The International Society for Bipolar Disorders (ISBD) Task Force Report on Antidepressant Use in Bipolar Disorders
Abstract
Objective
The risk-benefit profile of antidepressant medications in bipolar disorder is controversial. When conclusive evidence is lacking, expert consensus can guide treatment decisions. The International Society for Bipolar Disorders (ISBD) convened a task force to seek consensus recommendations on the use of antidepressants in bipolar disorders.
Method
An expert task force iteratively developed consensus through serial consensus-based revisions using the Delphi method. Initial survey items were based on systematic review of the literature. Subsequent surveys included new or reworded items and items that needed to be rerated. This process resulted in the final ISBD Task Force clinical recommendations on antidepressant use in bipolar disorder.
Results
There is striking incongruity between the wide use of and the weak evidence base for the efficacy and safety of antidepressant drugs in bipolar disorder. Few well-designed, long-term trials of prophylactic benefits have been conducted, and there is insufficient evidence for treatment benefits with antidepressants combined with mood stabilizers. A major concern is the risk for mood switch to hypomania, mania, and mixed states. Integrating the evidence and the experience of the task force members, a consensus was reached on 12 statements on the use of antidepressants in bipolar disorder.
Conclusions
Because of limited data, the task force could not make broad statements endorsing antidepressant use but acknowledged that individual bipolar patients may benefit from antidepressants. Regarding safety, serotonin reuptake inhibitors and bupropion may have lower rates of manic switch than tricyclic and tetracyclic antidepressants and norepinephrine-serotonin reuptake inhibitors. The frequency and severity of antidepressant-associated mood elevations appear to be greater in bipolar I than bipolar II disorder. Hence, in bipolar I patients antidepressants should be prescribed only as an adjunct to mood-stabilizing medications.
The efficacy and safety of antidepressant drug treatment in bipolar disorder is the subject of long-standing debate based on a scientific literature that is limited and inconsistent (1–6). The sparseness of high-quality clinical research hampers the formulation of sound clinical recommendations on the use of antidepressants in the treatment of bipolar disorder (7–12). We propose that a consensus formed by the experience and judgment of clinical and academic bipolar disorder experts, guided by the available research findings, may help in developing at least tentative treatment recommendations as additional research is awaited. Accordingly, the International Society for Bipolar Disorders (ISBD) appointed Dr. Vieta to assemble a task force of international experts to review the evidence base for benefits and risks of antidepressant treatment in bipolar disorder and to formulate clinical recommendations based on the consensus development process. This report represents a consensus statement from this endeavor.
Method
Consensus Methods
The ISBD Task Force was made up of a panel of global experts on bipolar disorder, selected according to an objective procedure based on a Scopus search of citations on the specific topic of antidepressant use in bipolar disorder (number of citations per candidate during the past 3 years). The most cited authors (including several ISBD nonmembers) and some additional authors from key geographical areas were identified and invited by e-mail to participate; 76% agreed to participate. An introductory meeting was held at the ISBD biennial congress (Istanbul, March 2012), where task force procedures were reviewed and agreed upon. These procedures focused on the discussion and integration of findings from peer-reviewed published research findings on the topic, including reviews and meta-analyses, as well as clinical trial reports. An expert coauthor (I.P.) was appointed to develop a first draft of a systematic review, to be circulated after initial review by the senior author (E.V.). The aims of the task force were to conduct a thorough and balanced review of research findings and to integrate them into an expert consensus, based on clinical experience and judgment, as well as research evidence, and to provide a synthesis of current knowledge supporting clinical recommendations for this important and timely topic. The final section of this report, which summarizes consensus statements, was achieved through a face-to-face meeting, personal and group e-mail correspondence, and serial iterative revisions of the report, in order to provide a final guide on the use of antidepressants in bipolar disorder. Funding for this international project was provided solely by the Spanish government.
Search Strategy
We performed an extensive literature search on PubMed, using the following search terms, limited to human studies: antidepressant* AND (mania[ti] OR manic[ti]); antidepressant* AND (bipolar[ti] AND depressi*[ti]); antidepressant* AND (mixed[ti] AND state*[ti]); antidepressant* AND bipolar disorder AND maintenance[ti]; antidepressant* AND bipolar disorder AND comorbid*[ti]; antidepressant* AND switch AND (manic OR mania OR hypomani*); antidepressant* AND (cycle acceleration OR phase shift OR cycle frequen*); and antidepressant* AND (suicid*[ti] OR self-kill*[ti] OR self-harm*[ti]).
We considered first-generation norepinephrine-serotonin reuptake inhibitors (SNRIs), including tricyclic and tetracyclic antidepressants; monoamine oxidase (MAO) inhibitors; and the modern antidepressants, including the dopamine-norepinephrine reuptake inhibitor bupropion, serotonin reuptake inhibitors (SSRIs), modern SNRIs, the norepinephrine-serotonin autoreceptor-specific antagonists mianserin and mirtazapine; the atypical, mixed monoamine-transporter inhibitors trazodone and nefazodone; and the melatonin M1 and M2 receptor agonist and 5-HT2c receptor antagonist agomelatine.
We address here only antidepressant medications, not the treatment of bipolar depression in general. Hence, we did not include alternative or experimental agents such as sulfoadenosine-l-methionine; Hypericum perforatum (St. John’s wort); various mood stabilizers and antipsychotics with some evidence of antidepressant efficacy (e.g., lithium, lamotrigine, quetiapine, olanzapine, lurasidone); glutamatergic modulators including glycine and its analogues; ketamine, memantine, and other N-methyl-d-aspartate (NMDA) inhibitors; azapirone anxiolytics (buspirone, gepirone); stimulants (e.g., amphetamines, methylphenidate); the antinarcolepsy agents modafinil and armodafinil; and direct dopamine receptor agonist antiparkinsonian agents such as pramipexole. We also did not include nonpharmacological treatments, such as ECT, transcranial magnetic stimulation, vagus nerve stimulation, deep brain stimulation, intense light therapy, or psychological interventions.
Systematic Review Methods
Each report considered was rated for methodological quality according to the Jadad scale (13) as poor (scores of 0–2) or acceptable-good (scores of 3–5; see the data supplement that accompanies the online edition of this article). Each report was rated A, B, C, or D for overall quality, as recommended by the Australian National Health and Medical Research Council (14), save for the applicability criterion (see the data supplement and Table 1). Included references may contain additional reports for particular questions and statements. Meta-analyses and reviews were used as evidence to support information that could not be drawn from individual studies. Figure 1 outlines how reports were selected.
| Topic | Jadad Score | Evidence Level |
|---|---|---|
| Antidepressant monotherapy | 3 | D |
| Adjunctive antidepressants: short-term efficacy in acute depression | 4 | B |
| Predictors of initial response to adjunctive antidepressants | 3 | D |
| Adjunctive antidepressants: maintenance studies | 3.5 | C |
| Predictors of long-term responsiveness to adjunctive antidepressant treatment | 3 | D |
| Antidepressant use in mania and mixed states | 3 | D |
| Antidepressants and affective switch (mania, hypomania, or mixed) | 4 | C |
| Are newly emerging or increasing irritability and agitation, subclinical mixed states during antidepressant treatment a form of mood switching? | 3.5 | D |
| Antidepressants and cycle acceleration | 3.5 | D |
| Antidepressants and suicidality | 3 | D |
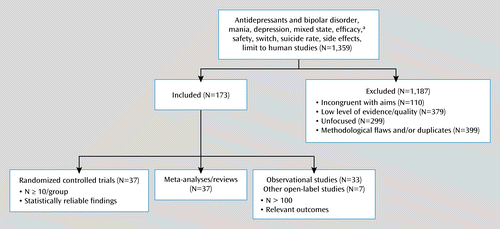
a Criteria for clinical response or remission were based on international standards, typically involving a decrease of ≥50% from baseline to a final score on a standard symptom rating scale (response) and a final depression score ≤7 (typically using the 17-item Hamilton Depression Rating Scale).
Delphi Method
To add a table of recommendations at the end of the systematic review, we conducted a survey using the Delphi method (15–17). Statements on antidepressant use in bipolar disorder that could be useful to clinicians were derived from the content of the literature search and classified into six common domains: acute treatment; maintenance treatment; monotherapy; switch to mania, hypomania, or mixed states and rapid cycling; use in mixed states; and drug class. Three survey rounds were conducted to develop consensus. The first survey included open-ended questions at the end of each section inviting participants to add comments and suggestions by e-mail. Later rounds were conducted online using eSurveysPro.com. The survey was sent to task force members for anonymous responses. Panel members rated survey items ranging from “essential” to “should not be included.” We calculated proportions of respondents rating each item. Survey items were classified as endorsed, rerated, or rejected.
Endorsed items.
Items rated by at least 80% of ISBD experts as essential or important were included in the ISBD recommendations.
Rerated items.
Items rated as essential or important by 65%−79% of panel experts were included in the next survey for rerating after considering feedback from first-round results. Panel members could decide whether they wanted to maintain or change their previous rating on these relatively controversial items. Items were rerated only once; if they did not achieve the criterion for endorsement, they were rejected.
Rejected items.
Items that were not included by at least 65% of panelists on the first round were rejected and excluded.
The initial survey included 25 items. The second survey included 23 items. The briefer third survey consisted of five items that needed rerating.
Twelve of the initial 25 items were endorsed and formed the section of the ISBD clinical recommendations of antidepressant use in bipolar disorder (Table 2).
| Domain | Recommendation |
|---|---|
| Acute treatment | 1. Adjunctive antidepressants may be used for an acute bipolar I or II depressive episode when there is a history of previous positive response to antidepressants. |
| 2. Adjunctive antidepressants should be avoided for an acute bipolar I or II depressive episode with two or more concomitant core manic symptoms in the presence of psychomotor agitation or rapid cycling. | |
| Maintenance treatment | 3. Maintenance treatment with adjunctive antidepressants may be considered if a patient relapses into a depressive episode after stopping antidepressant therapy. |
| Monotherapy | 4. Antidepressant monotherapy should be avoided in bipolar I disorder. |
| 5. Antidepressant monotherapy should be avoided in bipolar I and II depression with two or more concomitant core manic symptoms. | |
| Switch to mania, hypomania, or mixed states and rapid cycling | 6. Bipolar patients starting antidepressants should be closely monitored for signs of hypomania or mania and increased psychomotor agitation, in which case antidepressants should be discontinued. |
| 7. The use of antidepressants should be discouraged if there is a history of past mania, hypomania, or mixed episodes emerging during antidepressant treatment. | |
| 8. Antidepressant use should be avoided in bipolar patients with a high mood instability (i.e., a high number of episodes) or with a history of rapid cycling. | |
| Use in mixed states | 9. Antidepressants should be avoided during manic and depressive episodes with mixed features. |
| 10. Antidepressants should be avoided in bipolar patients with predominantly mixed states. | |
| 11. Previously prescribed antidepressants should be discontinued in patients currently experiencing mixed states. | |
| Drug class | 12. Adjunctive treatment with norepinephrine-serotonin reuptake inhibitors or tri- and tetracyclics should be considered only after other antidepressants have been tried, and should be closely monitored because of an increased risk of mood switch or destabilization. |
Results
Search Results
A summary of our literature search and review is presented in Figure 1.
Efficacy
Antidepressant monotherapy.
Antidepressant monotherapy is widely regarded as contraindicated for patients with bipolar disorder because of the weak evidence for efficacy and the potential risk for excessive mood elevation (switches). An elevated risk of mood switching was first observed in early trials that compared imipramine monotherapy, lithium monotherapy, lithium plus imipramine, and placebo (18, 19). Imipramine monotherapy was followed by more manic episodes than combination therapy with imipramine and lithium and was not superior to lithium in preventing depressive episodes.
To date, the largest randomized placebo-controlled trial assessing antidepressant monotherapy in bipolar depression has been the EMBOLDEN II (Efficacy of Monotherapy Seroquel in Bipolar Depression) study (20), in which paroxetine was included as an active comparator to quetiapine and placebo in 740 acutely depressed patients with bipolar I or II disorder. Compared with placebo, paroxetine did not result in significant symptomatic improvement as rated on the Montgomery-Åsberg Depression Rating Scale (MADRS) in either bipolar type, whereas quetiapine (at 300 and 600 mg/day) separated from placebo without evidence of a dose effect. The study is limited by its use of a moderate, fixed dose of paroxetine (20 mg/day). A small randomized controlled trial of escitalopram monotherapy (21), an uncontrolled trial of fluoxetine (22), and a randomized controlled trial of fluoxetine or lithium monotherapy and placebo (23) found some support for the efficacy of these drugs in depressed bipolar II patients, with no evidence of mood switching.
Conclusions.
Overall, available clinical trials do not provide adequate support for the efficacy of antidepressant monotherapy in acute bipolar depression, but the evidence base is poor (D) and inconclusive (Table 1). Although the evidence might be rated C for depression in bipolar II patients, the studies focusing on such cases are marred by methodological shortcomings and possibly selective reporting.
Adjunctive antidepressants: short-term efficacy in acute depression.
Short-term trials of adjunctive antidepressant treatment have reported mixed results, perhaps best exemplified by the contrasting findings in the two largest placebo-controlled trials carried out to date. The first of these (24) compared the efficacy and safety of olanzapine monotherapy (5–20 mg/day, N=370) to placebo (N=377) in depressed bipolar I patients in an 8-week randomized double-blind trial with a small exploratory arm with several dosages of olanzapine-fluoxetine combinations. The olanzapine-fluoxetine combinations were more effective than olanzapine alone or placebo in improving MADRS depression scores at weeks 4–8. Limitations of the study included its lack of a fluoxetine monotherapy arm and a substantial dropout rate (38.5%).
In the second trial (25), depressed bipolar I or II patients (N=366) receiving treatment with a mood stabilizer (lithium, valproate, carbamazepine, or other antimanic agents approved by the U.S. Food and Drug Administration, alone or in combination) were randomly assigned to receive adjunctive antidepressants (bupropion or paroxetine) or placebo for up to 26 weeks. Adjunctive antidepressants were no more effective than placebo at any time, and overall, 23.5% of patients given an antidepressant and 27.3% given placebo met criteria for enduring recovery. Limitations of the trial included its use of already well treated patients, its long duration, and the study requirement for sustained improvement.
A smaller placebo-controlled study (26) also found no significant differences in efficacy among paroxetine, imipramine, or placebo as adjuncts to mood stabilizers. Conversely, a single-blind randomized trial (27) of short duration and lacking a control found significant improvement after 6 weeks in depressed bipolar I and II patients treated with mood stabilizers plus paroxetine or venlafaxine (28).
Several meta-analyses have statistically combined data from some or all of these antidepressant studies. One such study (29) found higher response rates with an antidepressant compared with placebo and no increase in risk of manic switch (29), but it suffered from being heavily weighted by one large olanzapine-fluoxetine study (24). A later meta-analytic review (30) found nonsignificant differences between antidepressants and placebo in response and remission rates (p=0.06), which would have been even less favorable if the analysis had used a random-effects rather than a fixed-effects model (31). Another recent random-effects meta-analysis (32) found superiority of antidepressants over placebo (relative risk=1.43, 95% CI=1.11–1.84; z=2.76, p=0.006).
Finally, a large naturalistic study (33) found short-term antidepressant treatment (with or without mood stabilizers) in acute major depressive episodes to be similarly effective in a total of 1,036 patients with bipolar I disorder, bipolar II disorder, and unipolar depression.
Regarding possible predictors of short-term response to antidepressants in depressed bipolar patients, two naturalistic trials in acutely depressed bipolar I and II patients (34, 35) found that the most significant predictors of beneficial responses were previous response to antidepressants and a less severe illness course. In a naturalistic follow-up study (36), short-term recovery from depression in bipolar I or II disorder was neither hastened nor prolonged when antidepressants were added to a mood stabilizer in the presence of syndromal or subsyndromal hypomanic symptoms.
Conclusions.
Overall, the available evidence (category B) supports the efficacy of the olanzapine-fluoxetine combination in bipolar depression, indicates a lack of positive effects of paroxetine or bupropion added to mood stabilizers, and is otherwise inconsistent. The overall evidence quality of predictors of response to antidepressants is rated D, because of inconclusiveness and possible bias of findings (Table 1).
Adjunctive antidepressants: long-term maintenance studies.
For modern antidepressants, only two randomized controlled trials lacking placebo controls have examined the effects of continuing antidepressant treatment after favorable short-term responses in bipolar I depression (37, 38); notably, both trials involved patients who showed favorable short-term responses to antidepressant treatment. In one trial, depressed bipolar I or II patients who responded to initial treatment with venlafaxine, bupropion, or sertraline added to standard mood stabilizers after 2 months (50%−60% of each group) continued these treatments for up to 1 year, and only 15%−25% had no further episodes (37, 39). In the second trial (38), among 70 depressive bipolar I or II patients who had remitted for ≥8 weeks during treatment with a standard mood stabilizer plus an antidepressant (usually an SSRI, venlafaxine, or bupropion), antidepressant continuation showed nonsignificantly less severe depressive symptoms but a significant delay in recurrence of new depressive episodes, except that rapid-cycling patients experienced more depressive recurrences with an antidepressant.
A nonrandomized uncontrolled trial (40), again involving patients showing favorable short-term responses, examined the effect of continuing or discontinuing antidepressant treatment for bipolar I and II patients who had remitted from a depressive episode for ≥6 weeks after addition of an antidepressant to a mood stabilizer. Over 1 year of follow-up, patients who discontinued antidepressant treatment experienced a shorter latency to depressive relapse (χ2=9.63, p=0.002) and were more likely to relapse (70% compared with 36%).
In contrast to these findings, a meta-analysis of long-term antidepressant efficacy in bipolar depression (41) found that compared with mood stabilizer treatment alone, adjunctive antidepressants provided little protection from depression and tended to increased mania-hypomania, resulting in an unfavorable risk-benefit ratio for long-term antidepressant use in bipolar I disorder.
Examining possible predictors of long-term responsiveness to adjunctive antidepressants in the Stanley Foundation Bipolar Network study (42), the research team found that depressed bipolar patients who achieved an initial response during 10 weeks of adjunctive antidepressant treatment (bupropion, sertraline, or venlafaxine) were more likely to maintain response with the same continuation regimen. Similarly, in a randomized double-blind placebo-substitution study with fluoxetine monotherapy in bipolar II depression (23), initial response to antidepressant treatment was associated with fewer relapses if antidepressant treatment was continued for up to 50 weeks.
Conclusions.
Long-term trials involving addition of antidepressants to ongoing mood-stabilizing treatments are scant and have yielded ambiguous, inconclusive findings, despite a moderately favorable quality score (C) for the evidence and despite reliance on samples enriched for probable short-term antidepressant response. For predictors of response, the lack of adequate controls and reliance on enriched patient samples led to a D rating of available evidence (Table 1).
Antidepressant use in mania and mixed states.
Despite a lack of controlled trials of adjunctive antidepressants during manic, hypomanic, or mixed episodes, most clinicians avoid their use during mixed episodes for fear of worsening mania, and without improving mixed-state depressive symptoms (36, 43–46). Indeed, no evidence of efficacy is available for antidepressants in mania or mixed states.
Nevertheless, in an observational study, 21.9% of 2,416 manic patients were taking antidepressants at baseline. Predictors of continuing antidepressants for up to 24 months of follow-up were mixed states, more previous depressive episodes, rapid cycling, and higher rates of depressive and anxiety symptoms at baseline (47, 48).
Similarly, two retrospective observational studies (49, 50) found that a history of mixed episodes was associated with use of antidepressants, especially SNRIs and SSRIs—both lifetime and during the 6 months preceding an index mixed episode. Moreover, in a study of a large U.S. insurance claims database (51), antidepressant treatment was identified in 32% of 2,126 patients with bipolar I disorder following a recent manic or mixed episode, with more rehospitalizations within 12 months with than without an antidepressant.
Conclusions.
Overall, the lack of controlled studies of antidepressant use in mania and mixed states points to a D quality rating of available evidence (Table 1).
Safety
Antidepressants and mood switching.
The assessment of antidepressant-associated mood switches into hypomania, mania, or mixed states is a particularly controversial topic. The principal difficulty lies in attributing causality, as mood elevations and changes in cycle frequency occur unpredictably in the natural course of bipolar disorder, making it difficult to distinguish spontaneous from antidepressant-induced switching (52). Randomized trials evaluating the risk of switches with antidepressant treatment with and without a mood stabilizer are few and difficult to interpret, owing to methodological limitations (53–57). Moreover, the definition of mood switching lacks consensus among experts (58). In an attempt to clarify the terminology of antidepressant-associated mood elevations, the ISBD reached consensus in recommending use of the term “treatment-emergent affective switch” instead of antidepressant-induced switch (59), to emphasize association without implying causality. The ISBD definition of treatment-emergent affective switch provides operational criteria that consider factors such as timing, duration, and severity of mood changes in attributing probable causation to antidepressant treatment. Obviously, since these criteria are very recent, they have not yet been widely tested or adopted.
Despite these definitional shortcomings, a notably consistent finding from randomized controlled trials of antidepressants in bipolar patients is that of differences among types of antidepressants in their association with mood switches (54). For example, a small 8-week prospective double-blind trial comparing adjunctive bupropion or desipramine in bipolar depression (60) found hypomania or mania in five of 10 desipramine-treated patients but in only one of nine bupropion-treated patients. However, several placebo-controlled randomized controlled trials did not find elevated switch rates with SSRIs or bupropion, either as adjuncts to mood stabilizers or as monotherapy (54). In a previously described 6-month double-blind placebo-controlled trial (25), rates of mood elevations were indistinguishable (10.1% compared with 10.7%) between subjects receiving a mood stabilizer plus an antidepressant (bupropion or paroxetine, N=179) and those receiving a mood stabilizer plus placebo (N=197).
Even in monotherapy, it is not clear whether SSRIs increase the risk of mood switching. In one randomized controlled trial (20), paroxetine (20 mg/day) was not associated with more mood switching than placebo. In a 12-month trial (37), the risk of antidepressant-associated switching ranked was 9% for sertraline, 10% for bupropion, and 29% for venlafaxine. A short-term (6-week) study (27) also found more mood switching with venlafaxine than with paroxetine in depressed bipolar I and II patients. These findings suggest that mood switching may be limited to certain antidepressant classes, such as the tri- and tetracyclics and venlafaxine, and that dosages, co-treatments, exposure times, types of bipolar patients, and criteria for “switching” may each have a role. Several meta-analyses have evaluated antidepressant-associated switch risk. In an early meta-analysis (61), the rate of treatment-emergent switch occurred substantially more often with tri- and tetracyclics (11.2%) than with SSRIs (3.7%) or placebo (4.2%). A subsequent meta-analysis (29) found that, with the exception of tri- and tetracyclics and venlafaxine, switching was uncommon. Another review (54) found that treatment with or without antidepressants did not increase the risk of switching in bipolar patients (types I or II) compared with rates of spontaneous switching (15.3% and 13.8%, respectively). Nevertheless, tri- and tetracyclics carried a higher risk for new mania-hypomania than SSRIs or MAO inhibitors. Another review (30) found that SSRIs and bupropion were not associated with more switching than placebo during short-term treatment, but noted that studies employing sensitive criteria to define switching reported higher switch rates for tri- and tetracyclics and venlafaxine than for SSRIs and bupropion.
Regarding the issue of whether mood stabilizers protect against antidepressant treatment-associated mood switching, a comprehensive review (54) found a lack of evidence that treatment with mood stabilizers protects against mood elevation in bipolar patients, with or without antidepressant co-treatment, but noted a lack of appropriate controls or randomization with which to make an adequate assessment. However, one study (62) found a higher risk of mood switching in bipolar disorder than in unipolar depression (2.50%/week compared with 0.275%/week), even though 70% of the bipolar patients were receiving antidepressant-mood stabilizer combinations, whereas most unipolar patients received an antidepressant alone. Questions about antidepressant-associated switching include that of the risks associated with many specific antidepressants and effects of dosage, for which there is insufficient evidence. For example, most studies of SSRIs have involved sertraline or paroxetine, and effects of MAO inhibitors are virtually unknown (54, 63–65).
Besides the effect of antidepressant type, the risk of switch may also vary according to bipolar type. An early study (63) suggested that patients with bipolar I disorder are at higher risk of switching than those with bipolar II disorder, a finding that was supported in a post hoc analysis from the Stanley Foundation Bipolar Network study (66). Four previously discussed studies also suggested a low risk of switching for bipolar disorder II patients, even when treated with antidepressant monotherapy (21–23, 67).
A systematic meta-analytic review of 13 prospective studies (68) supported the impression that bipolar I patients have a higher antidepressant-associated switch rate than bipolar II patients (relative risk=1.78, p=0.002), although bipolar I cases involved mania as well as the hypomania found with bipolar II disorder. These findings suggest greater clinical safety with use of antidepressants in patients with bipolar II disorder (69), although even hypomania can be problematic.
Most information regarding clinical correlates of risk of mood switching during antidepressant treatment comes from retrospective or post hoc analyses. Results of two such studies (36, 45) suggest that even subsyndromal manic symptoms at the start of antidepressant treatment as an adjunct to mood stabilizer treatment were associated with a later increased risk of switching into hypomanic or manic episodes, worsening of manic symptoms, and higher rates of unsatisfactory response to antidepressants.
Conversely, another study (70) found that a history of suicide attempt and high scores on the aggressive-disruptive behavior item of the Young Mania Rating Scale, but not the presence of subsyndromal hypomanic symptoms, at study entry were associated with a higher risk of mood switching among antidepressant-treated depressed bipolar patients.
A large long-term study (71) found that among patients in a major depressive episode, those meeting criteria for bipolar features (72, 73) showed a higher risk of later episodes of hypomania or mania and greater mood lability during antidepressant treatment. Finally, a history of antidepressant-associated mood switching, lower rates of previous clinical benefit from antidepressant treatment, and multiple previous antidepressant trials have been associated with subsequent mood switches, generally more severe illness, and worse long-term outcomes (74–76).
Regarding antidepressant monotherapy and the risk of treatment-emergent affective switches, one study (77) found that depressed bipolar patients first exposed to antidepressant monotherapy had higher switch rates and more suicide attempts than those treated with antidepressant-mood stabilizer combinations. Finally, the picture regarding genetic characteristics as predictors of mood switching in bipolar patients treated with antidepressants (78–80) is still unclear.
Conclusions.
The risk of mood switching is considered to be higher and more severe in bipolar I than bipolar II patients and somewhat greater with tri- and tetracyclics (and perhaps some SNRIs) than with most modern antidepressants. The quality of available evidence on these topics is rated C (Table 1).
Are newly emerging or increasing irritability and agitation during antidepressant treatment a form of mood switching?.
Antidepressant treatment in bipolar disorder is sometimes associated with new or worsening irritability or agitation that may or may not be related to more fully expressed mood switching (58). Such responses have been termed “antidepressant-associated chronic irritable dysphoria” and may be more likely in bipolar patients with a history of antidepressant-related mood switching (81). In addition, many cases of agitated depression in bipolar patients, often with new-onset insomnia, impulsivity, and suicidal preoccupations, have been associated with new antidepressant treatment (44). Such states can be managed effectively by discontinuing antidepressants and using mood stabilizers or other antimanic agents (36, 82, 83). Other associations include increased risk of antidepressant-associated hypomania with previous episodes with mixed features (defined as three or more hypomanic symptoms) during antidepressant treatment (84).
Conclusions.
Despite interesting proposals, the question of whether worsening of depression, including emergence or worsening of irritability or agitation with antidepressants, is related to the phenomenon of antidepressant-associated mood switching remains unsettled. The overall evidence level for this topic is D (Table 1).
Antidepressants and cycle acceleration.
Another controversial question is whether antidepressants can accelerate episode frequency or induce rapid cycling in bipolar patients (52). Several anecdotal case series suggest that antidepressant treatment may induce or prolong rapid cycling in initially depressed bipolar patients (85–88). In a prospective longitudinal study comparing the frequency and pattern of mood changes between bipolar outpatients treated with antidepressants or not (89), those treated with antidepressants were depressed on twice as many days as those not so treated (29.0% compared with 14.8%).
In a large unblinded but randomized study of effects of receiving or not receiving continued antidepressant treatment after recovery from acute depression (38), a previous rapid cycling course predicted 3.1 times more depressive recurrences with continued antidepressant treatment (1.29 compared with 0.42 episodes per year; p=0.04).
One nonrandomized study (90) found that relatively longer exposure to antidepressants than to mood stabilizers was associated with fewer weeks in euthymia, more mixed symptoms in recurrences, and more polarity changes, but with uncertain cause-effect relationships. In contrast, a 1-year randomized controlled trial of treatment of bipolar II patients who responded initially to fluoxetine (91) found no statistical differences in risk of depressive relapse or of syndromal or subsyndromal mania or hypomania, or in mania ratings, with respect to prior rapid cycling or to continuation treatment with an SSRI, lithium, or placebo. However, the study has been criticized for selecting bipolar patients with a low apparent risk of excessive mood elevation with antidepressant treatment and for using questionable criteria for rapid cycling (92).
Conclusions.
The overall quality rating of the evidence on this topic is D. Limitations include the prevalent exclusion of rapid-cycling bipolar patients from controlled treatment trials, lack of control for cycling rates, and potential confounding by lack of randomization with treatment based on clinical need (Table 1).
Antidepressants and suicidal behavior.
The question of whether antidepressants may alter the risk of suicidal behavior in bipolar patients remains particularly uncertain, as the evidence concerning suicide-promoting or -preventing effects of antidepressants in bipolar disorder is limited and inconsistent (93). Two retrospective clinical studies (77, 94) found more suicidal behaviors in patients receiving antidepressants than in those receiving mood stabilizers with or without antidepressants. A prospective study (95) of 425 bipolar patients treated with antidepressants for a new major depressive episode found no evidence of more or less new suicidal ideation or behavior with antidepressant exposure. Similarly, in 789 mood disorder patients (unipolar major depression, N=605; bipolar disorder, N=184), antidepressant treatment was not associated with altered risk of suicidal thoughts or acts, based on changes in the suicide item of the Hamilton Depression Rating Scale at the start of and after 3.6 months of sustained antidepressant treatment (96).
However, in 757 patients with unipolar or bipolar depression, during periods in which patients were exposed to antidepressants, rates of suicidal behaviors appeared to be 35%−54% lower (more in bipolar I disorder) than without antidepressant treatment (97, 98). These observations are at odds with the proposal that antidepressant treatment may increase suicidal risk in some young patients with unipolar depressive or anxiety disorders—possibly by inducing agitated-dysphoric states in those with unrecognized bipolar disorder, or as a direct toxic effect of the treatment (33, 44, 57, 99).
In 1,380 depressed bipolar I or II patients (83), the presence of subsyndromal hypomanic symptoms was associated with more previous suicide attempts, and similar results were found in another large study (84). Three recent studies have found an association of lifetime mixed episodes and higher rates of antidepressant use with increased risk of suicide behaviors (49, 50, 100). In a study of 290 patients with bipolar I or II disorder (101), suicidal ideation or acts were found to be associated with more previous antidepressant trials (or more depressive morbidity). Also, in a study of 138 children and adolescents diagnosed with bipolar disorder (102), suicidal ideation and attempts were more likely in episodes of mixed agitated-depression.
Conclusions.
Overall, the evidence of an association of either decreased or increased suicidal risk with antidepressant treatment in bipolar patients is rated D (Table 1), largely because adequate designs are unfeasible because of the relative rarity of suicide, even among mood disorder patients with suicidal thoughts, and because of the challenge of designing ethical studies of effects of treatment on suicidal risk. Currently, it remains unclear whether antidepressants might decrease or increase suicidal risk.
Consensus Statements
Based on findings from the clinical studies considered above, the available evidence concerning both the value and the risks of antidepressant treatment in bipolar disorder is remarkably limited, and much of it is methodologically weak. Therefore, it is not currently possible to make firm clinical recommendations that are soundly evidence based. There was consensus in this ISBD Task Force that non-antidepressant treatments, including lithium, lamotrigine, olanzapine, quetiapine, and lurasidone, should be considered as monotherapy before using antidepressants in bipolar depression. If antidepressants are used in bipolar I disorder, they should be prescribed along with a mood-stabilizing treatment, even though the evidence for antidepressant-associated mood switching is mixed and the ability of mood stabilizers to prevent such responses to antidepressant treatment unproven. Antidepressants in the treatment of acute depression in bipolar II disorder appear to be relatively well tolerated but may or may not be effective. Evidence for the long-term prophylactic value of antidepressant treatment for patients with bipolar I or II disorder remains poorly studied, despite the common clinical use of antidepressants, ideally in combination with a mood stabilizer. There is little evidence to support the proposition that one type of antidepressant, at clinically equivalent doses, is more or less effective or dangerous than another. Exceptions are tri- and tetracyclics and venlafaxine, which appear to carry a particularly high risk of inducing pathologically elevated states of mood and behavior.
In short, we conclude that the use of antidepressants to treat depressive phases or components of bipolar disorder can neither be condemned nor endorsed without carefully evaluating individual clinical cases and circumstances. For these reasons, the intention of this ISBD Task Force consensus is to ascertain, as far as possible, how best to use antidepressants to treat patients with bipolar disorder. No simple guidelines can be provided at this time, but clinicians are encouraged to consult our consensus-based recommendations (Table 2). We strongly encourage further research to clarify the many remaining major and urgent clinical questions concerning optimally effective and safe treatment of depressive components of bipolar disorder.
1 : Treatment-resistant bipolar depression: towards a new definition. Acta Psychiatr Scand 2009; 120:429–440Crossref, Medline, Google Scholar
2 : Treatment options for acute depression in bipolar disorder. Bipolar Disord 2012; 14(suppl 2):37–50Crossref, Medline, Google Scholar
3 : Antidepressants: the scapegoat of poor outcome bipolar disorder? Aust N Z J Psychiatry 2012; 46:302–305Crossref, Medline, Google Scholar
4 : Antidepressants in bipolar depression: much confusion, many questions, few answers. Aust N Z J Psychiatry 2012; 46:295–297Crossref, Medline, Google Scholar
5 : Bipolar antidepressant agents: shaken not stirred. Aust N Z J Psychiatry 2012; 46:289–292Crossref, Medline, Google Scholar
6 : Antidepressants in bipolar depression: the clinical debate. Aust N Z J Psychiatry 2012; 46:298–301Crossref, Medline, Google Scholar
7 National Collaborating Centre for Mental Health: Bipolar Disorder: The Management of Bipolar Disorder in Adults, Children, and Adolescents, in Primary and Secondary Care. Leicester, UK, British Psychological Society, 2006Google Scholar
8 : International Consensus Group on the evidence-based pharmacologic treatment of bipolar I and II depression. J Clin Psychiatry 2008; 69:1632–1646Crossref, Medline, Google Scholar
9 ;
10 : Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013; 15:1–44Crossref, Medline, Google Scholar
11 ;
12 : New treatment guidelines for acute bipolar depression: a systematic review. J Affect Disord 2011; 129:14–26Crossref, Medline, Google Scholar
13 : Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12Crossref, Medline, Google Scholar
14 National Health and Medical Research Council (NHMRC) of the Australian Government: NHMRC Levels of Evidence and Grades for Recommendations for Developers of Guidelines. Canberra, NHMRC, Dec 2009Google Scholar
15 : Consensus methods for medical and health services research. BMJ 1995; 311:376–380Crossref, Medline, Google Scholar
16 : Is there consensus between breast cancer patients and providers on guidelines for breaking bad news? Behav Med 1999; 25:69–77Crossref, Medline, Google Scholar
17 ;
18 : Lithium carbonate and imipramine in prevention of affective episodes: a comparison in recurrent affective illness. Arch Gen Psychiatry 1973; 29:420–425Crossref, Medline, Google Scholar
19 : Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders: report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate-imipramine combination. Arch Gen Psychiatry 1984; 41:1096–1104Crossref, Medline, Google Scholar
20 ;
21 : SSRIs as mood stabilizers for bipolar II disorder? A proof of concept study. J Affect Disord 2006; 92:205–214Crossref, Medline, Google Scholar
22 : Efficacy and mood conversion rate of short-term fluoxetine monotherapy of bipolar II major depressive episode. J Clin Psychopharmacol 2010; 30:306–311Crossref, Medline, Google Scholar
23 : Efficacy and safety of long-term fluoxetine versus lithium monotherapy of bipolar II disorder: a randomized, double-blind, placebo-substitution study. Am J Psychiatry 2010; 167:792–800Link, Google Scholar
24 : Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003; 60:1079–1088Crossref, Medline, Google Scholar
25 : Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356:1711–1722Crossref, Medline, Google Scholar
26 : Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158:906–912Link, Google Scholar
27 : A randomized trial comparing paroxetine and venlafaxine in the treatment of bipolar depressed patients taking mood stabilizers. J Clin Psychiatry 2002; 63:508–512Crossref, Medline, Google Scholar
28 : Head to head comparisons as an alternative to placebo-controlled trials. Eur Neuropsychopharmacol 2012; 22:800–803Crossref, Medline, Google Scholar
29 : Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004; 161:1537–1547Link, Google Scholar
30 : Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry 2011; 72:156–167Crossref, Medline, Google Scholar
31 : Antidepressants in acute bipolar depression: an inconclusive meta-analysis. J Clin Psychiatry 2011; 72:415–416Crossref, Medline, Google Scholar
32 : Overview of antidepressant treatment of bipolar depression. Int J Neuropsychopharmacol 2013; 16:1673–1685Crossref, Medline, Google Scholar
33 : Clinical responses to antidepressants among 1036 acutely depressed patients with bipolar or unipolar major affective disorders. Acta Psychiatr Scand 2013; 127:355–364Crossref, Medline, Google Scholar
34 : Factors associated with initial treatment response with antidepressants in bipolar disorder. Eur Neuropsychopharmacol 2011; 21:362–369Crossref, Medline, Google Scholar
35 : Relationship of prior antidepressant exposure to long-term prospective outcome in bipolar I disorder outpatients. J Clin Psychiatry 2012; 73:924–930Crossref, Medline, Google Scholar
36 : Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry 2007; 164:1348–1355Link, Google Scholar
37 : Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion, and sertraline. Br J Psychiatry 2006; 189:124–131Crossref, Medline, Google Scholar
38 : Antidepressant discontinuation in bipolar depression: a Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry 2010; 71:372–380Crossref, Medline, Google Scholar
39 : Risk of switch in mood polarity to hypomania or mania in patients with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006; 163:232–239Link, Google Scholar
40 : Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003; 160:1252–1262Link, Google Scholar
41 : Long-term antidepressant treatment in bipolar disorder: meta-analyses of benefits and risks. Acta Psychiatr Scand 2008; 118:347–356Crossref, Medline, Google Scholar
42 : Impact of antidepressant continuation after acute positive or partial treatment response for bipolar depression: a blinded, randomized study. J Clin Psychiatry 2009; 70:450–457Crossref, Medline, Google Scholar
43 : Bipolar mixed states and their treatment. Expert Rev Neurother 2005; 5:63–68Crossref, Medline, Google Scholar
44 : Melancholia agitata and mixed depression. Acta Psychiatr Scand Suppl 2007; 115:50–57Crossref, Google Scholar
45 : Correlates of treatment-emergent mania associated with antidepressant treatment in bipolar depression. Am J Psychiatry 2009; 166:164–172Link, Google Scholar
46 : The treatment of mixed states and the risk of switching to depression. Eur Psychiatry 2005; 20:96–100Crossref, Medline, Google Scholar
47 : Mixed states vs pure mania in the French sample of the EMBLEM study: results at baseline and 24 months: European Mania in Bipolar Longitudinal Evaluation of Medication. BMC Psychiatry 2009; 9:33Crossref, Medline, Google Scholar
48 : Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry 2010; 71:1000–1006Crossref, Medline, Google Scholar
49 : Bipolar mixed episodes and antidepressants: a cohort study of bipolar I disorder patients. Bipolar Disord 2011; 13:145–154Crossref, Medline, Google Scholar
50 : Mania and depression: mixed, not stirred. J Affect Disord 2011; 133:105–113Crossref, Medline, Google Scholar
51 : The relationship between use of antidepressants and resource utilization among patients with manic or mixed bipolar disorder episodes: findings from a managed care setting. J Affect Disord 2012; 138:425–432Crossref, Medline, Google Scholar
52 : Are antidepressants safe in the treatment of bipolar depression? A critical evaluation of their potential risk to induce switch into mania or cycle acceleration. Acta Psychiatr Scand 2008; 118:337–346Crossref, Medline, Google Scholar
53 : Antidepressants in bipolar depression. Acta Psychiatr Scand 2008; 118:335–336Crossref, Medline, Google Scholar
54 : Mania associated with antidepressant treatment: comprehensive meta-analytic review. Acta Psychiatr Scand 2010; 121:404–414Crossref, Medline, Google Scholar
55 : Case for caution, case for action. Bipolar Disord 2003; 5:434–435Crossref, Medline, Google Scholar
56 : ECNP consensus meeting, Bipolar depression, Nice, March 2007. Eur Neuropsychopharmacol 2008; 18:535–549Crossref, Medline, Google Scholar
57 : Switching, induction of rapid cycling, and increased suicidality with antidepressants in bipolar patients: fact or overinterpretation? CNS Spectr 2008; 13:790–795Crossref, Medline, Google Scholar
58 : Do we need to flick the switch? The need for a broader conceptualization of iatrogenic course aggravation in clinical trials of bipolar disorder. Psychiatry Clin Neurosci 2010; 64:367–371Crossref, Medline, Google Scholar
59 : The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord 2009; 11:453–473Crossref, Medline, Google Scholar
60 : A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry 1994; 55:391–393Medline, Google Scholar
61 : Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994; 164:549–550Crossref, Medline, Google Scholar
62 : Comparison of antidepressant responses in patients with bipolar vs unipolar depression: a meta-analytic review. Pharmacopsychiatry 2011; 44:21–26Medline, Google Scholar
63 : Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991; 148:910–916Link, Google Scholar
64 : Tranylcypromine vs lamotrigine in the treatment of refractory bipolar depression: a failed but clinically useful study. Acta Psychiatr Scand 2007; 115:360–365Crossref, Medline, Google Scholar
65 ;
66 : Lower switch rate in depressed patients with bipolar II than bipolar I disorder treated adjunctively with second-generation antidepressants. Am J Psychiatry 2006; 163:313–315Link, Google Scholar
67 : Efficacy and safety of fluoxetine in treating bipolar II major depressive episode. J Clin Psychopharmacol 1998; 18:435–440Crossref, Medline, Google Scholar
68 : Antidepressant-associated mood elevations in bipolar II disorder compared with bipolar I disorder and major depressive disorder: a systematic review and meta-analysis. J Clin Psychiatry 2008; 69:1589–1601Crossref, Medline, Google Scholar
69 : Bipolar II Disorder: Modelling, Measuring, and Managing, 2nd ed. Cambridge, UK, Cambridge University Press, 2012Crossref, Google Scholar
70 : Transition to mania during treatment of bipolar depression. Neuropsychopharmacology 2010; 35:2545–2552Crossref, Medline, Google Scholar
71 ;
72 : Major depressive disorder with subthreshold bipolarity in the National Comorbidity Survey Replication. Am J Psychiatry 2010; 167:1194–1201Link, Google Scholar
73 : Diagnostic criteria for bipolarity based on an international sample of 5,635 patients with DSM-IV major depressive episodes. Eur Arch Psychiatry Clin Neurosci 2012; 262:3–11Crossref, Medline, Google Scholar
74 : Risk factors for antidepressant-related switch to mania. J Clin Psychiatry 2012; 73:e271–e276Crossref, Medline, Google Scholar
75 : Bipolar depression: clinical correlates of receiving antidepressants. J Affect Disord 2012; 139:89–93Crossref, Medline, Google Scholar
76 : Self-reported history of manic/hypomanic switch associated with antidepressant use: data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). J Clin Psychiatry 2007; 68:1472–1479Crossref, Medline, Google Scholar
77 : Differential outcome of bipolar patients receiving antidepressant monotherapy versus combination with an antimanic drug. J Affect Disord 2011; 129:321–326Crossref, Medline, Google Scholar
78 : Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry 2010; 15:473–500Crossref, Medline, Google Scholar
79 : The pharmacogenetics of antidepressant-induced mania: a systematic review and meta-analysis. Bipolar Disord 2010; 12:702–706Crossref, Medline, Google Scholar
80 : Pharmacogenomics of antidepressant induced mania: a review and meta-analysis of the serotonin transporter gene (5HTTLPR) association. J Affect Disord 2012; 136:e21–e29Crossref, Medline, Google Scholar
81 ;
82 : Why antidepressants are not antidepressants: STEP-BD, STAR*D, and the return of neurotic depression. Bipolar Disord 2008; 10:957–968Crossref, Medline, Google Scholar
83 : Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. Am J Psychiatry 2009; 166:173–181Link, Google Scholar
84 : Self-assessment and characteristics of mixed depression in the French national EPIDEP study. J Affect Disord 2012; 143:109–117Crossref, Medline, Google Scholar
85 : Course of the manic-depressive cycle and changes caused by treatment. Pharmakopsychiatr Neuropsychopharmakol 1980; 13:156–167Crossref, Medline, Google Scholar
86 : Do antidepressants induce rapid cycling? A gender-specific association. J Clin Psychiatry 2003; 64:814–818Crossref, Medline, Google Scholar
87 : Factors associated with rapid cycling in bipolar I manic patients: findings from a French national study. CNS Spectr 2008; 13:780–787Crossref, Medline, Google Scholar
88 : Rapid cycling in manic-depressives induced by tricyclic antidepressants. Arch Gen Psychiatry 1979; 36:555–559Crossref, Medline, Google Scholar
89 : Mood changes related to antidepressants: a longitudinal study of patients with bipolar disorder in a naturalistic setting. Psychiatry Res 2005; 133:73–80Crossref, Medline, Google Scholar
90 : Long-term worsening of bipolar disorder related with frequency of antidepressant exposure. Ann Clin Psychiatry 2011; 23:186–192Medline, Google Scholar
91 : Efficacy and mood conversion rate during long-term fluoxetine v lithium monotherapy in rapid- and non-rapid-cycling bipolar II disorder. Br J Psychiatry 2013; 202:301–306Crossref, Medline, Google Scholar
92 : Antidepressants and rapid-cycling bipolar II disorder: dogma, definitions, and deconstructing discrepant data. Br J Psychiatry 2013; 202:251–252Crossref, Medline, Google Scholar
93 : Antidepressant use and risk for suicide attempts in bipolar disorder (letter). J Clin Psychiatry 2011; 72:1697Crossref, Medline, Google Scholar
94 : Bipolar pharmacotherapy and suicidal behavior, part 2: the impact of antidepressants. J Affect Disord 2007; 103:13–21Crossref, Medline, Google Scholar
95 : Are antidepressants associated with new-onset suicidality in bipolar disorder? A prospective study of participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). J Clin Psychiatry 2006; 67:48–55Crossref, Medline, Google Scholar
96 : Suicidal status during antidepressant treatment in 789 Sardinian patients with major affective disorder. Acta Psychiatr Scand 2008; 118:106–115Crossref, Medline, Google Scholar
97 : Antidepressants and risks of suicide and suicide attempts: a 27-year observational study. J Clin Psychiatry 2011; 72:580–586Crossref, Medline, Google Scholar
98 : Treatment effectiveness and safety in the longitudinal course of mood disorders, in The Collaborative Depression Study: What Clinicians Need to Know. Edited by Keller MBCoryell WHEndicott JLeon AC. Washington, DC, American Psychiatric Publishing (in press)Google Scholar
99 : Antidepressant-resistant depression and antidepressant-associated suicidal behaviour: the role of underlying bipolarity. Depress Res Treat 2011; 2011:906462Medline, Google Scholar
100 : Predominant recurrence polarity among 928 adult international bipolar I disorder patients. Acta Psychiatr Scand 2012; 125:293–302Crossref, Medline, Google Scholar
101 : Suicidal risk factors in bipolar I and II disorder patients. J Clin Psychiatry 2012; 73:778–782Crossref, Medline, Google Scholar
102 : Suicidality in pediatric bipolar disorder: predictor or outcome of family processes and mixed mood presentation? Bipolar Disord 2011; 13:76–86Crossref, Medline, Google Scholar



