Disorder-Specific Dissociation of Orbitofrontal Dysfunction in Boys With Pure Conduct Disorder During Reward and Ventrolateral Prefrontal Dysfunction in Boys With Pure ADHD During Sustained Attention
Abstract
Objective: Among children, attention deficit hyperactivity disorder (ADHD) and conduct disorder are often comorbid and overlap clinically. Neuropsychological evidence suggests that children with conduct disorder demonstrate more prominent motivational problems and children with ADHD demonstrate more prominent attention deficits relative to healthy comparison subjects. The purpose of the present study was to investigate disorder-specific abnormalities in the neurobiological correlates of motivation and sustained attention in children and adolescents with pure conduct disorder and children and adolescents with pure ADHD. Method: Participants were male pediatric patients, ages 9–16 years, with noncomorbid conduct disorder (N=14) and noncomorbid ADHD, combined hyperactive-inattentive subtype (N=18), as well as age- and IQ-matched healthy comparison subjects (N=16). Both patient groups were medication naive. Event-related functional magnetic resonance imaging (fMRI) was used to compare brain activation during a rewarded continuous performance task that measured sustained attention as well as the effects of reward on performance. Results: During the sustained attention condition, patients with noncomorbid ADHD showed significantly reduced activation in the bilateral ventrolateral prefrontal cortex and increased activation in the cerebellum relative to patients with noncomorbid conduct disorder and healthy comparison subjects. Patients with noncomorbid conduct disorder showed decreased activation in paralimbic regions of the insula, hippocampus, and anterior cingulate as well as the cerebellum relative to patients with noncomorbid ADHD and healthy comparison subjects. However, during the reward condition, patients with noncomorbid conduct disorder showed disorder-specific underactivation in the right orbitofrontal cortex, while patients with noncomorbid ADHD showed disorder-specific dysfunction in the posterior cingulate gyrus. Conclusions: The findings revealed a process-related dissociation of prefrontal dysfunction in ADHD and conduct disorder patients. Attention-related dysfunction in the ventrolateral prefrontal cortex was seen in ADHD patients, and reward-related dysfunction in the orbitofrontal cortex was seen in conduct disorder patients. These findings, together with the pattern of paralimbic dysfunction demonstrated among children with conduct disorder during sustained attention, support theories of abnormalities in orbitofrontal-paralimbic motivation networks in individuals with conduct disorder and, in contrast, ventrolateral fronto-cerebellar attention network dysfunction in individuals with ADHD.
Conduct disorder is described as a disorder of persistent antisocial behavior and aggression. Among individuals with conduct disorder, there is high comorbidity with attention deficit hyperactivity disorder (ADHD). In addition, the notion of a separate neurobiological basis for conduct disorder has been questioned (1) .
One of the most consistent findings from studies of ADHD is that of deficits in sustained attention as measured on the Continuous Performance Test (2 , 3) . Neuroimaging studies of ADHD patients have associated abnormalities in fronto-striato-parietal neural networks with poor cognitive and attentional control (4 – 6) . However, to our knowledge, no functional magnetic resonance imaging (fMRI) study has directly examined sustained attention during a task on the Continuous Performance Test. Earlier positron emission tomography (PET) studies found abnormal glucose metabolism in frontal, striatal, and temporal brain regions during auditory continuous performance tasks, particularly among female adolescents with ADHD (7 – 9) .
Evidence for sustained attention deficits among individuals with conduct disorder and oppositional defiant disorder is controversial and has been associated with cases of comorbidity with ADHD (10 , 11) . However, conduct disorder but not ADHD has consistently been associated with reduced sensitivity to changes in reward contingencies, specifically to punishment (12 , 13) . Consequently, attention problems among individuals with conduct disorder have been postulated as secondary to motivational deficits (12) . Thus, neural substrates of motivation are good candidates for a distinct neurobiological basis for conduct disorder. In studies of adult antisocial behavior, neurobiological evidence has suggested that there are abnormalities in the paralimbic system, comprising orbitofrontal, superior temporal, cingulate, and limbic brain regions, which mediate the control of emotion and motivation (14) . However, there are no fMRI studies that have examined the theory of abnormal motivation networks in children with conduct disorder. To our knowledge, only three published fMRI studies of conduct disorder in children and adolescents are present in the literature. Two of these studies examined several comorbid cases and reported 1) abnormal anterior cingulate activation during the presentation of images with negative valence (15) and 2) reduced function of the amygdala and its interconnectivity to the orbitofrontal cortex during fearful face processing (16) . The other study, which we conducted, reported reduced activation in the posterior cingulate and temporo-parietal regions in patients with noncomorbid conduct disorder during error processing (17) .
Among individuals with ADHD, neuropsychological evidence for motivational deficits is inconsistent (18) . Few imaging studies have investigated reward functions. One study reported reduced activation in the insula in relation to a gambling task (19) . Another study found reduced activation in the nucleus accumbens during reward anticipation (20) .
Direct functional imaging comparisons between a pure ADHD patient group and a pure conduct disorder patient group found abnormalities in evoked potentials across frontal and parietal brain regions in both groups during a Continuous Performance Test, but no between-group differences were reported (21 , 22) . In our previous fMRI study, we found disorder-specific inferior prefrontal dysfunction among children with pure ADHD and disorder-specific abnormalities in parieto-temporal activation among children with pure conduct disorder during motor inhibition (17) .
The objective of the present study was to utilize fMRI to compare brain activation in a group of carefully selected male pediatric patients who met criteria for noncomorbid conduct disorder with brain activation in a group of male pediatric patients who had noncomorbid ADHD, combined hyperactive-inattentive subtype, and a male pediatric healthy comparison group. The three groups were assessed during a rewarded variant of the continuous performance task that measured 1) sustained attention and 2) the effects of reward. Based on relatively sparse evidence from imaging studies of ADHD during sustained attention (7 – 9 , 21) and reward function (19 , 20) , we hypothesized that relative to healthy comparison subjects, children with noncomorbid ADHD would show 1) reduced brain activation in lateral prefrontal and parieto-temporal regions during sustained attention and 2) abnormal activation in reward-related regions. Based on relatively scarce neurofunctional literature on conduct disorder (15 – 17 , 21 , 22) , we hypothesized that children with noncomorbid conduct disorder would be characterized by reduced brain activation in motivation mediating paralimbic areas during sustained attention as well as in response to reward.
Method
Subjects
Patients were 32 right-handed male children and adolescents, ages 9–16 years, who had a clinical diagnosis of either 1) conduct disorder without clinical ADHD (N=14) or 2) ADHD, combined hyperactive-inattentive subtype, without conduct disorder (N=18). Diagnoses were established using the standardized Maudsley Diagnostic Structural Interview (23) . Recruitment was conducted via parent support groups, clinics, and advertisements. Exclusion criteria were comorbidity with other psychiatric disorders; a learning disability, reading disorder, or neurological abnormality; epilepsy; substance abuse; and previous exposure to stimulant medication. All ADHD patients achieved a score above threshold on the hyperactivity subscale of the Strength and Difficulties Questionnaire (24) . All patients with conduct disorder also achieved a score above threshold on the conduct disorder subscale of this same questionnaire. ADHD patients were medication-naive by personal choice or were scanned prior to initial medication treatment. All patients with conduct disorder were medication naive, had early onset conduct disorder (<10 years of age), and met criteria for oppositional defiant disorder.
Healthy comparison subjects were 16 right-handed age- and IQ-matched male children and adolescents. In order to minimize differences in socioeconomic status, healthy subjects were recruited from the same local geographic area as ADHD and conduct disorder patients. Healthy subjects had no history of ADHD, conduct disorder, other mental or neurological disorder, neurotropic drug treatment, or substance abuse, and they scored below threshold on the Strength and Difficulties Questionnaire.
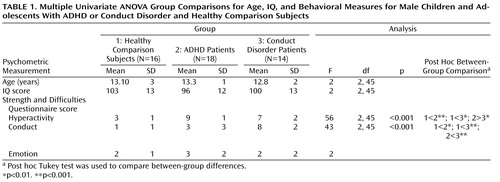
All participants scored above the fifth percentile (i.e., >75) on the Raven’s Standard Progressive Matrices Intelligence Questionnaire (25) ( Table 1 ). Written informed consent/assent from parents was given, and the study was approved by the local ethics committee.
One-way analysis of variance (ANOVA) showed no group differences in age and IQ. Post hoc t tests revealed that ADHD patients scored significantly higher than conduct disorder patients and healthy comparison subjects on the hyperactivity subscale of the Strength and Difficulties Questionnaire and conduct disorder patients scored significantly higher on the conduct disorder subscale of the questionnaire relative to ADHD patients and healthy comparison subjects ( Table 1 ). The three groups did not differ significantly on the emotion symptom subscale of the questionnaire.
fMRI Paradigm: Rewarded Continuous Performance Test
A rapid mixed-trial event-related fMRI design, along with jittered and randomized presentation, was used to optimize statistical efficiency. Patients and healthy subjects practiced the task one time prior to scanning.
The computerized fMRI adaptation of the rewarded Continuous Performance Test consists of a stream of 416 stimuli (letters), with a presentation time of 300 msec each (mean intertrial intervals: 900 msec) (3 , 26) . Forty-eight target stimuli, letters “X” and “O,” are included. Subjects were required to respond with the right-hand button box to target letters (“X” or “O”) only. For one of the two target letters (counterbalanced across subjects), a correct response resulted in a monetary reward (£1 for every three correct responses). The amount of money earned during the task (£8 for 100% correct responses) was displayed on the right side of the screen by one of two different rising colored score bars (red, blue). For the nonrewarded target letter, the other score bar would rise by one score for every three correct responses, but no monetary reward was given ( Figure 1 ).
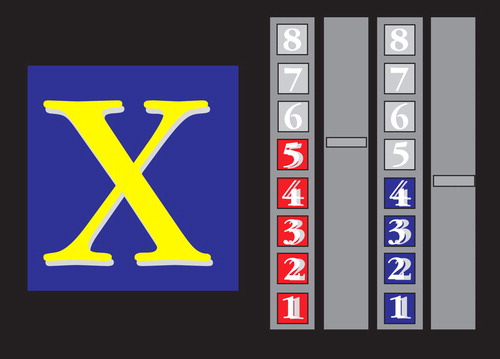
a Subjects respond to each target letter (“X” and “O”), ignoring other letters, and get rewarded for each response to one of the two target letters (red or blue bar). One correct score is equivalent to £1.
In order to measure effects of sustained attention, fMRI was used to contrast brain activation to nonrewarded target trials with brain activation to nontarget trials. The number of nonrewarded target trials was subtracted from the number of rewarded target trials in order to measure the effect of reward.
Analysis of Performance Data
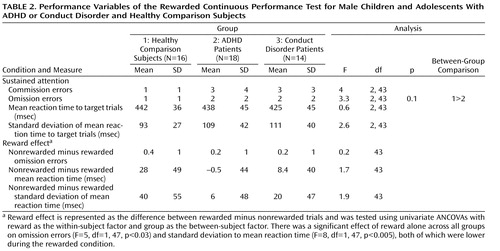
Although there were no significant differences in IQ or age, ADHD patients had slightly lower IQ scores relative to conduct disorder patients and healthy comparison subjects, and conduct disorder patients were, on average, 1 year younger than ADHD patients and healthy comparison subjects. Consequently, multiple univariate analyses of covariance (ANCOVAs), with IQ and age as covariates, were conducted to compare the following task performances between the three groups: omission errors to targets, commission errors (response to nontargets), mean reaction time to target trials, and intrasubject response variability of reaction time to targets (standard deviation). The reward effect was tested using univariate-repeated ANCOVAs, with reward as the within-subject factor and group as the between-subject factor. The false discovery rate was used to adjust p values for multiple testing.
fMRI Acquisition
Gradient-echo echo planar magnetic resonance imaging (MRI) data were acquired via the GE Signa 1.5T Horizon LX System using a semiautomated image quality control procedure. A quadrature birdcage head coil was used for radio frequency transmission and reception. In each of 16 noncontiguous planes parallel to the anterior-posterior commissural, 208 T2-weighted magnetic images, which depicted blood-oxygen-level-dependent (BOLD) contrast covering the whole brain, were acquired (TE=40 msec, time to repeat=1.8 sec, flip angle=90°, in-plane resolution=3.1 mm, slice thickness=7 mm, slice skip=0.7 mm).
fMRI Analysis
Time series analysis for each subject was established according to a previously published wavelet-based data resampling method for assessing fMRI data (27 , 28) . Using rigid body and affine transformation, individual maps were registered to Talairach standard space (29) . A group brain activation map was then produced for each experimental condition, and hypothesis testing was conducted at the cluster level. In essence, a voxel-wise test at p<0.05 was conducted to identify any voxels that might be activated by a subsequent test at a cluster level threshold of p<0.01 to remove false positive clusters produced by the voxel-level test. This combination (voxel/cluster) of tests, coupled with permutation testing, allows for excellent type I error control at the cluster level (27 , 28) . For each task, we expected less than one false positive activated cluster at a p value <0.05 at the voxel level and <0.01 at the cluster level. For between-group comparisons, one-way ANCOVAs, with group as a factor and age and IQ as covariates, were conducted using a randomized test to assess voxel- or cluster-wise differences (27 , 28) . For ANCOVAs, less than one false positive activated cluster was expected at a p value <0.05 for both voxel and cluster comparisons. In each significant cluster of the ANCOVAs, statistical measures of BOLD response for each participant were extracted, and post hoc least significance difference t tests (corrected for multiple comparisons) were conducted to identify between-group differences.
In large connected clusters, we identified local maxima that were farther apart than the upper bound of the likely Talairach mapping error (3 voxel radius:10 mm) (30) . Voxels were then assigned to the nearest local maximum with a statistic value that exceeded that of the voxels.
Results
Task Performance
ANCOVAs showed no significant group effects, with the exception of a tendency for omission errors, which were higher in ADHD patients ( Table 2 ). There was no significant group-by-reward effect. However, there was a significant effect of reward alone for all three groups on omission errors (F=5, df=1, 47, p<0.03) and standard deviation to mean reaction time (F=8, df=1, 47, p<0.005), both of which were lower during the rewarded condition.
Brain Activation
Motion
ANOVA showed no significant between-group differences for three-dimensional motion during task performance.
Sustained attention: nonrewarded target trials compared with nontarget trials
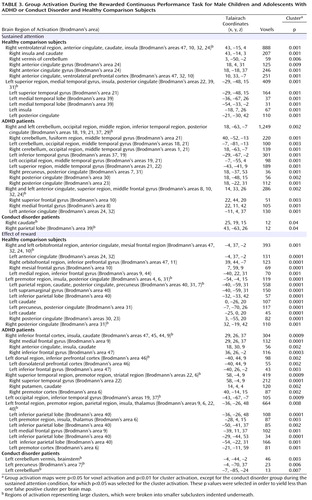
Group activations are detailed for all three groups in Table 3 and Figure 2 . ANCOVAs showed significant group effects in 1) right and left ventrolateral prefrontal activation, comprising inferior and orbital frontal gyri; 2) a large cluster in the cerebellum, occipital gyrus, thalamus, hippocampus, and left middle temporal gyrus; 3) a cluster comprising the right insula and hippocampus; and 4) the anterior cingulate gyrus ( Table 4 , Figure 3 ). Post hoc comparisons (corrected) revealed that ADHD patients showed reduced brain activation in the left and right ventrolateral prefrontal cortex relative to healthy comparison subjects (left ventrolateral prefrontal activation: p<0.02; right ventrolateral prefrontal activation: p<0.004) and conduct disorder patients (left ventrolateral prefrontal activation: p<0.003; right ventrolateral prefrontal activation: p<0.01). No difference was observed between conduct disorder patients and healthy comparison subjects. Post hoc comparisons (corrected) also revealed that ADHD patients showed increased activation in the cerebellar/temporal/occipital cluster (ADHD patients >healthy comparison subjects: p<0.02; ADHD patients >conduct disorder patients: p<0.02), which was reduced in conduct disorder patients relative to healthy comparison subjects (p<0.02 [ Figure 3 ]).
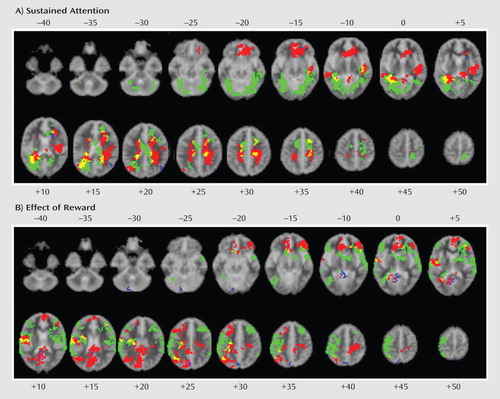
a The images in the top box illustrate the contrast of sustained attention (unrewarded target trials minus nontarget trials [voxel level: p<0.05; cluster level: p>0.01]). The images in the bottom box illustrate the contrast of effects of reward (rewarded minus nonrewarded target trials [voxel level: p<0.05; cluster level: p<0.01]). The three groups are indicated as follows: healthy comparison subjects (red); ADHD patients (green); conduct disorder patients (blue). Overlapping brain regions are indicated as follows: overlap between ADHD patients and healthy comparison subjects (yellow); overlap between conduct disorder patients and healthy comparison subjects (magenta); overlap between ADHD and conduct disorder patients (cyan); overlap between all groups (white). Talairach z coordinates are indicated for slice distance (mm) from the intercommissural line. The right side of each brain image corresponds with the right side of the brain.
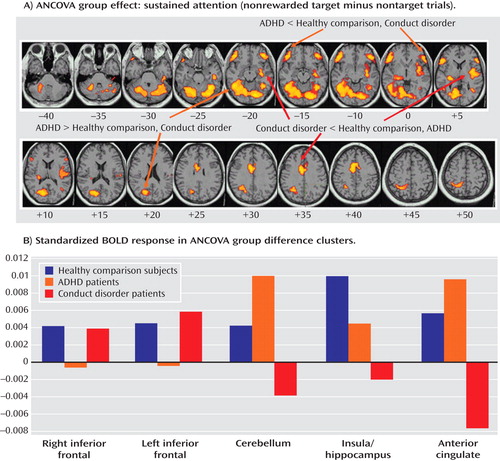
a The images in the top box illustrate the contrast of sustained attention (nonrewarded target minus nontarget trials [voxel and cluster levels: p<0.05]). Talairach z coordinates are indicated for slice distance (mm) from the intercommissural line. The bottom graph illustrates the standardized BOLD response in the brain regions of ANCOVA group effects for the three groups. The activation clusters in the left and right ventrolateral prefrontal cortex were reduced in ADHD patients relative to conduct disorder patients and healthy comparison subjects. The activation clusters in the cerebellum and occipital lobe were increased in ADHD patients relative to conduct disorder patients and healthy comparison subjects. The activation clusters in the anterior cingulate gyrus were decreased in conduct disorder patients relative to ADHD patients and healthy comparison subjects, and the clusters in the hippocampus and insula were reduced in conduct disorder patients relative to healthy comparison subjects and “trend-wise” relative to ADHD patients.
Patients with conduct disorder showed reduced brain activation in the cluster in the right insula and hippocampus relative to healthy comparison subjects (p<0.0001) and ADHD patients (p<0.03). No difference was found between ADHD patients and healthy comparison subjects. Conduct disorder patients also showed reduced brain activation in the anterior cingulate gyrus relative to healthy comparison subjects (p<0.005) and ADHD patients (p<0.001). ADHD patients did not differ from healthy comparison subjects ( Table 4 , Figure 3 ).
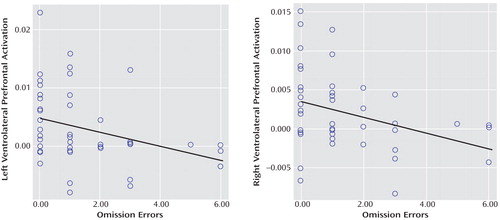
To test the hypothesis that the magnitude of activation in ventrolateral prefrontal clusters was related to better sustained attention, scalar measures of BOLD response were extracted for each participant in these clusters, and Pearson correlations (two-tailed) were calculated between these clusters and omission errors across all subjects. Omission errors were negatively correlated with left (r= –0.3, p<0.03) and right ventrolateral prefrontal activation (r=–0.3, p<0.02 [ Figure 4 ]).
Effects of reward (rewarded versus nonrewarded target trials)
Group activations are detailed in Table 3 and Figure 3 . Three-way ANCOVAs revealed significant group effects in a cluster comprising the right lateral and ventromedial orbitofrontal gyri and a cluster comprising the precuneus and posterior cingulate gyrus ( Table 4 , Figure 5 ). Post hoc comparisons showed that ADHD patients had significantly reduced brain activation in the cluster in the right precuneus and posterior cingulate gyrus relative to healthy comparison subjects (p<0.001) and conduct disorder patients (p<0.002). Healthy comparison subjects and conduct disorder patients did not differ. Conduct disorder patients showed reduced brain activation in the right orbitofrontal cortex relative to healthy comparison subjects (p<0.001) and ADHD patients (p<0.004). Healthy comparison subjects and ADHD patients did not differ ( Figure 5 ).
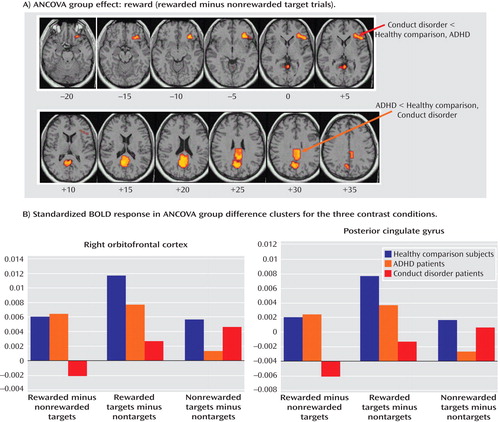
a The images in the top box illustrate significant ANCOVA group differences for the effects of reward (rewarded target minus nonrewarded target trials). Talairach z coordinates are indicated for slice distance (mm) from the intercommissural line. The two graphs at the bottom illustrate the standardized BOLD response in the areas of ANCOVA group effects for the three contrasts of the task for the three groups. The activation cluster in the right orbitofrontal cortex was decreased in conduct disorder patients relative to ADHD patients and healthy comparison subjects. The activation cluster in the posterior cingulate was decreased in ADHD patients relative to conduct disorder patients and healthy comparison subjects.
To test the hypothesis that activation in the orbitofrontal cortex was associated with a greater reward effect on performance measures, we correlated across all subjects the magnitude of BOLD response in this cluster with the reward effect on performance measures. There was a tendency for a positive correlation between the magnitude of reward effect on mean reaction time and orbitofrontal activation (r=0.2, p<0.09).
Discussion
Patients with noncomorbid conduct disorder and noncomorbid ADHD showed disorder-specific brain abnormalities during the two task conditions. During sustained attention, conduct disorder patients demonstrated disorder-specific dysfunction in paralimbic regions of the insula and hippocampus and the postgenual anterior cingulate gyrus. However, ADHD patients demonstrated disorder-specific underactivation in the right and left ventrolateral prefrontal cortex but increased activation in a cluster comprising the cerebellum, thalamus, and hippocampus. During the reward condition, conduct disorder patients showed disorder-specific dysfunction in the right orbitofrontal cortex, while ADHD patients showed disorder-specific reduced brain activation in the posterior cingulate and precuneus.
The underactivation in conduct disorder patients during sustained attention was in regions of the paralimbic system that lie at the interface between emotion and cognition. The postgenual anterior cingulate is connected to frontal-parietal attentional networks but is crucial for motivation and arousal (31 , 32) . A more anterior part of the insula has been shown to contribute to sustained attention (33) , and the hippocampus plays a role in selective visual attention to targets (34) . These findings suggest that conduct disorder is characterized by reduced brain activation in paralimbic regions that contribute to attention networks, presumably through their role in motivation.
The dorsal anterior cingulate gyrus has previously been found to be dysfunctional in boys with conduct disorder during the presentation of images with negative emotional valence (15) . Other limbic regions, such as the amygdala and its connectivity to the orbitofrontal cortex, have been found to be dysfunctional in callous-unemotional children with conduct disorder and/or oppositional defiant disorder relative to children with ADHD and comparison subjects during fearful face processing (16) . Structural studies have found abnormalities in orbitofrontal and temporal regions, including the amygdala and hippocampus, in boys with conduct disorder (35 , 36) . The findings of functional abnormalities in limbic structures during attention are thus consistent with the hypothesis that aggressive behavior is related to abnormalities in the paralimbic system, which mediates the control of motivation that may effect attention functions (14) .
The underactivation in ventrolateral prefrontal brain regions during sustained attention in ADHD patients was hypothesized. Lateral inferior and orbital prefrontal abnormalities have consistently been observed in boys with ADHD during a range of cognitive functions, including inhibition (4 , 5 , 17) and attention tasks during fMRI (6) , and in adolescent girls during an auditory Continuous Performance Test (7) . Left and right ventrolateral prefrontal activation was correlated across all subjects with omission errors, which were reduced at a tendency level in ADHD patients, suggesting that the underactivation in these regions may cause poor sustained attention capacity. The finding of disorder specificity of ventrolateral prefrontal underactivation is consistent with our previous findings of disorder-specific underactivation of left ventrolateral prefrontal activation in the same ADHD patient group relative to healthy comparison subjects and conduct disorder patients during a motor response inhibition task (17) . To our knowledge, this provides the first evidence that the consistently reported ventrolateral prefrontal dysfunction in ADHD patients during tasks of attention and inhibition may be specific to the disorder, at least when compared with conduct disorder.
The increased activation in posterior and subcortical regions in ADHD patients could have been a compensation for the poor frontal activation. The cerebellum, hippocampus, and thalamus are activated during the performance of sustained attention tasks (9 , 33 , 37) , and a prefrontal dysfunction in ADHD patients may have prompted the recruitment of posterior regions of a fronto-striato-thalamo-cerebellar attention network. The findings of cerebellar abnormalities in ADHD patients during attention are relatively novel in the context of functional imaging but are consistent with robust evidence for volumetric abnormalities of this brain area in structural imaging studies (38) . A possible alternative interpretation could be that ADHD patients showed lower activation in the cerebellum during nontarget trials, and thus the activation difference between target and nontarget trials in this region was enhanced in these patients. Interestingly, this cluster was reduced in patients with conduct disorder relative to healthy comparison subjects, suggesting that this region may be another area of neurofunctional differentiation between ADHD and conduct disorder.
The findings of underfunctioning of paralimbic contributions to attentional networks in conduct disorder patients and of lateral fronto-cerebellar network abnormalities in ADHD patients support the theory, based on neuropsychological studies, that motivational abnormalities mediated by the paralimbic system may be underlying conduct disorder, while attentional cognitive abnormalities mediated by fronto-striato-cerebellar networks may be underlying ADHD (4 , 5 , 14 , 17) . This is further supported by the findings in the present study of a process-dependent dissociation of prefrontal dysfunction in both patient groups, which was in a bilateral ventrolateral prefrontal location during sustained attention in ADHD patients and a more medial right hemispheric orbitofrontal location in conduct disorder patients.
Although the ventrolateral prefrontal cortex has been reported to be involved in sustained attention (33 , 37) , more medial regions of the orbitofrontal cortex are known to play a role in motivation and monetary reward (39 , 40) . The orbitofrontal cortex is also known to modulate paralimbic regions that mediate aggression (14) . The orbitofrontal cortex, together with temporal and limbic areas, was reduced in gray matter in boys with conduct disorder (35) . It has been hypothesized that abnormalities in reward computations that are mediated by the orbitofrontal cortex, leading to enhanced frustration, could trigger reactive aggression, which would explain the link between aggression, abnormalities in reward-related brain regions, and orbitofrontal abnormalities (14) . Our findings are consistent with this theory and, to our knowledge, provide the first functional evidence that aggressive and antisocial behaviors are related to a dysfunction of the orbitofrontal cortex and paralimbic areas during reward and attention.
The findings of underactivation in reward-related orbitofrontal regions in patients with conduct disorder only support the hypothesis of a disorder-specific hyposensitivity to reward in conduct disorder. This is consistent with findings of reduced autonomic responses in children with conduct disorder relative to children with ADHD and healthy comparison subjects during emotional stimuli (41) . Our findings do not support the theory of a hypersensitivity to reward in ADHD patients. To the contrary, our findings show reduced recruitment of the posterior cingulate and precuneus that are important for visual-spatial attention allocation to visually or motivationally salient events, such as reward (42) . This is consistent with evidence of a dopamine deficiency in ADHD patients (43 , 44) , which is associated with a blunted attentional response to saliency (45) . We previously found reduced posterior cingulate and precuneus activation in boys with ADHD during motor timing (4) , error detection (5 , 17) , and attention allocation (46) , which correlated with scores of hyperactivity on the Strength and Difficulties Questionnaire (5) . The posterior cingulate therefore appears to be an important—but thus far neglected—brain region of dysfunction in ADHD patients that could be the neural substrate for the reduced capacity in these patients for attention allocation to relevant targets.
In the present study, the three groups did not differ in performance, although there was a tendency for more omission errors among ADHD patients relative to healthy comparison subjects. This finding is not consistent with the majority of studies that reported deficits in children with ADHD during the continuous performance task (2 , 3) , although it is consistent with findings from several studies on conduct disorder (11) . ADHD adolescents are usually less impaired than ADHD children, and our fMRI adaptation of the Continuous Performance Test using single targets—designed to reduce working memory demands and confounds of performance differences between groups—was deliberately easier than more commonly used double-target versions (i.e., Continuous Performance Test [A–X]).
One common problem with fMRI adaptations of continuous performance tasks is that motor responses to targets are not controlled, since a motor response to nontargets would introduce unwanted attentional demands. Thus, some activation differences could have been potentially motor- rather than purely attention-related. However, the main ANCOVA findings were not in motor regions. Inferior prefrontal activation, which was reduced in ADHD patients, is known to mediate sustaining attention during motor-controlled vigilance and parametric-sustained attention tasks (33 , 37) , and this activation correlated with omission errors, which were the main attention measure of the task. Likewise, the paralimbic areas that were reduced in conduct disorder patients were not located in motor regions.
The careful selection of patients who differed from each other in conduct and ADHD problems is a strength of the present study. However, this selection limits generalizability to more commonly occurring comorbid cases. Future research should clarify the extent to which comorbidity results from the blending of conduct disorder and ADHD.
The findings of the present study suggest that conduct disorder and ADHD, two clinically overlapping disruptive disorders, may be based on different etiopathophysiological substrates implicating orbitofrontal-limbic motivational networks in patients with conduct disorder and fronto-cerebellar attention networks in patients with ADHD. Our results can be considered a first step toward the delineation of the specific biomarkers of the two diagnostic disorders, which will ultimately contribute to the development of more objective phenomenological differentiation and disorder-specific tailored treatment.
1. Banaschewski T, Hollis C, Oosterlaan J, Roeyers H, Rubia K, Willcutt E, Taylor E: Towards an understanding of unique and shared pathways in the psychopathophysiology of ADHD. Develop Sci 2005; 8:132–140Google Scholar
2. Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF: Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry 2005; 57:1336–1346Google Scholar
3. Rubia K, Smith A, Taylor E: Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychol 2007; 13:276–304Google Scholar
4. Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore E: Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study using functional MRI. Am J Psychiatry 1999; 156:891–896Google Scholar
5. Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E: Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry 2005; 162:1067–1075Google Scholar
6. Smith AB, Taylor E, Brammer M, Toone B, Rubia K: Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry 2006; 163:1044–1051Google Scholar
7. Ernst M, Liebenauer LL, King AC, Fitzgerald GA, Cohen RM, Zametkin AJ: Reduced brain metabolism in hyperactive girls. J Am Acad Child Adolesc Psychiatry 1994; 33:858–867Google Scholar
8. Ernst M, Cohen RM, Liebenauer LL, Jons PH, Zametkin AJ: Cerebral glucose metabolism in adolescent girls with attention deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:1399–1406Google Scholar
9. Zametkin AJ, Liebenauer LL, Fitzgerald GA, King AC, Minkunas DV, Herscovitch P, Yamada EM, Cohen RM: Brain metabolism in teenagers with attention-deficit hyperactivity disorder. Arch Gen Psychiatry 1993; 50:333–340Google Scholar
10. Dougherty DM, Bjork JM, Harper RA, Marsh DM, Moeller FG, Mathias CW, Swann AC: Behavioral impulsivity paradigms: a comparison in hospitalized adolescents with disruptive behavior disorders. J Child Psychol Psychiatry 2003; 44:1145–1157Google Scholar
11. Halperin JM, O’Brien JD, Newcorn JH, Healey JM, Pascualvaca DM, Wolf LE, Young JG: Validation of hyperactive, aggressive, and mixed hyperactive/aggressive childhood disorders: a research note. J Child Psychol Psychiatry 1990; 31:455–459Google Scholar
12. Dougherty DM, Bjork JM, Harper RA, Mathias CW, Moeller FG, Marsh DM: Validation of the immediate and delayed memory tasks in hospitalized adolescents with disruptive behavior disorders. Psychol Record 2003; 53:509–532Google Scholar
13. Matthys W, van Goozen SHM, Snoek H, van Engeland H: Response perseveration and sensitivity to reward and punishment in boys with oppositional defiant disorder. Eur Child Adolesc Psychiatry 2004; 13:362–364Google Scholar
14. Blair RJ: The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn 2004; 55:198–208Google Scholar
15. Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F: Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry 2005; 57:7–15Google Scholar
16. Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJR: Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 2008; 165:712–720Google Scholar
17. Rubia K, Halari R, Smith AB, Mohammad M, Scott S, Giampietro V, Taylor E, Brammer MJ: Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. Am J Psychiatry 2008; 165:889–897Google Scholar
18. Luman M, Oosterlaan J, Sergeant JA: The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev 2005; 25:183–213Google Scholar
19. Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Contoreggi C, Leff M, Bolla K: Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry 2003; 160:1061–1070Google Scholar
20. Scheres A, Milham MP, Knutson B, Castellanos FX: Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 61:720–724Google Scholar
21. Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A: Association of ADHD and conduct disorder: brain electrical evidence for the existence of a distinct subtype. J Child Psychol Psychiatry 2003; 44:356–376Google Scholar
22. Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A: Questioning inhibitory control as the specific deficit of ADHD: evidence from brain electrical activity. J Neural Transm 2004; 111:841–864Google Scholar
23. Goldberg D, Murray R (eds): The Maudsley Handbook of Practical Psychiatry. New York, Oxford University Press, 2002Google Scholar
24. Goodman R, Scott S: Comparing the Strength and Difficulties Questionnaire and the Child Behavior Checklist: Is small beautiful? J Abnorm Child Psychol 1999; 27:17–24Google Scholar
25. Raven J: Guide to the Standard Progressive Matrices. London, HK Lewis, 1960Google Scholar
26. Schmitz N, Rubia K, Daly E, Smith A, van Amelsvoort T, Murphy D: The neural substrates of monetary reward in autism. Br J Psychiatry 2008; 192:19–25Google Scholar
27. Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, Carpenter TA, Brammer M: Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp 2001; 12:61–78Google Scholar
28. Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ: Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 1999; 18:32–42Google Scholar
29. Talairach T, Tournoux P: Co-Planar Stereotaxic Atlas of the Brain. New York, Thieme Medical Publishers, 1988Google Scholar
30. Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB: Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage 2007; 35:105–120Google Scholar
31. Critchley HD: Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 2005; 493:154–166Google Scholar
32. Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S: The role of the medial frontal cortex in cognitive control. Science 2004; 306:443–447Google Scholar
33. Voisin J, Bidet-Caulet A, Bertrand O, Fonlupt P: Listening in silence activates auditory areas: a functional magnetic resonance imaging study. J Neurosci 2006; 26:273–278Google Scholar
34. Wu Z, Guo A: Selective visual attention in a neurocomputational model of phase oscillators. Biol Cybern 1999; 80:205–214Google Scholar
35. Huebner T, Vloet TD, Marx I, Konrad K, Fink GR, Herpertz SC, Herpertz-Dahlmann B: Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry 2008; 47:540–547Google Scholar
36. Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A: Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res 2004; 132:1–11Google Scholar
37. Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA: Multiple neuronal networks mediate sustained attention. J Cogn Neurosci 2003; 15:1028–1038Google Scholar
38. Castellanos FX, Tannock R: Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci 2002; 3:617–628Google Scholar
39. Kringelbach ML, Rolls ET: The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004; 72:341–372Google Scholar
40. Elliott R, Deakin B: Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: evidence from functional magnetic resonance imaging studies in healthy human subjects. Int Rev Neurobiol 2005; 65:89–116Google Scholar
41. Herpertz SC, Mueller B, Qunaibi M, Lichterfeld C, Konrad K, Herpertz-Dahlmann B: Response to emotional stimuli in boys with conduct disorder. Am J Psychiatry 2005; 162:1100–1107Google Scholar
42. Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM: The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage 2003; 18:633–641Google Scholar
43. Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM: High midbrain [ 18 F]dopa accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry 1999; 156:1209–1215 Google Scholar
44. Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, Logan J, Wong C, Ma YM, Swanson JM, Schulz K, Pradhan K: Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage 2007; 34:1182–1190Google Scholar
45. Volkow ND, Wang GJ, Ma YM, Fowler JS, Wong C, Jayne M, Telang F, Swanson JM: Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neuroimage 2006; 32:1782–1792Google Scholar
46. Rubia K, Smith AB, Brammer MJ, Taylor E: Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry 2007; 62:999–1006Google Scholar