Clinical Phenotypes of Psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP)
Abstract
Objective
Developing categorical diagnoses that have biological meaning within the clinical phenotype of psychosis (schizophrenia, schizoaffective disorder, and bipolar I disorder with psychosis) is as important for developing targeted treatments as for nosological goals. The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) was formed to examine a broad array of intermediate phenotypes across psychotic disorders and to test the hypothesis that intermediate phenotype characteristics are homogeneous within phenomenologically derived DSM-IV diagnoses.
Method
The consortium recruited 933 stable probands with schizophrenia, schizoaffective disorder, or psychotic bipolar I disorder, 1,055 of their first-degree relatives, and 459 healthy comparison subjects for clinical characterization and dense phenotyping. Clinical, psychosocial, and family characteristics were contrasted.
Results
All proband groups showed lower psychosocial functioning than the relatives or comparison group. On average, schizophrenia probands showed more symptoms and lower psychosocial functioning than probands with psychotic bipolar disorder, but there was considerable overlap in clinical manifestations. The characteristics of schizoaffective disorder were more often similar to schizophrenia than to psychotic bipolar disorder. The rates of lifetime suicide attempts were high across all proband groups, with the highest reported frequencies in the schizoaffective and bipolar groups. Proband family lineages included both families with “pure” psychosis diagnoses and families with mixed schizophrenia-bipolar diagnoses.
Conclusions
Symptoms, psychosocial functioning, and familial lineage overlap across the three DSM-IV psychosis diagnoses used in B-SNIP. The comingling of psychosis diagnoses within families suggests overlapping genetic determinants across psychoses. These data provide scant evidence for distinct phenotypic clustering around traditional phenomenological diagnoses.
Attempts to identify the fundamental mechanisms of major psychiatric disorders, including their etiological underpinnings, have been remarkably unsuccessful. Defining the molecular and structural determinants of psychiatric disease is important for establishing biologically based diagnostic definitions and supporting novel targeted treatments (1). At present, psychiatric diagnoses are based entirely on phenomenological descriptions (2), with no assay-based biological criteria underlying diagnoses; this has resulted in syndromal categories within serious mental illness that have overlapping symptom fields (3). This may be especially prominent among psychotic diagnoses, including schizophrenia and bipolar I disorder with psychosis. The literature documents genetic and biological overlap between schizophrenia and bipolar disorder (4), including common risk genes (4, 5) and overlapping family lineages with mixed psychosis diagnoses (6–8). To accommodate that overlap in clinical diagnosis, schizoaffective disorder is used variably in practice as an intermediate condition, with clinical manifestations of both psychosis and mood instability but with variable outcome (9–11). An important clinical feature shared by these diagnoses is psychosis. For the purpose of creating a cohort for genetic and molecular characterization that addresses this diagnostic overlap, psychosis provides an ideal clinical phenotype because it cuts broadly across traditional diagnostic groups (including, but not limited to, schizophrenia, schizoaffective disorder, and bipolar disorder), has a typical symptomatic phenomenology with clear and measurable manifestations, and is plausibly associated with specific anatomical circuits (12–15).
Intermediate phenotypes, sometimes called endophenotypes, are defined as quantifiable, overt measures of discrete brain functions that are state independent, are heritable, cosegregate with the illness in families, and are expressed in some unaffected family members. The intermediate phenotypes may be more closely associated with genes than are complex clinical syndromes, and hence they have utility in genetic and molecular discovery in psychiatry (16, 17). The strategy of attempting to link genes with intermediate phenotypes has shown modest promise within schizophrenia cohorts (18–22). Therefore, we have extended the use of intermediate phenotypes to psychosis as a clinical phenotype, in order to test the hypothesis that intermediate phenotypes are relatively homogeneous in clinical, familial, and phenotypic characteristics across categorical diagnoses and that diagnoses will segregate within families.
The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) was established as a multisite consortium to characterize intermediate phenotypes of the psychosis dimension, with the expectation that some phenotypes would segregate by categorical diagnosis and others would be expressed across the target psychotic disorders (23). Distinguishing these characteristics can define more biologically homogeneous groups of individuals, generate the molecular, cellular, and systems knowledge that will lead to a biological understanding of psychosis and psychotic disorders (2), and thereby sharpen conceptualizations about common and distinct aspects of psychosis pathophysiology, risk pathways, and clinical boundaries between psychotic illnesses. The transition from traditional phenomenological classification, as developed by early pioneers in the field of psychiatric nosology (2, 24, 25), to brain-based disease entities has been helped by the Research Domain Criteria (RDoC) project initiated by the National Institute of Mental Health, an approach that organizes brain disorders as deviations from normal patterns of behavior within the brain’s neuronal networks (26, 27).
The B-SNIP consortium includes five sites in the United States that were organized to carry out parallel recruitment and phenotyping procedures and that have demonstrated scientific expertise across intermediate phenotypes of psychosis. The process for collecting data on clinical and intermediate phenotypes was standardized across all sites with rigorous quality assurance controls in place to document site-to-site consistency in data acquisition. The B-SNIP consortium has been successful in this intensive psychosis phenotyping effort, as described here in regard to clinical characterization, using frequent face-to-face and audio conferencing and taking advantage of internal technical expertise. In this article, we present the characteristics of the clinical psychosis study group and its analysis, and we report on clinical and demographic characteristics of the patient groups (by categorical diagnoses) and their first-degree relatives across a range of psychotic diagnoses.
Method
B-SNIP Phenotyping Consortium
B-SNIP included sites in Baltimore (G.K.T.), Chicago (J.A.S.), Dallas (C.A.T.), Detroit and Boston (M.S.K.), and Hartford, Conn. (G.D.P.). All sites had preexisting experience with the recruitment, study, and care of psychotic patients with serious mental illness. The sites used identical diagnostic and clinical assessment techniques, and they used similar approaches to recruitment. The study protocol was approved by the institutional review board at each local site. After complete description of the study to the volunteers, written informed consent was obtained. In addition to the clinical characterization, each volunteer underwent comprehensive phenotypic assessment. The Brief Assessment of Cognition in Schizophrenia battery (28) was used to characterize cognition. Neurophysiologic phenotyping included ocular motor testing with smooth-pursuit and saccade paradigms; resting-state EEG, and auditory-event-related potentials. Structural, diffusion tensor, and resting-state functional brain imaging was conducted. In addition, a blood sample from each volunteer was collected and stored for genetic analysis.
Recruitment
Each site committed to recruit 200 probands with diagnoses of schizophrenia, schizoaffective disorder, or bipolar disorder, at least one of each proband’s first-degree relatives, and 100 healthy comparison subjects. All sites recruited within 20% of the goal. The probands in the study were clinically stable and in a nonacute symptom state. The broad geographical span of B-SNIP facilitated enrichment of the study group by local geographical characteristics. Sites used a combination of newspaper and community advertising for probands and comparison subjects, with the groups similarly recruited across all five sites. Proband recruitment was carried out by dimension (psychosis) within cases of serious mental illness in the schizophrenia-bipolar disorder spectrum, from which all potential participants with a history of psychosis and suspected schizophrenia, schizoaffective disorder, or bipolar disorder were recruited. These probands and comparison subjects are a research sample and neither a clinical nor epidemiological sample; nonetheless, the large study numbers and broad geographical recruitment enhance the generalizability of data from the B-SNIP cohort. This strategy generated a more inclusive study group than is typical in studies focusing on specific disorders, with the aim of having a representative sample of the spectrum of psychotic serious mental illness. Psychosis probands were limited to schizophrenia, schizoaffective disorder, and bipolar disorder because these are the diagnoses with the highest prevalences of psychosis and studying more diagnostic categories was deemed unfeasible as a first approach.
Clinical Characterization of Study Group
Proband volunteers were assessed phenomenologically, with the Hollingshead Index of Social Position, psychiatric and medical histories, a modified family psychiatric history interview (29), the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P) including the Global Assessment of Functioning (GAF) scale (30), the Positive and Negative Syndrome Scale (PANSS) (31), the Young Mania Rating Scale (32), the Montgomery-Åsberg Depression Rating Scale (MADRS) (33), the Schizo-Bipolar Scale (23), and the Birchwood Social Functioning Scale (34). Relatives were also assessed with the Structured Interview for DSM-IV Personality Disorders (35) to evaluate personality traits, especially those relevant to the psychosis spectrum, represented by the cluster A personality disorders (36). Similar evaluation of the healthy comparison subjects assured the absence of a personal history of psychotic or bipolar disorder or recurrent major depressive disorder or a family history of schizophrenia-bipolar spectrum disorders in first- and second-degree relatives.
The extensive clinical information on each volunteer was reviewed in a best-estimate diagnostic meeting with at least two experienced research clinicians, to establish the consensus diagnosis. Cross-site diagnostic conference calls were carried out monthly; they were chaired by two senior primary investigators (C.A.T., M.S.K.) and attended by the 2–4 trained clinical assessors at each site. At study start, there was a face-to-face training session for all raters, with a requirement for reliability above 0.85. Each month, diagnostic conferences were held with in-depth diagnostic discussions. Each year, rater training was repeated to reestablish reliability. A detailed description of the clinical rater training and maintenance, as well as data management and family history methods, is presented in the supplementary methods section in the data supplementappearing with the online version of this article.
Statistical analysis of the sociodemographic and clinical data was done descriptively by using NCSS software (http://www.ncss.com/software/ncss/). A one-way analysis of variance, with a subsequent Tukey-Kramer multiple comparison test, and the Yates-corrected chi-square test were used, as appropriate. Given the large study group, alpha was set at 0.01 for all statistical analyses.
Results
The B-SNIP recruitment and phenotype evaluations began in 2008, with study assessments finalized through 2012. The data reported here include all subjects recruited through September 2012. To date, 933 probands (see Table 1 for diagnostic distribution), 1,055 relatives (with an overall average of 41.8% parents, 39.7% siblings, and 18.5% children), and 459 healthy comparison subjects have been recruited, phenotyped, analyzed, and entered into the B-SNIP database. The schizoaffective disorder group included 153 probands with the bipolar type, 71 with the depressed type, and their relatives (N=192 and N=88, respectively); because these were small groups, they were combined in these analyses.
| Probands | Relatives | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Schizophrenia (N=397) | Schizoaffective Disorder (N=224) | Bipolar Disorder (N=312) | Schizophrenia (N=415) | Schizoaffective Disorder (N=280) | Bipolar Disorder (N=360) | Healthy Comparison Subjects (N=459) | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years)b | 35.7 | 12.7 | 36.6 | 11.9 | 36.4 | 12.9 | 43.3 | 15.0 | 40.1 | 16.0 | 40.0 | 15.9 | 36.5 | 12.7 |
| Education (years)c | 12.7 | 2.3 | 13.1 | 2.2 | 14.1 | 2.4 | 13.9 | 2.4 | 13.6 | 2.9 | 14.4 | 2.8 | 14.8 | 2.5 |
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Male genderd | 267 | 67.3 | 93 | 41.5 | 110 | 35.4 | 132 | 31.8 | 83 | 30.0 | 130 | 36.5 | 210 | 46.0 |
| Hispanic ethnicity | 32 | 8.1 | 21 | 9.4 | 24 | 7.7 | 34 | 8.2 | 25 | 9.0 | 26 | 7.3 | 39 | 8.5 |
| Racee | ||||||||||||||
| Caucasian | 182 | 45.8 | 121 | 54.0 | 227 | 72.8 | 219 | 52.8 | 161 | 57.5 | 278 | 77.2 | 283 | 61.7 |
| African American | 181 | 45.6 | 90 | 40.2 | 67 | 21.5 | 173 | 41.7 | 102 | 36.4 | 62 | 17.2 | 132 | 28.8 |
| Other | 34 | 8.6 | 13 | 5.8 | 18 | 5.8 | 23 | 5.5 | 17 | 6.1 | 20 | 5.6 | 44 | 9.6 |
| Handedness | ||||||||||||||
| Right-handed | 309 | 85.1 | 193 | 86.5 | 251 | 84.2 | 353 | 88.9 | 236 | 87.1 | 303 | 86.1 | 379 | 88.3 |
| Left-handed | 46 | 12.7 | 22 | 9.9 | 42 | 14.1 | 39 | 9.8 | 29 | 10.7 | 45 | 12.8 | 44 | 10.3 |
| Ambidextrous | 8 | 2.2 | 8 | 3.6 | 5 | 1.7 | 5 | 1.3 | 6 | 2.2 | 4 | 1.1 | 6 | 1.4 |
| Marital statusf | ||||||||||||||
| Married | 28 | 7.6 | 25 | 11.2 | 50 | 16.7 | 145 | 36.5 | 99 | 36.8 | 142 | 40.6 | 114 | 26.0 |
| Widowed | 4 | 1.1 | 1 | 0.4 | 8 | 2.7 | 16 | 4.0 | 10 | 3.7 | 7 | 2.0 | 8 | 1.8 |
| Divorced/separated | 45 | 12.3 | 61 | 27.4 | 74 | 24.7 | 77 | 19.4 | 41 | 15.2 | 47 | 13.4 | 64 | 14.6 |
| Never married | 290 | 79.0 | 136 | 61.0 | 167 | 55.9 | 159 | 40.1 | 119 | 44.2 | 154 | 44.0 | 252 | 57.5 |
| Hollingshead Index of Social Position | ||||||||||||||
| Participantg | ||||||||||||||
| Class 1 | 1 | 0.3 | 3 | 1.5 | 7 | 2.5 | 15 | 4.1 | 18 | 7.1 | 32 | 9.9 | 23 | 5.5 |
| Class 2 | 29 | 8.7 | 21 | 10.3 | 60 | 21.1 | 98 | 26.6 | 62 | 24.5 | 93 | 28.7 | 136 | 32.8 |
| Class 3 | 79 | 23.8 | 69 | 34.0 | 108 | 37.9 | 130 | 35.3 | 73 | 28.9 | 111 | 34.3 | 169 | 40.7 |
| Class 4 | 128 | 38.6 | 72 | 35.5 | 78 | 27.4 | 85 | 23.1 | 58 | 22.9 | 45 | 13.9 | 64 | 15.4 |
| Class 5 | 95 | 28.6 | 38 | 18.7 | 32 | 11.2 | 40 | 10.9 | 42 | 16.6 | 43 | 13.3 | 23 | 5.5 |
| Family of originh | ||||||||||||||
| Class 1 | 32 | 12.5 | 15 | 10.1 | 40 | 17.2 | 42 | 13.5 | 17 | 9.3 | 45 | 16.7 | 53 | 14.6 |
| Class 2 | 56 | 21.8 | 38 | 25.5 | 70 | 30.0 | 61 | 19.7 | 52 | 28.6 | 71 | 26.4 | 96 | 26.4 |
| Class 3 | 71 | 27.6 | 41 | 27.5 | 60 | 25.8 | 73 | 23.5 | 45 | 24.7 | 65 | 24.2 | 98 | 26.9 |
| Class 4 | 72 | 28.0 | 37 | 24.8 | 43 | 18.5 | 102 | 32.9 | 48 | 26.4 | 70 | 26.0 | 97 | 26.6 |
| Class 5 | 26 | 10.1 | 18 | 12.1 | 20 | 8.6 | 32 | 10.3 | 20 | 11.0 | 18 | 6.7 | 20 | 5.5 |
Sociodemographic Characteristics
The sociodemographic characteristics of the study participants are presented in Table 1.
Age and gender.
Age differed across groups, with the groups of relatives being older than the comparison and proband groups, as predicted. The relatives’ age differed across relative subgroups; the mean ages were 54.3 years for parents, 36.2 for siblings, and 22.9 for children. The proband ages were similar and did not differ significantly from the age of the comparison group.
Gender distribution also differed across groups (Table 1). There was a higher proportion of men in the schizophrenia probands than in the other proband groups, all relative groups, and the comparison group.
Ethnicity and race.
No differences in ethnicity were found; high proportions of non-Hispanic subjects were recruited into all groups (Table 1). Racial characteristics did differ across groups, accounted for by differences in the ratio of Caucasian to African American subjects. The ratios were greater than 2:1 in the bipolar probands, their relatives, and the comparison subjects, but the probands with schizophrenia and schizoaffective disorder and their respective relatives had lower percentages of Caucasian subjects.
Handedness.
No differences in handedness were found among the probands, relatives, and comparison subjects (Table 1); all groups had high proportions of right-handed individuals.
Marital status.
Marital status characteristics differed across groups (Table 1), with higher proportions of never-married individuals among the probands than among relatives.
Education and social class.
The groups also differed in years of education (Table 1). The comparison group had the highest education level; it was significantly higher than the levels for all proband groups and for the relatives of the schizophrenia and schizoaffective disorder relatives, but not for the relatives of the bipolar group. The bipolar probands had more education than the probands with either schizophrenia or schizoaffective disorder, and relatives had more education than probands.
Social class as determined with the Hollingshead index for the family of origin head of household did not differ across groups (Table 1). Classes 2–4 reflect the middle socioeconomic level, and they were consistently predominant in all proband, relative, and comparison groups. However, there was a between-group difference in social class for the probands, who had smaller proportions in the higher classes (classes 1 and 2) than did relatives and comparison subjects.
Clinical Characteristics
Proband symptoms and history.
There was an overall difference in the PANSS total score (Figure 1), with the schizophrenia and schizoaffective disorder probands having higher scores than the bipolar probands. Analysis of the PANSS subscale scores reflected similar differences between the proband groups on the positive, negative, and general symptom subscales. However, there was a highly extensive overlap in symptom expression across proband groups, despite these significant contrasts (Figure 2 and Table S1 in the data supplement accompanying the online version of this article). In addition, there was an across-group difference in the scores on the Young Mania Rating Scale (Figure 1), accounted for by higher scores in the probands with schizoaffective disorder than in those with schizophrenia, whereas the bipolar probands had intermediate scores and did not statistically differ from either of the other proband groups. Likewise, an across-group difference was found in the MADRS scores, and the probands with schizoaffective disorder had higher scores than both the probands with schizophrenia and those with bipolar disorder (Figure 1). Nevertheless, a substantial overlap in the distribution of the affect characteristics was observed (see Table S1 in the data supplement associated with the online version of this article).
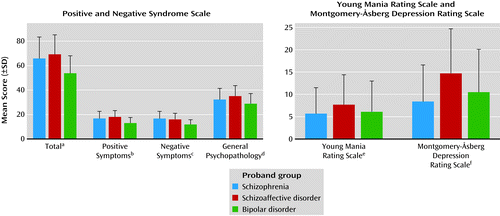
a Significant difference across groups (F=68.22, df=2, 835, p<0.001). Tukey-Kramer post hoc test results (p<0.01): schizophrenia and schizoaffective disorder probands different from bipolar probands.
b Significant difference across groups (F=67.88, df=2, 836, p<0.001). Tukey-Kramer post hoc test results (p<0.01): schizophrenia and schizoaffective disorder probands different from bipolar probands.
c Significant difference across groups (F=76.57, df=2, 836, p<0.001). Tukey-Kramer post hoc test results (p<0.01): schizophrenia and schizoaffective disorder probands different from bipolar probands.
d Significant difference across groups (F=32.07, df=2, 837, p<0.001). Tukey-Kramer post hoc test results (p<0.01): schizophrenia and schizoaffective disorder probands different from bipolar probands.
e Significant difference across groups (F=6.69, df=2, 828, p=0.001). Tukey-Kramer post hoc test results (p<0.01): schizoaffective disorder probands different from schizophrenia probands.
f Significant difference across groups (F=31.00, df=2, 834, p<0.001). Tukey-Kramer post hoc test results (p<0.01): schizoaffective disorder probands different from schizophrenia and bipolar disorder probands.

a The whisker plots show the group median within the box, with the limits of the box demonstrating the 25th and 75th percentiles of values and the vertical bars indicating the 5th and 95th percentiles, thus demonstrating the overlap of the distributions.
b On the PANSS total rating, 98.9% of the schizoaffective disorder probands and 100.0% of the bipolar probands had scores within 2 standard deviations of the scores for the probands with schizophrenia.
c On the Birchwood Social Functioning Scale, 90.1% of the schizoaffective disorder probands and 94.6% of the bipolar probands had scores within 2 standard deviations of the scores for the schizophrenia probands.
No significant differences were found in the age at illness onset, age at first psychiatric hospitalization, and total number of lifetime hospitalizations among the proband groups (Table 2). Exceptionally high numbers of probands in all groups reported previous suicide attempts, but the lifetime frequency was higher among those with schizoaffective disorder (51.1%) or bipolar disorder (42.4%) than among schizophrenia probands (31.9%).
| Probands | Relatives | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Schizophrenia (N=361) | Schizoaffective Disorder (N=224) | Bipolar Disorder (N=295) | Schizophrenia (N=379) | Schizoaffective Disorder (N=262) | Bipolar Disorder (N=339) | Healthy Comparison Subjects (N=420) | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Estimated IQ (Wide Range Achievement Test reading subtest score)b | 94.8 | 33.2 | 95.8 | 14.7 | 100.0 | 14.6 | 96.3 | 15.9 | 98.1 | 16.1 | 102.4 | 15.3 | 102.7 | 13.9 |
| Global Assessment of Functioning scorec | 49.4 | 12.5 | 48.9 | 11.6 | 60.8 | 12.8 | 74.5 | 14.0 | 73.8 | 14.1 | 75.9 | 13.2 | 85.7 | 7.3 |
| Age at illness onset (years)d | 20.9 | 7.8 | 19.8 | 9.2 | 19.6 | 8.6 | ||||||||
| Age at first hospitalization (years)e | 22.5 | 6.8 | 23.0 | 9.2 | 24.0 | 9.7 | ||||||||
| Lifetime number of hospitalizationsf | 5.6 | 7.7 | 6.5 | 6.6 | 5.8 | 7.0 | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Lifetime suicide attemptg | 113 | 31.9 | 112 | 51.1 | 125 | 42.4 | — | — | — | — | — | — | — | — |
Functioning.
Significant between-group differences were found in the total score on the Birchwood Social Functioning Scale, as well as in the seven subscales (Figure 3). The pattern of differences was consistent across all subscales, with the comparison subjects showing the highest scores (best psychosocial functioning), as expected, whereas the probands with schizophrenia or schizoaffective disorder demonstrated the lowest scores (poorest social functioning); the bipolar probands scored higher than the other two proband groups and just below the relative groups. The relative groups had social functioning scores intermediate between the probands and comparison group; the relatives of the bipolar probands were closest to the comparison subjects. However, considerable overlap was found among the proband groups in levels of social functioning (Figure 3).
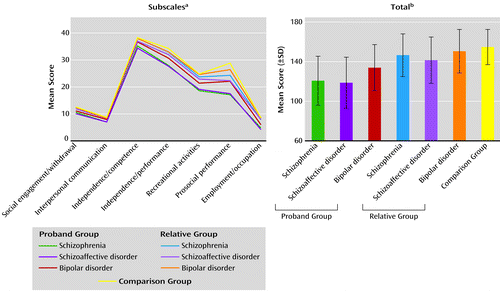
a Significant difference among groups (one-way ANOVA) for social engagement/withdrawal (F=67.44, df=6, 2172, p<0.001), interpersonal communication (F=77.80, df=6, 2166, p<0.001), independence/competence (F=35.84, df=6, 1921, p<0.001), independence/performance (F=74.39, df=6, 2103, p<0.001), recreational activities (F=37.37, df=6, 2118, p<0.001), prosocial performance (F=55.66, df=6, 2093, p<0.001), and employment/occupation (F=103.38, df=6, 2097, p<0.001).
b Significant difference (one-way ANOVA) among groups (F=99.97, df=6, 1755, p<0.001).
The GAF scores differed across groups, owing to lower scores in the probands than in the relatives and comparison group (Table 2). The probands with schizophrenia and those with schizoaffective disorder had lower scores than the bipolar probands, whereas no differences were found between the relative groups.
Estimated IQ.
Scores on the reading subtest of the Wide Range Achievement Test differed across groups (Table 2). The schizophrenia probands had lower scores than the bipolar probands, whereas the probands with schizoaffective disorder had intermediate scores that statistically were not different from those of either other proband group. Likewise, the schizophrenia relatives had lower scores than the relatives of the bipolar probands, and the schizoaffective disorder relatives had intermediate scores.
Medication history.
Antipsychotic use was highest in the proband groups with schizophrenia and schizoaffective disorder (91.6% and 86.7%, respectively) and lower but still appreciable in the bipolar proband group (70.1%). Reported use of mood stabilizers was higher in the bipolar and schizoaffective disorder groups (71.1% and 51.4%, respectively) than in the schizophrenia group (21.7%). Antidepressant use was highest for schizoaffective disorder (56.9%) and lower in the schizophrenia and bipolar disorder groups (38.9% and 44.0%, respectively). All group psychotropic medication use is detailed in Table S2 in the data supplement appearing with the online version of the article.
Family History
The frequencies of DSM-IV axis I and II lifetime diagnoses, derived from the SCID-I/P and the Structured Interview for DSM-IV Personality Disorders, respectively, in the relative groups are presented in Table 3; all between-group comparisons are reported descriptively. Approximately one-third of the relatives of the probands in each group showed no axis I or II diagnosis. The frequencies of schizophrenia and schizoaffective disorder were higher in the schizophrenia and schizoaffective disorder relatives than in the bipolar disorder relatives, whereas bipolar diagnoses, both psychotic and nonpsychotic, were more prevalent in the bipolar and schizoaffective disorder relatives than in the schizophrenia relatives. Major depressive disorder and substance use disorders were the most frequent diagnoses in all relative groups and had similar rates across the three groups (22.1%−26.5%). The frequencies of paranoid and schizoid personality disorders were higher in the schizophrenia and schizoaffective disorder relatives than in the bipolar disorder relatives, whereas borderline personality disorder was more frequent among the bipolar and schizoaffective disorder relatives. Other cluster B and cluster C personality disorders were equally distributed across the relative groups (Table 3).
| Diagnosis | Relatives of Probands With Schizophrenia (N=386) | Relatives of Probands With Schizoaffective Disorder (N=267) | Relatives of Probands With Bipolar Disorder (N=339) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| No axis I or II diagnosis | 147 | 38.1 | 89 | 33.3 | 116 | 34.2 |
| Axis I diagnosesa | ||||||
| Psychotic disorders | ||||||
| Schizophrenia | 26 | 6.7 | 11 | 4.1 | 4 | 1.2 |
| Schizoaffective disorder | 6 | 1.6 | 2 | 0.7 | 1 | 0.3 |
| Psychotic bipolar I disorder | 2 | 0.6 | 6 | 2.2 | 9 | 2.7 |
| Psychotic major depressive disorder | 4 | 1.0 | 6 | 2.2 | 4 | 1.2 |
| Other | 6 | 1.6 | 5 | 1.9 | 4 | 1.2 |
| Nonpsychotic disorders | ||||||
| Nonpsychotic bipolar disorder | 4 | 1.0 | 14 | 5.2 | 28 | 8.3 |
| Nonpsychotic major depressive disorder | 91 | 23.6 | 59 | 22.1 | 85 | 25.1 |
| Substance use disorder | 98 | 25.4 | 65 | 24.3 | 90 | 26.5 |
| Anxiety disorder | 76 | 19.7 | 62 | 23.2 | 69 | 20.4 |
| Other | 39 | 10.1 | 26 | 9.7 | 40 | 11.8 |
| Axis II diagnosesb | ||||||
| Cluster A personality disorders | ||||||
| Paranoid | 22 | 5.7 | 19 | 7.1 | 12 | 3.5 |
| Schizoid | 9 | 2.3 | 8 | 3.0 | 4 | 1.2 |
| Schizotypal | 3 | 0.8 | 3 | 1.1 | 6 | 1.8 |
| Cluster B personality disorders | ||||||
| Borderline | 5 | 1.3 | 11 | 4.1 | 11 | 3.2 |
| Antisocial | 8 | 2.1 | 6 | 2.2 | 8 | 2.4 |
| Narcissistic | 7 | 1.8 | 5 | 1.9 | 6 | 1.8 |
| Histrionic | 1 | 0.3 | 1 | 0.4 | 1 | 0.3 |
| Cluster C personality disorders | ||||||
| Dependent | 3 | 0.8 | 2 | 0.7 | 1 | 0.3 |
| Avoidant | 21 | 5.4 | 15 | 5.6 | 16 | 4.7 |
| Obsessive-compulsive | 26 | 6.7 | 19 | 7.1 | 24 | 7.1 |
| Mean | SD | Mean | SD | Mean | SD | |
| Number of axis I diagnoses | 1.9 | 1.1 | 2.0 | 1.3 | 1.8 | 1.2 |
| Number of axis II diagnoses | 1.4 | 0.8 | 1.5 | 0.9 | 1.4 | 0.9 |
Family history of psychiatric illness in first-degree, second-degree, and distant relatives was assessed on the basis of all available historical information from the proband and his or her relative(s). The frequencies of reported psychiatric illnesses in the pedigrees are listed in Table 4; all between-group comparisons are reported descriptively. Of the probands with schizophrenia, 17.6% had a first-degree relative and 22.4% had a second-degree relative with schizophrenia or schizoaffective disorder; these rates were decreased in the relatives of the bipolar probands to 13.5% and 14.4%, respectively. Of the bipolar probands, 44.6% had a first-degree and 30.5% reported a second-degree relative with bipolar disorder; these were decreased in the schizophrenia probands to 15.4% and 12.1%, respectively. Within the schizophrenia probands, 27.0% had relatives with schizophrenia but not bipolar disorder, 11.5% had relatives with bipolar disorder but not schizophrenia, and 17.3% reported both types among their relatives. Within the bipolar probands, 39.8% had relatives with bipolar disorder but not schizophrenia, 9.9% had relatives with schizophrenia but not bipolar disorder, and 25.4% had a mixed lineage, with both bipolar disorder and schizophrenia present in their pedigrees. In addition, the frequency of depression was noticeably higher in the pedigrees of the probands with bipolar disorder or schizoaffective disorder than in the pedigrees of the schizophrenia probands (Table 4). Alcohol and illicit substance use were frequently reported in all relative groups, with the highest rates among the relatives of the schizoaffective disorder probands. Across groups, the rates of psychiatric diagnoses in distant relatives were lower overall than the rates for first- and second-degree relatives.
| Probands With Schizophrenia (N=397) | Probands With Schizoaffective Disorder (N=224) | Probands With Bipolar Disorder (N=312) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First-Degree Relatives | Second-Degree Relatives | First-Degree Relatives | Second-Degree Relatives | First-Degree Relatives | Second-Degree Relatives | |||||||
| Relative’s Diagnosis | N | % | N | % | N | % | N | % | N | % | N | % |
| Schizophrenia or schizoaffective disorder | 70 | 17.6 | 89 | 22.4 | 46 | 20.5 | 62 | 27.7 | 42 | 13.5 | 45 | 14.4 |
| Bipolar disorder | 61 | 15.4 | 48 | 12.1 | 81 | 36.2 | 75 | 33.5 | 139 | 44.6 | 95 | 30.5 |
| Depressive disorder | 112 | 28.2 | 79 | 19.9 | 121 | 54.0 | 83 | 37.1 | 172 | 55.1 | 119 | 38.1 |
| Suicide or suicide attempt | 30 | 7.6 | 46 | 11.6 | 24 | 10.7 | 34 | 15.2 | 53 | 17.0 | 48 | 15.4 |
| Alcoholism | 129 | 32.5 | 147 | 37.0 | 100 | 44.6 | 110 | 49.1 | 131 | 42.0 | 148 | 47.4 |
| Illicit substance use | 81 | 20.4 | 73 | 18.5 | 81 | 36.2 | 67 | 29.9 | 105 | 33.7 | 66 | 21.2 |
| Other psychiatric disorder | 73 | 18.4 | 79 | 19.9 | 46 | 20.5 | 59 | 26.3 | 99 | 31.7 | 103 | 33.0 |
Discussion
A distinction between the two major psychotic diagnoses, schizophrenia and bipolar disorder, began with Kraepelin (24, 37) and Bleuler (25) and persisted throughout the last century. Though Kraepelin himself acknowledged the overlap between these disorders (37), the dichotomy was reified within APA’s Diagnostic and Statistical Manual editions (38), even now in the 5th edition. Schizoaffective disorder was first introduced with its own criteria in DSM-III-R, although it was mentioned as a schizophrenia subgroup in previous DSM editions (9). Previous studies have attempted to test the validity of these categories and, while interesting, have been insufficiently powered (4, 6) or only conceptual (2), leaving questions of commonality and distinctiveness unresolved. In the current study, probands with schizophrenia, contrasted with bipolar probands, had a high degree of overlap in clinical characteristics without categorical distinctions, but they generally showed modestly greater psychosis severity and somewhat poorer psychosocial functioning, even during stable illness periods. Clinical and demographic characteristics of the schizoaffective disorder proband group were often more similar to those of the schizophrenia probands than those of the bipolar group: For example, 1) schizophrenia and schizoaffective disorder proband groups both had lower proportions of Caucasians than the bipolar probands, an observation also reflected in the relative groups; 2) the schizophrenia and schizoaffective disorder probands both had less education than the bipolar proband group, but only the schizophrenia probands had a lower reading level, which reflects education and IQ; 3) PANSS scores (total and all subscales) were higher in both the schizophrenia and schizoaffective disorder probands than in the bipolar probands; and 4) the scores on the social functioning scales were lower in both the schizophrenia and schizoaffective disorder probands than in the bipolar probands. Yet, in some cases, the characteristics of schizoaffective disorder mirrored those of psychotic bipolar disorder: 1) the reports of previous suicide attempts were similarly high among these two proband groups but lower in schizophrenia and 2) the schizoaffective and bipolar disorder proband groups had similarly high proportions of women, whereas the schizophrenia probands had a lower number of women, which is an often-cited characteristic of schizophrenia itself.
The frequency of reported lifetime suicide attempts was disturbingly high in all groups of probands in this B-SNIP cohort, underscoring the immense public health importance of psychosis and its high medical need. It is interesting that the frequency was highest in the probands with schizoaffective disorder. Suicide is among the 10 leading causes of death worldwide; both schizophrenia (hazard ratio=1.87) and mood disorders (hazard ratio=1.72) are associated with suicide (39). The absolute lifetime risk of completed suicide has been reported to be 7.77% in bipolar disorder and 5.85% in schizophrenia (40). The evidence that preserved cognitive function is associated with greater suicide ideation (41) suggests that these suicides are driven by rational considerations along with depressed mood. Both genetic (42, 43) and molecular (44) correlates of suicide in schizophrenia and bipolar disorder have been established. The prevalence of this single severe outcome across groups is consistent with the low overall level of psychosocial functioning seen in psychosis.
Epidemiological studies characteristically use family history as the primary measure of genetic liability for disease (45). Early family studies in schizophrenia found pure schizophrenia lineages in the probands with positive family histories (46–48). However, these early observations are now challenged by outcomes from registry-based national cohort studies (7, 49), which show higher rates of both bipolar disorder and other psychiatric diagnoses within schizophrenia families. Support for this complex inheritance is also seen in population-based studies (6, 7), extending to the identification of autism (50) and epilepsy (51) in schizophrenia lineages. In the current B-SNIP cohort, we verified both “pure” schizophrenia and bipolar disorder pedigrees as well as “mixed” lineages with both schizophrenia and bipolar disorder. Of the probands with schizophrenia, 17.3% had mixed pedigrees (27.0% had schizophrenia-only pedigrees), and of those with bipolar disorder, 25.4% had mixed pedigrees (39.8% had bipolar-only pedigrees). These rates support the existence of genetic overlap between these serious mental illnesses and implicate common genetic mechanisms. Current family and genetic evidence broadens this idea even further, to suggest that many axis I and II psychiatric diagnoses in probands are risk factors for psychotic disorders (49, 52). Here, among the relatives of the psychosis probands, only 33%−38% were free of any axis I or II diagnosis. These outcomes suggest a high burden of psychiatric morbidity in families with psychosis, a morbidity that is similar across the proband diagnoses.
The B-SNIP study has been motivated by the strong need in serious mental illness research to connect biological characteristics of psychotic illness, through intermediate phenotypes, with diagnostic categories, molecular characteristics, and therapeutic outcomes. The developments in complex disease genetics (53) and the burgeoning neuroscience knowledge base in our field (54) provide an excellent opportunity to translate the biological correlates of serious mental illness to disease understanding. The B-SNIP consortium has focused on a single clinical phenotype—psychosis within the schizophrenia-bipolar spectrum—and has attempted to characterize this phenotype biologically. Several meaningful outcomes of such a project can be realized, one of which is to test the distribution of clinical and biological phenotypes across categorical diagnoses, evaluating their biological heterogeneity. Another potential outcome is to determine experimentally whether biologically homogeneous populations emerge out of such a broadly defined clinical phenotype. Not without importance is the goal of understanding how these disparate clinical and intermediate phenotypes covary and segregate, an outcome that could inform their use as clinical biomarkers.
Such transformative goals will need to be rigorously tested in biologically based dense phenotyping protocols, with oversampling, in large numbers of individuals with serious mental illness (1). The multisite B-SNIP consortium provides sufficient recruiting strength to approach this task, with 933 psychotic probands, along with relatives and healthy comparison subjects. Moreover, the dense phenotyping protocol, with its high cross-site phenotype reliability and single-site expert processing, provides the requisite study group size and phenotype methods to tackle these goals. Previous multisite intermediate-phenotype protocols were models (18, 19). The intermediate-phenotype reports from B-SNIP will build on this clinical database, contrasting cognitive, neurophysiological, and imaging characteristics of these proband and relative groups, to examine the biological characteristics of these serious mental illnesses within the clinical phenotype of psychosis.
1 : Grouping diagnoses of mental disorders by their common risk factors. Am J Psychiatry 2011; 168:1–3Link, Google Scholar
2 : Toward reformulating the diagnosis of schizophrenia. Am J Psychiatry 2000; 157:1041–1050Link, Google Scholar
3 : Phenomenology of first-episode psychosis in schizophrenia, bipolar disorder, and unipolar depression: a comparative analysis. Clin Schizophr Relat Psychoses 2012; 6:145–151Crossref, Medline, Google Scholar
4 McDonald C (ed): The Maudsley Family Study of Psychosis: A Quest for Intermediate Phenotypes. Abingdon, UK, Psychology Press, 2008Google Scholar
5 : Genetics and intermediate phenotypes of the schizophrenia–bipolar disorder boundary. Neurosci Biobehav Rev 2010; 34:897–921Crossref, Medline, Google Scholar
6 : Bipolar disorder, schizoaffective disorder, and schizophrenia overlap: a new comorbidity index. J Clin Psychiatry 2009; 70:1432–1438Crossref, Medline, Google Scholar
7 : Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet 2009; 373:234–239Crossref, Medline, Google Scholar
8 ;
9 : Examining the validity of DSM-III-R schizoaffective disorder and its putative subtypes in the Roscommon Family Study. Am J Psychiatry 1995; 152:755–764Link, Google Scholar
10 : Schizophrenia, schizoaffective and bipolar disorder within an epidemiologically complete, homogeneous population in rural Ireland: small area variation in rate. Schizophr Res 2004; 67:143–155Crossref, Medline, Google Scholar
11 : Does schizoaffective disorder really exist? a systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affect Disord 2008; 106:209–217Crossref, Medline, Google Scholar
12 : The hippocampal formation in schizophrenia. Am J Psychiatry 2010; 167:1178–1193Link, Google Scholar
13 : High classification accuracy for schizophrenia with rest and task FMRI data. Front Hum Neurosci 2012; 6:145Crossref, Medline, Google Scholar
14 : De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry 2012; 17:142–153Crossref, Medline, Google Scholar
15 : Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry 2012; 169:1082–1091Link, Google Scholar
16 : Genetic theorizing and schizophrenia. Br J Psychiatry 1973; 122:15–30Crossref, Medline, Google Scholar
17 : Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry 2009; 66:988–989Crossref, Medline, Google Scholar
18 : The Consortium on the Genetics of Endophenotypes in Schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr Bull 2007; 33:33–48Crossref, Medline, Google Scholar
19 : Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophr Bull 2007; 33:21–32Crossref, Medline, Google Scholar
20 : Initial heritability analyses of endophenotypic measures for schizophrenia: the Consortium on the Genetics of Schizophrenia. Arch Gen Psychiatry 2007; 64:1242–1250Crossref, Medline, Google Scholar
21 : Group and site differences on the California Verbal Learning Test in persons with schizophrenia and their first-degree relatives: findings from the Consortium on the Genetics of Schizophrenia (COGS). Schizophr Res 2011; 128:102–110Crossref, Medline, Google Scholar
22 : Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry 2011; 168:930–946Link, Google Scholar
23 : A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res 2011; 133:250–254Crossref, Medline, Google Scholar
24 : Dementia Praecox and Paraphrenia. Edinburgh, Scotland, ES Livingstone, 1919Google Scholar
25 : Dementia Praecox or the Group of Schizophrenias. New York, International Universities Press, 1950Google Scholar
26 : Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull 2010; 36:1061–1062Crossref, Medline, Google Scholar
27 : Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167:748–751Link, Google Scholar
28 : The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004; 68:283–297Crossref, Medline, Google Scholar
29 : The family history approach to diagnosis: how useful is it? Arch Gen Psychiatry 1986; 43:421–429Crossref, Medline, Google Scholar
30 : Structured Clinical Interview for DSM-IV Axis I Disorders. Arlington, Va, American Psychiatric Publishing, 1997Google Scholar
31 : Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS). Schizophr Res 2000; 42:231–239Crossref, Medline, Google Scholar
32 : A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
33 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
34 : The Social Functioning Scale: the development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry 1990; 157:853–859Crossref, Medline, Google Scholar
35 : The Diagnostic Interview for DSM-IV Personality Disorders (DIP DIV). Belmont, Mass, McLean Hospital, 1996Google Scholar
36 : Structured Interview for DSM-IV Personality (SIDP). Iowa City, University of Iowa, Department of Psychiatry, 1997Google Scholar
37 : Manic-depressive illness: evolution in Kraepelin’s Textbook, 1883–1926. Harv Rev Psychiatry 2005; 13:155–178Crossref, Medline, Google Scholar
38
39 : Determinants and outcomes of serious attempted suicide: a nationwide study in Finland, 1996–2003. Am J Epidemiol 2008; 167:1155–1163Crossref, Medline, Google Scholar
40 : Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry 2011; 68:1058–1064Crossref, Medline, Google Scholar
41 : Preserved cognitive function is associated with suicidal ideation and single suicide attempts in schizophrenia. Schizophr Res 2012; 140:232–236Crossref, Medline, Google Scholar
42 : Suicide candidate genes associated with bipolar disorder and schizophrenia: an exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genomics 2007; 8:413Crossref, Medline, Google Scholar
43 : Genetic polymorphisms in the SCN8A gene are associated with suicidal behavior in psychiatric disorders in the Chinese population. World J Biol Psychiatry 2010; 11:956–963Crossref, Medline, Google Scholar
44 : CSF kynurenic acid and suicide risk in schizophrenia spectrum psychosis. Psychiatry Res 2013; 205:165–167Crossref, Medline, Google Scholar
45 : Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull 2008; 34:1066–1082Crossref, Medline, Google Scholar
46 : A controlled family study of chronic psychoses: schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 1988; 45:328–336Crossref, Medline, Google Scholar
47 : The Roscommon Family Study, I: methods, diagnosis of probands, and risk of schizophrenia in relatives. Arch Gen Psychiatry 1993; 50:527–540Crossref, Medline, Google Scholar
48 : Resolving genetic models for the transmission of schizophrenia. Genet Epidemiol 1985; 2:99–110Crossref, Medline, Google Scholar
49 : Psychiatric family history and schizophrenia risk in Denmark: which mental disorders are relevant? Psychol Med 2010; 40:201–210Crossref, Medline, Google Scholar
50 : Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch Gen Psychiatry 2012; 69:1099–1103Crossref, Medline, Google Scholar
51 : Evidence for shared susceptibility to epilepsy and psychosis: a population-based family study. Biol Psychiatry 2012; 71:836–839Crossref, Medline, Google Scholar
52 : Psychiatric illnesses in families of subjects with schizophrenia-spectrum personality disorders: high morbidity risks for unspecified functional psychoses and schizophrenia. Am J Psychiatry 1993; 150:66–71Link, Google Scholar
53 ;
54 : Neurobiology of Mental Illness, 2nd ed. New York, Oxford University Press, 2004Google Scholar



