Neural and Cognitive Correlates of the Common and Specific Variance Across Externalizing Problems in Young Adolescence
Abstract
Objective:
The authors sought to model the unique and common variance across conduct disorder, substance misuse, and attention deficit hyperactivity disorder (ADHD) and to investigate the neurocognitive factors that relate generally or uniquely to externalizing problems in adolescence.
Method:
Personality and behavioral measures and functional imaging responses to reward sensitivity and response inhibition tasks were assessed in 1,778 European adolescents at age 14 and, using structural equation modeling, were related to the unique and common variance across externalizing problems assessed and modeled at ages 14 and 16.
Results:
Externalizing problems best fit a general-specific model made up of a specific factor representing ADHD and conduct disorder symptoms, a specific factor representing substance misuse symptoms, and a common externalizing factor representing the variance shared among all symptoms. Common variance across externalizing problems was associated with high impulsivity and delay discounting as well as low blood-oxygen-level-dependent (BOLD) response in the substantia nigra and subthalamic nucleus but high BOLD response in the presupplementary motor area and precentral gyrus during successful inhibition. Unique variance for ADHD/conduct disorder was associated with impulsivity, poor response inhibition, and high delay discounting, as well as low BOLD response in frontal brain areas bilaterally during failed inhibition. In contrast, unique variance for substance misuse was associated with high sensation seeking and delay discounting, as well as differential brain response to reward anticipation: high BOLD response in the left orbitofrontal cortex but low BOLD response in the left inferior frontal gyrus.
Conclusions:
Personality, behavioral, and fMRI findings suggest that abnormalities in response inhibition, error processing, and reward processing are differentially implicated in underlying vulnerability specific to ADHD/conduct disorder and substance misuse and general to externalizing problems.
High rates of comorbidity between adult substance use and antisocial personality disorders on the one hand and childhood conduct disorder, oppositional defiant disorder, attention deficit hyperactivity disorder (ADHD), and substance misuse on the other suggest a shared etiology across such externalizing problems. These and other common comorbidities have inspired a trend in psychiatry to reformulate diagnostic categories from a dimensional and neuroscience perspective (e.g., the Research Domain Criteria [1]).
Accordingly, quantitative modeling of variance across externalizing problems (2, 3) suggests that much of the variance is common or shared and dimensional in nature (4). This research consistently supports a general-specific model of externalizing problems, with a latent general factor representing variance common to all externalizing symptoms and two specific factors representing variance that is unique to some conduct disorder symptoms (e.g., vandalism in adolescents) and substance misuse (e.g., binge drinking and frequency of drug use in adolescents). However, the role of ADHD, despite a high rate of comorbidity with conduct disorder and substance use disorders (5), was not investigated in these previous studies assessing the general-specific structure of externalizing problems.
Furthermore, while it is generally agreed that there is overlap in brain processes linked to substance misuse, conduct disorder, and ADHD, few studies have attempted to combine multivariate models of behavior with multivariate models of cognitive or brain function. There are several reasons for this, including small sample sizes and the fact that neuroimaging studies tend to work with a relatively simplistic phenotypic characterization (e.g., presence or absence of a particular diagnosis) and rarely account for other forms of comorbidity. In the investigation of the neurobiological correlates of externalizing problems, it is important that we accurately characterize the shared and the unique nature of these symptoms and investigate this sophisticated phenotypic characterization with state-of-the-art neuroimaging probes designed to engage the cortical and subcortical brain regions implicated in relevant brain functions. Doing so may reveal neurobiological profiles that explain underlying latent dimensions reflecting vulnerability to unique or broad psychiatric outcomes.
For example, while impulsivity has been identified as a risk factor for a variety of externalizing problems (6), it is known not to be a unitary construct (7, 8) but one that can be subdivided into at least two subfactors, one reflecting impulsive action (related to deficits in response inhibition) and another reflecting impulsive choice or decision making (often measured using a delay discounting task) (9). However, there is some debate as to whether impulsive choice involves two additional dissociable processes: 1) reward processing and 2) temporal perception or decision making (9). Deficits in impulsive action (poor response inhibition) have been reported in various externalizing problems, including ADHD (10), conduct disorder (11), and substance abuse (12), and are measured behaviorally using go/no-go and stop-signal tasks. Studies have shown that aggressive adolescent males and children with ADHD and conduct disorder make more commission errors in these tasks compared with control subjects (13, 14). The few studies that have investigated the neuropsychological correlates of externalizing problems from a dimensional perspective showed that self-reported impulsivity and poor response inhibition on a stop-signal task (impulsive action) prospectively predicted variance common to externalizing problems and specific to conduct disorder, whereas sensation seeking and reward sensitivity predicted variance specific to substance misuse (3, 13).
Functional neuroimaging studies involving response inhibition tasks implicate a number of brain regions involved in successfully inhibiting behavior, including frontal regions; frontal-striatal regions; left and right putamen; caudate; globus pallidus and thalamus; inferior frontal gyrus; insula and anterior cingulate; substantia nigra and subthalamic nucleus; orbitofrontal and medial gyri; presupplementary motor area; and a parietal network (15). Adolescents with ADHD show reduced responses in frontal and striatal regions of the brain when performing go/no-go and stop-signal tasks (5, 14). A study that controlled for comorbid cases (14, 16) found that abnormal blood-oxygen-level-dependent (BOLD) response patterns specific to ADHD are seen in the prefrontal cortex and abnormalities common to ADHD and conduct disorder are seen in regions such as the insula and cingulate on trials of failed inhibition. By contrast, delay discounting (and the simple task of thinking about the future) involves the ventromedial prefrontal cortex and ventral striatum (17). Functional MRI (fMRI) studies investigating individual differences in brain response to reward magnitude (such as the monetary incentive delay task) also implicate the ventral striatum, but more recent studies using newer methodologies, such as functional connectivity, also highlight the role of the orbitofrontal cortex and its connectivity with the ventral striatum in reward processing (e.g., 18).
While few neuroimaging studies of substance-misusing individuals have controlled for comorbid personality or attentional problems, there is emerging evidence that individuals specifically prone to substance misuse can be distinguished from other clinically disinhibited groups based on impulsive choice and reward sensitivity, rather than general deficits in response inhibition (impulsive action). For example, high-functioning drug users (19) and adolescents with pure substance misuse profiles (13) show impulsivity and behavioral activation only when anticipating reward, but are otherwise quite controlled. While several studies suggest a relationship between substance misuse generally and poor delay discounting (e.g., 20), to our knowledge no study to date has investigated how these processes are linked to specific sets of symptoms within complex models of psychopathology that include latent constructs of shared and specific vulnerability.
In this study, using a large sample of adolescents assessed longitudinally through the IMAGEN study (21), we sought to determine whether the pattern of shared and unique variance across externalizing problems is best represented by a general-specific hierarchical structure (2, 3), whether that structure has predictive validity, and whether these latent factors can be dissociated from a neurocognitive endophenotype perspective (6). Similarly, based on previous theoretical and empirical research demonstrating that impulsivity is a complex construct represented by at least three cognitive processes, we hypothesized that impulsive action, impulsive choice, and reward processing would dissociate the core dimensions of externalizing problems, with impulsive action (as measured by response inhibition and the brain areas most implicated in stopping behavior, particularly frontal networks [15]) being associated with the variance specific to ADHD and/or conduct disorder symptoms (that does not covary with substance use symptoms), and sensation seeking and reward processing (implicating the ventral striatum and the orbitofrontal cortex) being most associated with the variance specific to substance misuse. Impulsive choice and related brain functions were hypothesized to be implicated in variance common to all externalizing problems.
Method
Participants
A total of 2,232 participants across eight European sites were recruited through high schools. Parents gave written informed consent and adolescents gave written assent to the study procedures before enrollment. All procedures were approved by each local institutional ethics committee. Further details on the study design, sample, and recruitment procedure, as well as data storage and safety, may be found elsewhere (21; see also the data supplement that accompanies the online edition of this article).
After data quality control, complete and reliable data sets were available for 1,778 participants with a mean age of 14.4 years (SD=0.35) and a balanced gender ratio (51% of them girls [N=912]). Follow-up data on externalizing symptoms were gathered at age 16 for 1,210 (68%) of these participants and were used to test the stability of externalizing symptoms and their structure across adolescence. Participants who attended the assessments at age 16 did not differ significantly from those who did not in demographic, behavioral, or cognitive variables, except in language (English, German, and French; English speakers were more likely to participate in the follow-up: odds ratio=3.44, 95% CI=2.55–4.64) and self-reported ADHD symptoms (those scoring higher on ADHD symptoms were less likely to participate: odds ratio=0.91, 95% CI=0.85–0.98).
Of the 1,778 adolescents, 4.4% (N=78) were identified as having a diagnosis of conduct disorder (N=37), ADHD (N=30), or both (N=11) according to the Development and Well-Being Assessment interview (22); 3.7% (N=65) reported problematic alcohol use, and 10.6% (N=189) reported drug use. At age 16, 6.3% (N=66/1210) were identified as having a diagnosis of conduct disorder (N=25), ADHD (N=31), or both (N=10); 18.0% (N=218) reported problematic alcohol use, and 27.1% (N=328) reported drug use.
Measures
All measures were selected on the basis of brevity, age-appropriateness, and validity in their variant forms (English, German, and French). They are described briefly below; for a more detailed description, see the online data supplement.
Externalizing problems.
Self-report and parent-report behavioral and clinical measures were assessed through an online computer platform provided by Psytools (Delosis Research Technology, London) and administered at participants’ homes, as well as the Development and Well-Being Assessment interview (22; www.dawba.com) and the Strengths and Difficulties Questionnaire (23), administered at the research site. Substance misuse was assessed using the Alcohol Use Disorders Identification Test (24) and the European School Survey Project on Alcohol and Drugs (25). Only externalizing symptoms were assessed at both ages 14 and 16.
Personality traits were assessed with the impulsivity and sensation-seeking subscales of the self-reported Substance Use Risk Profile Scale (26). (For a discussion of the dissociation between impulsivity and sensation seeking and their psychometric properties, see references 3 and 13.)
IQ and behavioral measures.
To control for the general effects of intelligence on behavior and cognitive performance, estimates of intelligence were derived from the vocabulary and similarities subtests (verbal IQ) and block design and matrix reasoning subtests (performance IQ) of the Wechsler Intelligence Scale for Children, 4th Edition (27). Response inhibition was measured as number of commission errors in a go/no-go passive avoidance learning paradigm (28). In this task, participants are asked to learn, by trial and error, to respond to “correct” numbers and to withhold a response for “wrong” numbers by rewarding correct or punishing wrong go and no-go responses. Commission errors were used as an index of response inhibition. Delay discounting was assessed with the Kirby Delay Discounting Questionnaire (20), with larger k values indicating greater delay discounting of value for delayed options.
Neuroimaging tasks.
Two MRI sessions were conducted, lasting 45 minutes each, each including a combination of structural and functional scans. Before each session, participants familiarized themselves with the scanner and tasks in a practice session. (Further information about the imaging procedure, with links to task specifications, is available at http://www.imagen-europe.com/en/Publications_and_SOP.php; see also reference 21.)
The stop-signal task (14) was used to measure brain response during successful and failed inhibition. Participants responded to regularly presented visual go stimuli (arrows pointing left or right) and were instructed to withhold their response when the go stimulus was followed (unpredictably) by a stop signal (arrow pointing upward). Stopping difficulty was manipulated across trials by varying the delay between the onset of the go arrow and the stop arrow (stop-signal delay) using a previously described tracking algorithm (14). There were two contrasts of interest, in which go trials were used as “implicit baseline”: successful inhibition–go trial, and failed inhibition (commission error)–go trial. A comprehensive report on this task by Whelan et al. (15) identified, through factor analysis, seven brain networks involved in stop success in this sample of adolescents (basal ganglia; parietal cortex; orbitofrontal cortex; medial orbitofrontal cortex; substantia nigra and subthalamic nucleus; right frontal cortex; and presupplementary motor area) and six networks involved in stop failure (left and right frontal cortex; parietal cortex; posterior cingulate and medial orbitofrontal cortex; orbitofrontal cortex; substantia nigra and subthalamic nucleus; and basal ganglia). Factor scores from that analysis were used in the present study.
A modified version of the monetary incentive delay task (29) was used to assess brain response to reward anticipation. In the anticipation phase of this task, participants were presented with cues (which varied between 4 and 4.5 seconds) signaling the amount of reward that could be won in a given trial (large reward, small reward, or no reward). We focused on three regions of interest, in which mean activity levels for each contrast were extracted during the reward anticipation phase involving the contrast of large reward anticipation versus no reward anticipation. The regions of interest selected were the left and right ventral striatum, orbitofrontal cortex, and inferior frontal gyrus. As BOLD responses in the left and right ventral striatum were highly correlated, they were averaged to create a bilateral ventral striatum activation score.
Data Analysis
A series of a priori structural equation models on substance misuse, ADHD, and conduct disorder symptom scores (incorporating symptom severity and impact) were analyzed using MPlus, version 6.12 (www.statmodel.com). Based on previously reported analyses (2, 3), several models were assessed for goodness of fit.
| 1. | A single “externalizing problems” factor loading on all symptom scores. | ||||
| 2. | Two hierarchical models, in which variables assessing conduct disorder symptoms (scaled likelihood of diagnosis, parent- and self-reported symptoms, self-reported bullying behavior), ADHD symptoms (scaled likelihood of diagnosis, parent- and self-reported symptoms), and substance misuse variables (age at onset of drinking, number of drugs used, frequency of drunkenness, frequency of bingeing, the interaction of quantity and frequency of drinking, and drinking-related problems) loading onto specific conduct disorder, ADHD, and substance misuse subfactors, respectively, which then load onto a higher-order “externalizing problems” factor (model 2a) and a modified version of this model where conduct disorder and ADHD symptoms load onto the same specific ADHD/conduct disorder factor (model 2b). | ||||
| 3. | Two general-specific models, in which a general externalizing factor was added at the same level as the specific conduct disorder, ADHD, and substance misuse factors from the previous (2a) model (model 3a), or the specific ADHD/conduct disorder and substance misuse factors from the previous (2b) model (model 3b). In these last general-specific models, factors were not allowed to covary (see Figure 1) 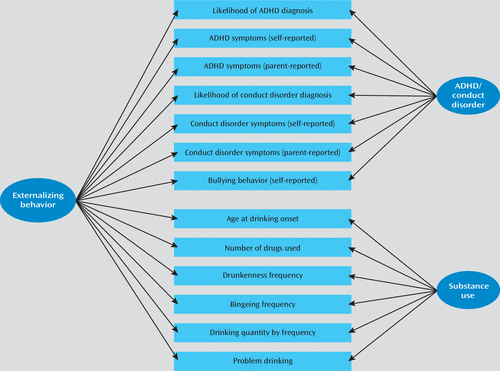 FIGURE 1. General-Specific Model of Unique and Common Variance Across Externalizing Problems in Young Adolescencea a ADHD=attention deficit hyperactivity disorder. Likelihood of ADHD and conduct disorder diagnoses refers to scaled likelihood of DSM-IV diagnosis according to band scores from the Development and Well-Being Assessment (22). | ||||
All models were fitted using a complex random-effects design to control for site as a cluster variable and using robust maximum-likelihood estimation. Unlike other studies on this topic, we incorporated variance in behavior across the entire population, not simply severity of symptoms in participants with diagnoses, which allowed us to investigate the extent to which continuous models of liability also represent variance with normal adolescent populations.
Once the best-fitting model was established, four sets of covariates (personality, cognitive indices, and brain response scores during the stop task and during the monetary incentive delay tasks) were each entered into the model separately.
Finally, stability of externalizing problems across adolescence was tested by fitting the model using follow-up data at age 16 (N=1,210) and assessing correlations between factor scores across time. All analyses controlled for gender, verbal and performance IQ, and language. The Benjamini-Hochberg procedure was used to correct for multiple testing in all analyses. See the online data supplement for further discussion of the analytic approach.
Results
Externalizing Problems Model Fit
Of the models tested, the general-specific model with the combined ADHD/conduct disorder and substance misuse specific factors (model 3b) fit the data best (Table 1). Factor loadings for this model are presented in Table 2. The substance misuse factor reflected mostly variance unique to drinking. The ADHD/conduct disorder factor captured variance unique to ADHD with some loading from parent-reported conduct disorder symptoms. With the exception of parent-reported ADHD symptoms, all substance misuse, ADHD, and conduct disorder variables loaded significantly on the general externalizing factor, with conduct disorder variables loading the strongest on this factor.
| Model | χ2 | df | SCF | CFI | RMSEA | SRMR | AIC | BIC | Δχ2 | Δdf | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Models without covariates | |||||||||||
| Model 1: one factor | 1952.20 | 65 | 1.39 | 0.59 | 0.13 | 0.11 | 75489 | 75846 | 1158.5 | 13 | <0.001 |
| Model 2a: hierarchical, three subfactors | 389.57 | 62 | 1.53 | 0.92 | 0.06 | 0.04 | 73372 | 73745 | 51.7 | 10 | <0.001 |
| Model 2b: hierarchical, two subfactors | 567.57 | 64 | 1.55 | 0.88 | 0.07 | 0.06 | 73658 | 74020 | 157.4 | 12 | <0.001 |
| Model 3a: general-specific (three specific) | 336.88 | 52 | 1.55 | 0.94 | 0.06 | 0.04 | 73317 | 73745 | |||
| Model 3b: general-specific (two specific) | 341.25 | 52 | 1.25 | 0.94 | 0.05 | 0.03 | 73221 | 73648 | |||
| Models with covariates | |||||||||||
| Model 3b and IQ covariatesb | 374.31 | 75 | 0.94 | 0.05 | 0.03 | ||||||
| Model 3b and personality covariatesb | 487.72 | 97 | 0.93 | 0.05 | 0.04 | ||||||
| Model 3b and behavioral covariates (go/no-go and delay discounting task data)b | 418.01 | 97 | 0.94 | 0.04 | 0.03 | ||||||
| Model 3b and stop-signal task datab | 679.91 | 232 | 0.92 | 0.03 | 0.03 | ||||||
| Model 3b and monetary incentive delay task data (reward anticipation)b | 554.08 | 136 | 0.92 | 0.04 | 0.03 |
TABLE 1. Fit Indices for Successive Structural Equation Models of Externalizing Problems in Young Adolescencea
| Factor | ||||||
|---|---|---|---|---|---|---|
| ADHD/Conduct Disorder | Substance Misuse | General Externalizing Behavior | ||||
| Predictor | Load | p | load | p | Load | p |
| Likelihood of conduct disorder diagnosis | 0.27 | <0.001 | 0.55 | <0.001 | ||
| Conduct disorder symptoms, self-reported | 0.14 | 0.303 | 0.68 | <0.001 | ||
| Conduct disorder symptoms, parent-reported | 0.46 | <0.001 | 0.41 | <0.001 | ||
| Likelihood of ADHD diagnosis | 0.70 | <0.001 | 0.19 | 0.039 | ||
| ADHD symptoms, self-reported | 0.39 | <0.001 | 0.35 | 0.016 | ||
| ADHD symptoms, parent-reported | 0.79 | <0.001 | 0.21 | 0.191 | ||
| Bullying behavior, self-reported | 0.08 | 0.002 | 0.26 | <0.001 | ||
| Age at drinking onset | 0.36 | <0.001 | 0.33 | <0.001 | ||
| Number of drugs used | 0.30 | <0.001 | 0.30 | <0.001 | ||
| Drunkenness frequency | 0.70 | <0.001 | 0.32 | <0.001 | ||
| Bingeing frequency | 0.77 | <0.001 | 0.33 | <0.001 | ||
| Drinking quantity by frequency | 0.73 | <0.001 | 0.36 | <0.001 | ||
| Problem drinking symptoms | 0.45 | <0.001 | 0.31 | <0.001 | ||
TABLE 2. Factor Loadings for General-Specific Model 3b at Age 14 (N=1,778)a
Personality, Cognitive, and Neural Covariates
All predictor models with covariates showed good model fit (Table 1). Table 3 presents regression paths between covariates and the general-specific factors of externalizing problems from model 3b, showing that lower verbal and performance IQ were significantly related to the ADHD/conduct disorder factor only. Impulsivity was significantly associated with the ADHD/conduct disorder and general externalizing factors, but not substance misuse, while sensation seeking was significantly associated with the substance misuse factor only. Commission errors on the go/no-go task were associated with the ADHD/conduct disorder factor only, while steeper delay discounting was associated with all factors. In terms of regional brain response variables, the ADHD/conduct disorder factor was associated with lower BOLD response in frontal areas bilaterally during failed inhibition, the substance misuse factor was not significantly associated with BOLD response during this task, and the general externalizing factor was associated with lower BOLD response during successful inhibition in the substantia nigra and subthalamic nucleus, and in the presupplementary motor area and precentral gyrus. Only the substance misuse factor was associated with differential BOLD response during reward anticipation on the monetary incentive delay task: higher BOLD response in the left orbitofrontal cortex and lower BOLD response in the left inferior frontal gyrus.
| Factor | ||||||
|---|---|---|---|---|---|---|
| ADHD/Conduct Disorder | Substance Misuse | General Externalizing Behavior | ||||
| Predictor | Estimate | p | Estimate | p | Estimate | p |
| IQ | ||||||
| Verbal | –0.17 | <0.001 | –0.02 | 0.376 | 0.03 | 0.756 |
| Performance | –0.16 | <0.001 | –0.10 | 0.023 | –0.04 | 0.345 |
| Personality measures | ||||||
| Impulsivity | 0.27 | <0.001 | –0.03 | 0.549 | 0.53 | <0.001 |
| Sensation seeking | 0.01 | 0.746 | 0.11 | 0.009 | 0.06 | 0.241 |
| Behavioral measures | ||||||
| Delay discounting task (k values) | 0.06 | <0.001 | 0.07 | 0.006 | 0.11 | 0.001 |
| Go/no-go task (commission errors) | 0.09 | 0.007 | 0.02 | 0.410 | 0.04 | 0.230 |
| fMRI region-of-interest factors for stop-signal task success and failure | ||||||
| Stop success | ||||||
| Basal ganglia | –0.02 | 0.826 | –0.06 | 0.132 | –0.08 | 0.100 |
| Parietal cortex | –0.01 | 0.783 | 0.00 | 0.936 | 0.01 | 0.849 |
| Orbitofrontal cortex | 0.03 | 0.394 | 0.01 | 0.767 | –0.01 | 0.787 |
| Medial orbitofrontal cortex | –0.02 | 0.467 | 0.02 | 0.700 | 0.05 | 0.038 |
| Substantia nigra and subthalamic nucleus | 0.08 | 0.043 | 0.07 | 0.062 | –0.12 | <0.001 |
| Right frontal cortexb | 0.04 | 0.273 | 0.02 | 0.709 | 0.05 | 0.260 |
| Presupplementary motor area, precentral gyrusb | –0.01 | 0.839 | 0.06 | 0.020 | 0.14 | 0.001 |
| Stop failure | ||||||
| Basal ganglia | 0.09 | 0.121 | 0.01 | 0.892 | 0.06 | 0.475 |
| Orbitofrontal cortex | –0.01 | 0.804 | –0.01 | 0.806 | 0.05 | 0.098 |
| Precentral cortex, medial orbitofrontal cortex | 0.04 | 0.398 | –0.05 | 0.180 | 0.03 | 0.317 |
| Parietal cortexb | –0.02 | 0.561 | 0.03 | 0.348 | 0.02 | 0.489 |
| Left and right frontal cortexb | –0.09 | 0.003 | 0.01 | 0.861 | –0.05 | 0.446 |
| Substantia nigra and subthalamic nucleus | –0.01 | 0.881 | 0.05 | 0.345 | –0.01 | 0.412 |
| fMRI regions of interest for reward anticipation (monetary incentive delay task) | ||||||
| Left and right ventral striatum | –0.09 | 0.017 | –0.02 | 0.429 | 0.00 | 0.945 |
| Right orbitofrontal cortex | 0.02 | 0.595 | 0.02 | 0.573 | –0.01 | 0.844 |
| Left orbitofrontal cortex | –0.02 | 0.653 | 0.05 | 0.010 | 0.00 | 0.987 |
| Right inferior frontal gyrus | 0.04 | 0.417 | 0.01 | 0.770 | 0.04 | 0.293 |
| Left inferior frontal gyrus | –0.03 | 0.534 | –0.09 | <0.001 | 0.04 | 0.305 |
TABLE 3. Standardized Parameter Estimates for Concurrent Associations Between Covariates and ADHD/Conduct Disorder, Substance Misuse, and General Externalizing Behavior Factors as Established in General-Specific Model 3b at Age 14 (N=1,778)a
Predicting Externalizing Problems at Age 16
Externalizing problems data at age 16 best fit a general-specific model as well, represented by ADHD/conduct disorder-specific, substance misuse-specific, and common externalizing factors, which were correlated strongly with corresponding factors at age 14 (see Table 4 for fit indices, factor loadings, and 2-year test-retest correlations between factors). When covariates at age 14, including externalizing problem factor scores, were added to the model at age 16, impulsivity at age 14 predicted the general externalizing factor at age 16 (b=0.15, p<0.05), sensation-seeking at age 14 predicted substance misuse at age 16 (b=0.07, p<0.01), and go/no-go commission errors as well as lower BOLD response in frontal areas bilaterally during failed inhibition at age 14 predicted ADHD/conduct disorder at age 16 (for both, b=0.09, p<0.001). No other significant associations were observed between covariates (cognitive or brain imaging) at age 14 and externalizing problem factors at age 16.
| Factor | ||||||
|---|---|---|---|---|---|---|
| Predictor | ADHD/Conduct Disorder at Age 16 | Substance Misuse at Age 16 | General Externalizing Behavior at Age 16 | |||
| Load | p | Load | p | Load | p | |
| Likelihood of conduct disorder diagnosis | 0.19 | 0.316 | 0.39 | <0.001 | ||
| Conduct disorder symptoms, self-reported | –0.15 | 0.647 | 0.82 | <0.001 | ||
| Conduct disorder symptoms, parent-reported | 0.35 | 0.001 | 0.48 | <0.001 | ||
| Likelihood of ADHD diagnosis | 0.44 | <0.001 | 0.35 | 0.007 | ||
| ADHD symptoms, self-reported | 0.09 | 0.446 | 0.52 | <0.001 | ||
| ADHD symptoms, parent-reported | 0.64 | <0.001 | 0.49 | 0.004 | ||
| Bullying behavior, self-reported | 0.03 | 0.393 | 0.18 | <0.001 | ||
| Age at drinking onset | 0.36 | <0.001 | 0.23 | <0.001 | ||
| Drug use frequency | 0.51 | <0.001 | 0.23 | <0.001 | ||
| Drunkenness frequency | 0.85 | <0.001 | 0.22 | <0.001 | ||
| Bingeing frequency | 0.83 | <0.001 | 0.26 | <0.001 | ||
| Drinking quantity by frequency | 0.84 | <0.001 | 0.24 | <0.001 | ||
| Problem drinking symptoms | 0.56 | <0.001 | 0.16 | <0.001 | ||
| r | p | r | p | r | p | |
| Correlations between factors | ||||||
| ADHD/conduct disorder at age 14 | 0.56 | <0.001 | –0.01 | 0.800 | 0.21 | 0.004 |
| Substance misuse at age 14 | 0.00 | 0.814 | 0.45 | <0.001 | –0.03 | 0.392 |
| General externalizing behavior at age 14 | –0.09 | 0.178 | –0.07 | 0.062 | 0.61 | <0.001 |
TABLE 4. Factor Loadings for General-Specific Model 3b at Age 16 (N=1,210)a
Discussion
This study provides further support for the latent trait model of externalizing problems previously proposed by Krueger et al. (2) and validated in an adolescent sample by Castellanos-Ryan and Conrod (3), and it extends the model to include early-adolescent ADHD symptoms (as also recently demonstrated by Callagher et al. in adults [4]) and to demonstrate developmental stability. When such problems are modeled hierarchically, personality, cognitive, and neural measures of disinhibition dissociate in their relationship to the latent variables generated by the model, extending the validity and utility of the dimensional-spectrum conceptualization of externalizing problems. The findings suggest that while risk-taking behavior is observed in normal adolescents, personality and neurocognitive abnormalities appear to be associated with the tendency to commit these behaviors across multiple behavioral indicators, contexts, and time points, as reflected by the latent dimensions and their stability across time. At least three neurocognitive profiles were shown to dissociate dimensions of externalizing problems: self-reported sensation seeking (and reward sensitivity), impulsive action tendencies (poor response inhibition), and impulsive choice (delay discounting). Understanding a young person’s dominant learning and motivational profile from this perspective might help in individualizing treatments and have an impact on a variety of behavioral outcomes.
The latent factor previously identified by Castellanos-Ryan and Conrod (3) as representing variance unique to conduct disorder was shown in this study to also include ADHD symptoms, and it was correlated with self-reported impulsivity scores, lower verbal and performance IQ, poor response inhibition, higher delay discounting, and a weaker BOLD response in the frontal cortex bilaterally (including the anterior cingulate, rostral caudate, and inferior frontal gyrus) during failed inhibition in a stop task. These findings are all consistent with a profile of poor frontal executive control involving impairments in both impulsive action and impulsive choice. Our findings are similar to those reported by Rubia et al. (31) showing that adolescents with a pure ADHD symptom profile had low BOLD response in prefrontal cortical brain regions during failed inhibition but not during successful inhibition. While a number of studies have also reported reduced right inferior frontal cortex activation in ADHD patients during successful stop trials (32), these previous findings may reflect co-occurrence of general externalizing tendencies, rather than the variance specific to ADHD/conduct disorder. This clinical profile appears best characterized by deficits in motor impulsivity regulated by prefrontal brain circuits, rather than reward sensitivity, and related subcortical brain circuits.
The general externalizing factor, which accounted for much of the variance in externalizing problems, was also strongly associated with self-reported impulsivity but was dissociated from the other externalizing dimensions on cognitive and neuroimaging measures of poor impulsive choice, low BOLD response in the substantia nigra and subthalamic nucleus, and high BOLD response in the presupplementary motor area/precentral gyrus during successful inhibition. These brain regions have been implicated in inhibitory control (33), with some findings suggesting that both networks have a more specific role in the motivation of action and the capacity to slow down to evaluate conflicting choices (34). The presupplementary motor area is thought to be associated with motivation for movement initiation by linking expected reward with specific actions, rather than controlling whether an action should be made (35). Findings from the present study add to previous findings by showing that high BOLD response in the presupplementary motor area/precentral gyrus may be associated with vulnerability to externalizing problems in general, rather than substance use specifically (15).
The substance misuse-specific factor, reflecting mostly early onset and frequency and quantity of drinking, was associated with self-reported sensation seeking, high delay discounting, and high left orbitofrontal response and low left inferior frontal gyrus response when anticipating reward. These findings of a double dissociation in the neurocognitive profiles of ADHD/conduct disorder and substance misuse are consistent with previous reports (13) showing that sensation seeking and individual differences in reward response (previously measured using behavioral tasks) specifically predict vulnerability to binge drinking in adolescence. The orbitofrontal cortex has consistently been identified as a key part of the reward-processing network (36), with high BOLD response in this brain area being associated with heightened attention to reward (37). The present findings are consistent with reports of frontolimbic (including the orbitofrontal cortex and inferior frontal gyrus) reward-processing deficits involved in alcohol problems (38) and further suggest a reward-related deficit in adolescents prone to early-onset frequent alcohol misuse, independent of other externalizing problems.
This study also demonstrates that the latent dimensions of externalizing problems are moderately stable and that personality, cognitive, and neural correlates of such dimensions also have longitudinal predictive validity. Considering the age of our participants at baseline (age 14) and their relatively minor substance use histories, these findings likely reflect underlying etiologic mechanisms rather than the long-term effects of substance use and suggest that prevention efforts directed at reducing underlying motivational and trait dimensions of risk (39, 40) could benefit from incorporating training components that target these brain functions (41). Furthermore, in line with recent proposals to shift our research strategy in psychopathology toward the study of cognitive and neural endophenotypes (1), our findings suggest that the dissociation of impulsive action, impulsive choice, and reward sensitivity is relevant to understanding the common and shared variance across externalizing problems. Furthermore, these findings suggest that new intervention strategies targeting these endophenotypes of risk, either at the personality, cognitive, or neural level, have the potential to affect a number of clinical outcomes concurrently. The fact that they can be measured before the emergence of psychiatric symptoms suggests that they might also be good candidates for novel prevention strategies in mental health, such as has been demonstrated by the personality-targeted approach to drug and alcohol prevention (40), which recently was also shown to concurrently reduce emotional and behavioral problems in youths (39).
1 : Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 2013; 11:126Crossref, Medline, Google Scholar
2 : Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol 2007; 116:645–666Crossref, Medline, Google Scholar
3 : Personality correlates of the common and unique variance across conduct disorder and substance misuse symptoms in adolescence. J Abnorm Child Psychol 2011; 39:563–576Crossref, Medline, Google Scholar
4 : ADHD and the externalizing spectrum: direct comparison of categorical, continuous, and hybrid models of liability in a nationally representative sample. Soc Psychiatry Psychiatr Epidemiol (Epub ahead of print, Oct 1, 2013)Google Scholar
5 : Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Dev Sci 2005; 8:132–140Crossref, Medline, Google Scholar
6 : Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci 2012; 16:81–91Crossref, Medline, Google Scholar
7 : Varieties of impulsivity. Psychopharmacology (Berl) 1999; 146:348–361Crossref, Medline, Google Scholar
8 : Dimensions of impulsive behavior: personality and behavioral measures. Pers Individ Dif 2006; 40:305–315Crossref, Google Scholar
9 : Impulsive choice behavior in four strains of rats: evaluation of possible models of attention-deficit/hyperactivity disorder. Behav Brain Res 2013; 238:10–22Crossref, Medline, Google Scholar
10 : A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol 2005; 114:216–222Crossref, Medline, Google Scholar
11 : Investigation of cool and hot executive function in ODD/CD independently of ADHD. J Child Psychol Psychiatry 2011; 52:1035–1043Crossref, Medline, Google Scholar
12 : Behavioral disinhibition and the development of early-onset addiction: common and specific influences. Annu Rev Clin Psychol 2008; 4:325–348Crossref, Medline, Google Scholar
13 : Response inhibition and reward response bias mediate the predictive relationships between impulsivity and sensation seeking and common and unique variance in conduct disorder and substance misuse. Alcohol Clin Exp Res 2011; 35:140–155Crossref, Medline, Google Scholar
14 : Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry 2005; 162:1067–1075Link, Google Scholar
15 ;
16 : Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am J Psychiatry 2009; 166:83–94Link, Google Scholar
17 : Brain activity in valuation regions while thinking about the future predicts individual discount rates. J Neurosci 2013; 33:13150–13156Crossref, Medline, Google Scholar
18 : Reward sensitivity modulates connectivity among reward brain areas during processing of anticipatory reward cues. Eur J Neurosci 2013; 38:2399–2407Crossref, Medline, Google Scholar
19 : Individual differences in the response to forgone payoffs: an examination of high functioning drug abusers. J Behav Decis Mak 2005; 18:97–110Crossref, Google Scholar
20 : Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 1999; 128:78–87Crossref, Medline, Google Scholar
21 ;
22 : The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000; 41:645–655Crossref, Medline, Google Scholar
23 : Predicting type of psychiatric disorder from Strengths and Difficulties Questionnaire (SDQ) scores in child mental health clinics in London and Dhaka. Eur Child Adolesc Psychiatry 2000; 9:129–134Crossref, Medline, Google Scholar
24 : Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons With Harmful Alcohol Consumption–II. Addiction 1993; 88:791–804Crossref, Medline, Google Scholar
25 : The 2007 ESPAD Report: Substance Use Among Students in 35 European Countries. Stockholm, Swedish Council for Information on Alcohol and Other Drugs, 2009Google Scholar
26 : The Substance Use Risk Profile Scale: a scale measuring traits linked to reinforcement-specific substance use profiles. Addict Behav 2009; 34:1042–1055Crossref, Medline, Google Scholar
27 : Wechsler Intelligence Scale for Children, 4th ed. San Antonio, Tex, Psychological Corp, 2003Google Scholar
28 : Diverse pathways to deficient self-regulation: implications for disinhibitory psychopathology in children. Clin Psychol Rev 1993; 13:699–720Crossref, Google Scholar
29 ;
30 : Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling 1999; 6:1–55Crossref, Google Scholar
31 : Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry 2011; 70:255–262Crossref, Medline, Google Scholar
32 : Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 2013; 70:185–198Crossref, Medline, Google Scholar
33 : Dissociating the role of the pre-SMA in response inhibition and switching: a combined online and offline TMS approach. Front Hum Neurosci 2013; 7:150Crossref, Medline, Google Scholar
34 : Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 2007; 318:1309–1312Crossref, Medline, Google Scholar
35 : Medial frontal cortex motivates but does not control movement initiation in the countermanding task. J Neurosci 2010; 30:1968–1982Crossref, Medline, Google Scholar
36 : Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 2011; 35:1219–1236Crossref, Medline, Google Scholar
37 : Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci 2003; 23:303–307Crossref, Medline, Google Scholar
38 : Neural and behavioral mechanisms of impulsive choice in alcohol use disorder. Alcohol Clin Exp Res 2011; 35:1209–1219Crossref, Medline, Google Scholar
39 : Two-year impact of personality-targeted, teacher-delivered interventions on youth internalizing and externalizing problems: a cluster-randomized trial. J Am Acad Child Adolesc Psychiatry 2013; 52:911–920Crossref, Medline, Google Scholar
40 : Effectiveness of a selective, personality-targeted prevention program for adolescent alcohol use and misuse: a cluster randomized controlled trial. JAMA Psychiatry 2013; 70:334–342Crossref, Medline, Google Scholar
41 : Changes in cortical activity after training of working memory: a single-subject analysis. Physiol Behav 2007; 92:186–192Crossref, Medline, Google Scholar



