipolar Disorder (High-Risk Offspring) and Offspring of Community Comparison Parents
Offspring for whom follow-up assessment data were available were more likely to be white, to be living with both biological parents at baseline, and to have a lifetime diagnosis of a major depressive episode, anxiety disorder, and a substance use disorder at baseline, compared with those who did not complete any follow-up assessments. The groups did not differ in sex distribution or in rates of bipolar spectrum disorders, ADHD, oppositional defiant disorder, or conduct disorder (see Table S1 in the data supplement that accompanies the online edition of this article). Offspring with follow-up data had higher socioeconomic status on average, and their mothers and fathers were older on average at the time of the offspring’s birth.
Offspring Demographic Characteristics
Compared with community comparison offspring, high-risk offspring were less likely to be living with both biological parents, and their mothers’ mean age at the time of the offspring’s birth was significantly lower (Table 1).
Parent Factors
Relative to the comparison parents, the parents with bipolar disorder were more likely to be white, were less likely to be married, were younger, and were of lower socioeconomic status (see Table S2 in the data supplement). Parents with bipolar disorder were more likely to have a history of a major depressive episode and of a non-mood axis I disorder, including anxiety disorders, ADHD, disruptive behavior disorders (oppositional defiant or conduct disorder), and substance use disorders. These factors were eligible for inclusion in final multivariate models comparing offspring groups.
The biological coparents of the high-risk offspring were more likely to have an axis I diagnosis, depression, and a substance use disorder (see Table S3 in the data supplement). There was no difference between offspring groups in the proportion of coparents who were assessed using the SCID compared with the family history method.
Offspring Lifetime Diagnoses and Mood Episodes at Last Assessment
Relative to comparison offspring, high-risk offspring had significantly higher lifetime rates of all of the disorders and mood episodes examined except obsessive-compulsive disorder, posttraumatic stress disorder, conduct disorder, and unipolar major and minor depressive disorders (Table 2). After adjustment for parent and coparent lifetime axis I diagnoses (Table 3), there was little change in the results, except that unipolar major depressive disorder was significantly higher in the high-risk offspring. When the comparison was limited to high-risk offspring compared with offspring of comparison parent probands who had a lifetime axis I disorder, there were no differences between groups in unipolar depression, ADHD, and disruptive behavior disorders.
| High-Risk Offspring (N=391) | Comparison Offspring (N=248) | Analysisa | ||||
|---|---|---|---|---|---|---|
| Diagnoses | N | % | N | % | F | p |
| Any axis I disorder | 290 | 74.2 | 120 | 48.4 | 29.9 | <0.0001 |
| Any mood disorder | 189 | 48.3 | 56 | 22.6 | 30.2 | <0.0001 |
| Any bipolar spectrum disorder | 75 | 19.2 | 5 | 2.0 | 31.5 | <0.0001 |
| Bipolar I disorder | 15 | 3.8 | 1 | 0.4 | 6.84 | 0.009 |
| Bipolar II disorder | 18 | 4.6 | 1 | 0.4 | 9.39 | 0.002 |
| Bipolar disorder not otherwise specified | 42 | 10.7 | 3 | 1.2 | 18.2 | <0.0001 |
| Any unipolar depressive disorder | 114 | 29.2 | 51 | 20.6 | 4.40 | 0.04 |
| Major depressive disorder | 74 | 18.9 | 34 | 13.7 | 2.68 | 0.10 |
| Minor depressive disorderb | 40 | 10.2 | 17 | 6.9 | 0.94 | 0.33 |
| Mood episodes | 0 | |||||
| Manic, mixed, or hypomanic | 36 | 9.2 | 2 | 0.8 | 13.2 | 0.0003 |
| Subthreshold manic or hypomanicc | 52 | 13.3 | 3 | 1.2 | 23.5 | <0.0001 |
| Any depressive episode | 181 | 46.3 | 54 | 21.8 | 29.5 | <0.0001 |
| Major depressive | 125 | 32.0 | 37 | 14.9 | 20.3 | <0.0001 |
| Minor depressive | 108 | 27.6 | 25 | 10.1 | 26.0 | <0.0001 |
| Any non-mood axis I disorder | 263 | 67.3 | 106 | 42.7 | 26.2 | <0.0001 |
| Any anxiety disorder | 156 | 39.9 | 54 | 21.8 | 20.2 | <0.0001 |
| Generalized anxiety disorder | 80 | 20.5 | 17 | 6.9 | 25.8 | <0.0001 |
| Social phobia | 49 | 12.5 | 17 | 6.9 | 5.48 | 0.02 |
| Separation anxiety disorder | 67 | 17.1 | 17 | 6.9 | 11.6 | 0.001 |
| Panic disorder | 27 | 6.9 | 6 | 2.4 | 5.02 | 0.03 |
| Obsessive-compulsive disorder | 21 | 5.4 | 6 | 2.4 | 2.79 | 0.10 |
| Posttraumatic stress disorder | 33 | 8.4 | 14 | 5.6 | 0.75 | 0.39 |
| Attention deficit hyperactivity disorder | 120 | 30.7 | 45 | 18.1 | 6.06 | 0.01 |
| Disruptive behavior disorders | 107 | 27.4 | 38 | 15.3 | 4.85 | 0.03 |
| Oppositional defiant disorder | 99 | 25.3 | 32 | 12.9 | 5.87 | 0.02 |
| Conduct disorder | 37 | 9.5 | 14 | 5.7 | 0.91 | 0.34 |
| Substance use disorder | 78 | 19.9 | 25 | 10.1 | 10.1 | 0.002 |
TABLE 2. Lifetime Diagnoses at Last Assessment Among Offspring of Parents With Bipolar Disorder (High-Risk Offspring) and Offspring of Community Comparison Parents
| High-Risk Offspring (N=391) Versus Offspring of All Comparison Parents (N=248)a | High-Risk Offspring (N=391) Versus Offspring of Comparison Parents Who Had a Lifetime Axis I Disorder (N=149)a | High-Risk Offspring (N=391) Versus Offspring of Healthy Comparison Parents (N=99)b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnoses | Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p |
| Any axis I disorder | 2.15 | 1.39, 3.34 | 0.0006 | 2.13 | 1.31, 3.44 | 0.002 | 3.94 | 2.30, 6.74 | <0.0001 |
| Any mood disorder | 2.68 | 1.64, 4.37 | <0.0001 | 2.60 | 1.53, 4.42 | 0.0004 | 4.13 | 2.15, 7.93 | <0.0001 |
| Bipolar spectrum disorders | 10.58 | 4.15, 26.93 | <0.0001 | 10.94 | 3.34, 35.81 | <0.0001 | 10.03 | 2.37, 42.46 | 0.002 |
| Bipolar I and II disorders | 11.34 | 2.69, 47.82 | 0.001 | 13.64 | 1.84, 101.05 | 0.01 | 9.03 | 1.22, 67.14 | 0.03 |
| Unipolar depressive disorders | 1.47 | 0.9, 2.40 | 0.1 | 1.38 | 0.82, 2.32 | 0.2 | 2.22 | 1.17, 4.23 | 0.02 |
| Unipolar major depressive disorder | 1.70 | 1.05, 2.77 | 0.03 | 1.53 | 0.89, 2.65 | 0.1 | 1.79 | 0.87, 3.69 | 0.1 |
| Any anxiety disorder | 1.67 | 1.02, 2.72 | 0.04 | 1.83 | 1.07, 3.14 | 0.03 | 2.69 | 1.45, 4.97 | 0.002 |
| Social phobia, separation anxiety, and/or generalized anxiety disorder | 2.03 | 1.23, 3.33 | 0.005 | 2.18 | 1.25, 3.81 | 0.006 | 2.80 | 1.49, 5.25 | 0.002 |
| Attention deficit hyperactivity disorder | 1.63 | 1.08, 2.47 | 0.02 | 1.25 | 0.79, 1.97 | 0.3 | 3.68 | 1.75, 7.72 | 0.0006 |
| Disruptive behavior disorders | 1.90 | 1.11, 3.25 | 0.02 | 1.73 | 0.93, 3.20 | 0.08 | 2.50 | 1.11, 5.60 | 0.03 |
| Oppositional defiant disorder | 2.20 | 1.25, 3.86 | 0.006 | 1.79 | 0.95, 3.35 | 0.07 | 3.86 | 1.47, 10.10 | 0.006 |
| Conduct disorder | 1.75 | 0.68, 4.52 | 0.2 | 1.34 | 0.55, 3.25 | 0.5 | 0.82 | 0.33, 2.00 | 0.7 |
| Substance use disorders | 4.49 | 1.86, 10.81 | 0.0009 | 4.36 | 1.74, 10.92 | 0.002 | 2.13 | 0.88, 5.17 | 0.09 |
TABLE 3. Comparison of Diagnostic Rates Between Offspring of Parents With Bipolar Disorder (High-Risk Offspring) and Offspring of Community Comparison Parents
Onset of Bipolar Disorder
The high-risk offspring had a substantially higher likelihood of having a bipolar spectrum disorder and mania/hypomania than the comparison offspring (Figure 1). The cumulative rate of bipolar spectrum disorders at age 21 estimated by the Kaplan-Meier analysis was 23.0% in the high-risk offspring and 3.2% in the comparison offspring. The estimated cumulative rate of mania/hypomania at age 21 was 12.7% in the high-risk offspring and 1.5% in the comparison offspring.
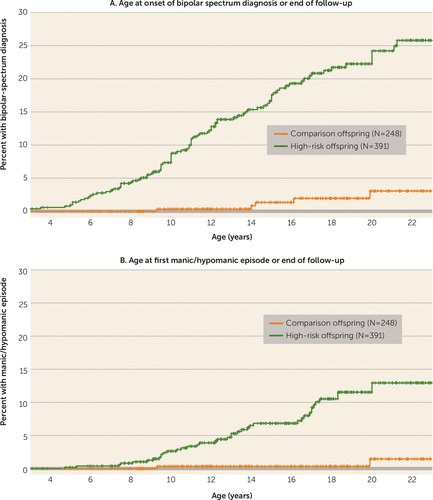
FIGURE 1. Cumulative Rates of Bipolar Spectrum Disorders and Mania/Hypomania, by Age at Onset, in Offspring of Parents With Bipolar Disorder (High-Risk Offspring) and Offspring of Community Comparison Parentsa
a Mania/hypomania includes bipolar I and II disorders and bipolar disorder not otherwise specified. For bipolar spectrum diagnoses (panel A), high-risk offspring > comparison offspring; log-rank χ2 = 40.2; p=2.3×10−10; for first manic or hypomanic episode (panel B), high-risk offspring > comparison offspring; log-rank χ2=19.2; p=1.2×10−5.
In the high-risk offspring, the mean age at onset of mania/hypomania was 13.4 years (SD=3.8) and the first onset of a bipolar spectrum disorder was at a mean age of 12.1 years (SD=4.0). The mean age at onset of the first major depressive episode was 13.7 years (SD=4.0), and the first onset of any depressive episode (major depressive episode or minor depression) was at a mean age of 12.5 years (SD=4.6). Of the 15 high-risk offspring who had a manic episode, five (33%) had the first episode before age 10 and eight (53%) before age 12, with the earliest at 8.1 years of age.
Diagnoses Preceding First Manic or Hypomanic Episode in Offspring of Parents With Bipolar Disorder
Of the 391 offspring of parents with bipolar disorder, 36 had a lifetime history of a manic/hypomanic episode. Of these, 25 (69.4%) had a depressive episode and 20 (55.6%) had a major depressive episode prior to the onset of mania/hypomania. A subthreshold manic or hypomanic episode preceded the onset of mania/hypomania in 13 (36.1%) of those with a lifetime history of mania/hypomania (Table 4).
| Lifetime Mania/Hypomania (N=36) | No Mania/Hypomania (N=355) | Analysis | |||||
|---|---|---|---|---|---|---|---|
| Episode or Diagnosis | Onset Prior to Manic/Hypomanic Episode (%) | Lifetime at Last Assessment (%) | Lifetime at Last Assessment (%) | Hazard Ratioa | 95% CI | χ2 | p |
| Any mood episode | 77.8 | 100.0 | 43.1 | 3.56 | 1.62, 7.81 | 10.02 | 0.002 |
| Any depressive episode | 69.4 | 91.7 | 41.7 | 2.49 | 1.23, 5.07 | 6.37 | 0.01 |
| Major depressive episode | 55.6 | 80.6 | 27.0 | 2.58 | 1.34, 4.98 | 7.96 | 0.005 |
| Subthreshold manic or hypomanic episode | 36.1 | —b | 11.0 | 3.76 | 1.90, 7.44 | 14.47 | <0.001 |
| Any non-mood disorder | 80.6 | 97.2 | 64.2 | 2.30 | 1.01, 5.26 | 3.92 | 0.05 |
| Attention deficit hyperactivity disorder | 41.7 | 44.4 | 28.7 | 2.04 | 1.05, 3.98 | 4.42 | 0.04 |
| Disruptive behavior disorder | 47.2 | 61.1 | 23.9 | 2.88 | 1.49, 5.59 | 9.84 | 0.002 |
| Anxiety disorder | 52.8 | 72.2 | 36.6 | 1.86 | 0.97, 3.59 | 3.47 | 0.06 |
| Substance use disorder | 5.6 | 44.4 | 17.5 | 0.20 | 0.05, 0.83 | 4.90 | 0.03 |
TABLE 4. Association of Prior Mood Episodes and Diagnoses With Hazard of Developing Subsequent Manic/Hypomanic Episodes in Offspring of Parents With Bipolar Disorder (High-Risk Offspring)
Cox regressions showed that any depressive episode, major depressive episode, subthreshold manic or hypomanic episode, ADHD, and disruptive behavior disorder were associated with an elevated hazard of developing mania/hypomania, while substance use disorders were associated with a reduced hazard (Table 4). The associations between potential confounding variables and risk of developing mania/hypomania are listed in Table S4 in the online data supplement. The multivariate Cox model retained three clinical diagnostic factors: subthreshold manic or hypomanic episode (hazard ratio=2.29, 95% CI=1.10–4.78, p=0.03), disruptive behavior disorders (hazard ratio=2.12, 95% CI=1.09–4.14, p=0.03), and major depressive episode (hazard ratio=1.99, 95% CI=0.99–3.97, p=0.05). It also included one confounder covariate that met criteria for significant impact: proband age at intake assessment (hazard ratio=0.94, 95% CI=0.89–0.99, p=0.02).
We also examined the association of two comorbid conditions (subthreshold mania or hypomania and ADHD) with onset of mania/hypomania (see Table S5 in the data supplement). No comorbid pair of diagnoses was more significantly associated with onset of mania/hypomania than one of the diagnoses alone.
Sensitivity Analyses: Diagnoses Preceding Prospectively Observed Mania/Hypomania in High-Risk Offspring
When the analysis was restricted to the 344 high-risk offspring who had prospective follow-up data and did not have a history of mania/hypomania at intake, 21 (6.1%) had a new onset of mania/hypomania during follow-up, eight of them with manic episodes and 13 of them with hypomanic episodes only. Of these 21 offspring, 12 (57.1%) were diagnosed with a subthreshold manic or hypomanic episode at an assessment prior to the assessment when mania/hypomania was first diagnosed, while 66.7% had a prior depressive episode, 52.4% had a prior major depressive episode, 42.9% had a prior ADHD diagnosis, 38.1% had a prior disruptive behavior disorder diagnosis, 42.9% had a prior anxiety disorder diagnosis, and 9.5% had a prior substance use disorder diagnosis. Multivariate models showed that only subthreshold manic or hypomanic episodes were associated with prospectively observed onset of mania/hypomania (hazard ratio=7.57, 95% CI=3.09–18.6, p<0.0001), with proband age at onset of bipolar disorder retained as a confounder covariate (hazard ratio=0.95, 95% CI=0.89–1.02, p=0.1).
Discussion
This study demonstrates that bipolar spectrum disorders—bipolar I, bipolar II, and bipolar disorder not otherwise specified—are significantly more prevalent in high-risk offspring as compared with community comparison offspring, even before the offspring have reached young adulthood. The high-risk offspring in this study had much higher rates of depressive episodes relative to comparison offspring, but the rate of unipolar depressive disorders (depression with no history of full-threshold or subthreshold manic or hypomanic episodes) was only marginally higher. Nearly all non-mood axis I disorders were more prevalent in the high-risk offspring than in the community offspring. Mania/hypomania in the high-risk offspring was almost always preceded by identifiable mood episodes and non-mood disorders. Distinct subthreshold episodes of mania or hypomania were highly specific to the high-risk offspring and were the strongest predictor of progression to full-threshold mania/hypomania in the high-risk cohort. Identifiable depressive episodes preceded the onset of mania/hypomania in about two-thirds of the cases, but only major depressive episodes specifically predicted the onset of mania/hypomania above and beyond subthreshold manic or hypomanic episodes and the presence of a disruptive behavior disorder. Sensitivity analyses using only prospective data, in which the effects of recall bias were minimized, showed that only subthreshold manic or hypomanic episodes were associated with prospectively observed mania/hypomania.
When we examined the higher rates of non-mood axis I disorders in the high-risk offspring, we found that controlling for parental non-bipolar axis I disorders did not substantially change the results. However, when the comparison group was limited to offspring of community comparison parents who had a lifetime axis I disorder, ADHD and disruptive behavior disorders no longer differed between groups. Given that the rates of ADHD and disruptive behavior disorders were significantly higher in the high-risk offspring as compared with all comparison offspring when parental non-bipolar axis I diagnoses were included in the model, these results must be interpreted with caution.
Before considering the implications of these findings, it is important to consider the study’s limitations. Although this was a prospective study, all diagnoses and estimates of onset age were made retrospectively for the interval between assessments. Offspring were recruited across a broad age range (6–18 years), so most offspring were not through the age of risk for onset of bipolar illness; only 29% were older than age 21 at their last assessment. Although this is the largest longitudinal study of offspring of parents with bipolar disorder, only 36 offspring were diagnosed with full-threshold mania/hypomania. Only a small proportion of the biological coparents had direct diagnostic interviews. Lastly, the bipolar parents were not systematically recruited from clinical or epidemiological populations, so it is unclear whether these findings are generalizable to the offspring of all bipolar parents.
The unexpected finding that presence of a substance use disorder was associated with a lower risk of developing mania/hypomania was likely caused by the conservative procedure used for diagnosing mania/hypomania in the presence of a substance use disorder. It may not be a valid result, particularly in light of the higher rate of lifetime substance use disorders in high-risk offspring who developed mania/hypomania compared with those who did not (44.0% compared with 17.5%).
The lifetime prevalence of any axis I disorder was substantial in both the high-risk offspring (74.2%) and the comparison offspring (48.4%). However, the prevalence of disorders in the comparison offspring is similar to that of other community studies of adolescents and young adults. It is nearly identical to that of the National Comorbidity Survey Adolescent Supplement study (49.5%), which was a large cross-sectional study of adolescents in the community (22). The lifetime prevalence in the comparison offspring matches the cumulative rate of psychiatric disorders at age 21 (49.2%) reported in the Great Smoky Mountains Study, which, like BIOS, used multiple assessments over time (23). The lifetime prevalence rates in the high-risk offspring are similar to those reported in the Dutch longitudinal high-risk study (72%) and by Duffy et al. (76%) (15, 24).
The rate of bipolar I disorder in the BIOS high-risk sample (3.8%) was comparable to that reported in other longitudinal offspring studies (range, 3%–7%), while the rate of either bipolar I or II disorder was slightly lower (8.4% compared with 10.1%–18.8%) (15–17, 25). This may be due to the younger age at last assessment in BIOS, as overall rates of bipolar I or II disorder in BIOS continue to climb after age 18. A recent meta-analysis of epidemiologic studies of adolescents found the rates of bipolar I disorder (1.2%) and bipolar spectrum disorders (1.8%) to be similar to those in the BIOS offspring of community comparison parents (bipolar I disorder, 0.4%; bipolar spectrum disorders, 2.0%) (26).
The results from BIOS differ substantially from those of other longitudinal offspring studies in three ways. First, the mean age at onset of manic/hypomanic episodes was considerably younger in BIOS (13.4 years compared with 17–19 years), with manic episodes occurring before age 12 in half of the BIOS bipolar I offspring, compared with none reported in other studies. Second, a major depressive episode preceded the onset of mania/hypomania only about 50% of the time in BIOS, as compared with nearly all cases in other studies. Third, the BIOS offspring had significantly higher rates of psychopathology meeting criteria for one or more non-mood DSM-IV disorders prior to the onset of full-threshold bipolar illness. Age at first assessment may be a contributing factor for the first issue; BIOS offspring were 11.9 years old on average, as compared with 16.5 years old in the two largest longitudinal studies. The mean age at onset of first manic/hypomanic episode was significantly lower in the offspring who were under age 12 at intake (N=16; mean age, 11.3 years [SD=3.3]) than in those who were 12–18 years old at intake (N=20; mean age, 15.0 years [SD=3.3]) (t=−3.3, p=0.002). The age at onset of first manic/hypomanic episode was about 12 years in the National Comorbidity Survey Adolescent Supplement study, so BIOS is not unique in observing onset of mania and hypomania before adolescence (27). However, there was no significant difference between the younger and older age groups at intake in the rate of major depressive episodes (50% compared with 60%), subthreshold manic or hypomanic episodes (38% compared with 35%), or non-mood axis I disorders (81% compared with 80%) preceding the onset of mania/hypomania in the BIOS offspring, so the reasons for these findings are not clear.
The finding that subthreshold manic or hypomanic episodes often precede manic/hypomanic episodes has been found in one other longitudinal offspring study. Duffy et al. (16) noted in their cohort that 48% of offspring with bipolar disorder would have met criteria for bipolar disorder not otherwise specified prior to the onset of mania/hypomania. The BIOS results demonstrate that this is not a result of recall bias, as prospectively observed manic/hypomanic episodes were preceded in 55% of the cases by subthreshold episodes identified at prior assessments. The prognostic significance of subthreshold manic or hypomanic episodes has also been demonstrated in a pediatric clinical population. Youths diagnosed with bipolar disorder not otherwise specified at intake in the COBY study developed full-threshold bipolar I or II disorder at a rate of 45% over an average of 5 years of follow-up (28). Those with a family history of bipolar disorder had a significantly higher rate of diagnostic progression compared with those who did not (56% compared with 33%). The prognostic significance of subthreshold mania or hypomania may not be limited to youth, as subthreshold symptoms of hypomania have been found to be predictive of future conversion to bipolar disorder in adults diagnosed with unipolar depression (29).
Anxiety disorders were present at significantly higher rates in the high-risk offspring relative to comparison offspring in BIOS and have been associated with the presence of mood disorders in high-risk offspring in other studies (14, 30). However, presence of an anxiety disorder was not associated with future onset of mania/hypomania in BIOS, although it was close to statistical significance in the univariate analysis. The lack of association of anxiety disorder with risk of future bipolar disorder is consistent with what was found by Duffy et al. (31). Anxiety disorders were not associated in multivariate models, likely because of their high comorbidity with depression and subthreshold mania or hypomania.
In summary, these results indicate that in the offspring of parents with bipolar disorder, mania and hypomania do not come “out of the blue” but instead are almost always preceded by identifiable mood episodes and non-mood disorders. Major depression is the index episode only half of the time, and subthreshold episodes of mania or hypomania are the only specific diagnostic antecedent to mania/hypomania when using prospectively obtained data. Clinicians should carefully assess for subthreshold manic or hypomanic episodes and differentiate them from symptoms of depression and other disorders in the offspring of parents with bipolar disorder, as they carry prognostic significance for the future development of full-threshold bipolar illness. This may provide an opportunity for early intervention to improve the course of illness. For instance, a recent study demonstrated benefits from a family-focused approach for symptomatic youths who had a first-degree relative with bipolar disorder (32). Given the clear prognostic significance of subthreshold mania or hypomania in youths at familial risk for bipolar disorder, we need additional treatment research for these youths. As both major depressive episodes and disruptive behavior disorders also indicate risk of future bipolarity, they are indications for close clinical monitoring in high-risk offspring. Given the high prevalence of both mood and non-mood disorders in the high-risk offspring and the difficulty of differentiating subthreshold mania or hypomania from other disorders, it would be worthwhile for clinicians who treat bipolar adults to encourage them to have their children evaluated by specialty pediatric mental health services if the parent is concerned about their child’s mood or behavior.
1 : Retrospective age at onset of bipolar disorder and outcome during two-year follow-up: results from the STEP-BD study. Bipolar Disord 2009; 11:391–400Crossref, Medline, Google Scholar
2 : The National Depressive and Manic-Depressive Association (DMDA) survey of bipolar members. J Affect Disord 1994; 31:281–294Crossref, Medline, Google Scholar
3 : Relationship of birth cohort and early age at onset of illness in a bipolar disorder case registry. Am J Psychiatry 2003; 160:1636–1642Link, Google Scholar
4 : The poor prognosis of childhood-onset bipolar disorder. J Pediatr 2007; 150:485–490Crossref, Medline, Google Scholar
5 : Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression, 2nd ed. New York, Oxford University Press, 2007Google Scholar
6 : The genetics of bipolar disorder. Neuroscience 2009; 164:331–343Crossref, Medline, Google Scholar
7 : Review of studies of child and adolescent offspring of bipolar parents. Bipolar Disord 2001; 3:325–334Crossref, Medline, Google Scholar
8 : Children of parents with bipolar disorder: a metaanalysis of risk for mental disorders. Can J Psychiatry 1997; 42:623–631Crossref, Medline, Google Scholar
9 : Psychopathology in the offspring of parents with bipolar disorder: a controlled study. Biol Psychiatry 2005; 58:554–561Crossref, Medline, Google Scholar
10 : Psychiatric phenomenology of child and adolescent bipolar offspring. J Am Acad Child Adolesc Psychiatry 2000; 39:453–460Crossref, Medline, Google Scholar
11 : Psychiatric disorders in preschool offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study (BIOS). Am J Psychiatry 2010; 167:321–330Link, Google Scholar
12 : Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry 2009; 66:287–296Crossref, Medline, Google Scholar
13 : Mental disorders in offspring of parents with bipolar and major depressive disorders. Bipolar Disord 2012; 14:641–653Crossref, Medline, Google Scholar
14 : A high-risk study of bipolar disorder. Childhood clinical phenotypes as precursors of major mood disorders. Arch Gen Psychiatry 2011; 68:1012–1020Crossref, Medline, Google Scholar
15 : The Dutch bipolar offspring study: 12-year follow-up. Am J Psychiatry 2013; 170:542–549Link, Google Scholar
16 : Early stages in the development of bipolar disorder. J Affect Disord 2010; 121:127–135Crossref, Medline, Google Scholar
17 : A 16-year prospective study of prodromal features prior to BPI onset in well Amish children. J Affect Disord 2012; 142:186–192Crossref, Medline, Google Scholar
18 : Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry 2006; 63:175–183Crossref, Medline, Google Scholar
19 : Clinical, demographic, and familial correlates of bipolar spectrum disorders among offspring of parents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:388–396Medline, Google Scholar
20 : The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry 1977; 34:1229–1235Crossref, Medline, Google Scholar
21 : Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
22 : Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2010; 49:980–989Crossref, Medline, Google Scholar
23 : Cumulative prevalence of psychiatric disorders by young adulthood: a prospective cohort analysis from the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry 2011; 50:252–261Crossref, Medline, Google Scholar
24 : The early manifestations of bipolar disorder: a longitudinal prospective study of the offspring of bipolar parents. Bipolar Disord 2007; 9:828–838Crossref, Medline, Google Scholar
25 : A prospective study of the association among impaired executive functioning, childhood attentional problems, and the development of bipolar disorder. Dev Psychopathol 2004; 16:461–476Crossref, Medline, Google Scholar
26 : Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry 2011; 72:1250–1256Crossref, Medline, Google Scholar
27 : Mania with and without depression in a community sample of US adolescents. Arch Gen Psychiatry 2012; 69:943–951Crossref, Medline, Google Scholar
28 : Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry 2011; 50:1001.e3–1016.e3Crossref, Google Scholar
29 : Subthreshold hypomanic symptoms in progression from unipolar major depression to bipolar disorder. Am J Psychiatry 2011; 168:40–48Link, Google Scholar
30 : The developmental trajectory of bipolar disorder. Br J Psychiatry 2014; 204:122–128Crossref, Medline, Google Scholar
31 : Childhood anxiety: an early predictor of mood disorders in offspring of bipolar parents. J Affect Disord 2013; 150:363–369Crossref, Medline, Google Scholar
32 : Early intervention for symptomatic youth at risk for bipolar disorder: a randomized trial of family-focused therapy. J Am Acad Child Adolesc Psychiatry 2013; 52:121–131Crossref, Medline, Google Scholar



