Association of Genetic Variation in the MET Proto-Oncogene With Schizophrenia and General Cognitive Ability
Abstract
Objective
Despite increased exposure to cancer risk factors, several studies have demonstrated a lower incidence of cancer in schizophrenia patients than in the general population. Lower cancer rates in first-degree relatives of schizophrenia patients suggest that the inverse relationship between cancer and schizophrenia may be related to genetic factors. Few studies of schizophrenia have focused on cancer-related genes. The MET proto-oncogene is primarily linked to tumor metastasis, but MET is also involved in neurodevelopment and influences risk for autism. Thus, MET may be of particular interest as a candidate gene for neuropsychiatric diseases with a developmental etiology, including schizophrenia.
Method
The authors examined the relationship between 21 single-nucleotide polymorphisms in MET and schizophrenia in 173 Caucasian patients and 137 comparison subjects. They then genotyped a second independent sample (107 patients and 112 comparison subjects) for replication. Finally, they tested for MET's effects on general cognitive ability (g).
Results
In the initial cohort, the authors identified four haplotype blocks and found one block to be globally associated with schizophrenia. In block 3, the most common haplotype was overrepresented in comparison subjects (frequency, 47%) relative to schizophrenia patients (frequency, 33%) (p=4.0×10–4; odds ratio=0.56). The authors replicated the block 3 finding in the second sample with similar frequencies: 46% in comparison subjects and 36% in schizophrenia patients (p=0.03; odds ratio=0.66). Moreover, the protective haplotype was associated with a higher g in the combined comparison sample.
Conclusion
These data suggest that MET variation influences schizophrenia risk and neurocognition, supporting a neurodevelopmental role across CNS-relevant phenotypes. These results add to the growing evidence suggesting an intriguing relationship between cancer-related genes and schizophrenia susceptibility.
Several studies have demonstrated a significantly lower incidence of cancer in schizophrenia patients relative to the general population. This association is counterintuitive, given health risks in the majority of schizophrenia patients, such as heavy smoking, poor dietary habits, obesity, and substance abuse (1).
Hippisley-Cox et al. (2) conducted a study to determine the risk for six common cancers in schizophrenia patients. In a sample of 40,441 incident cases of cancer, odds ratios for cancer risk associated with schizophrenia and bipolar disorder were calculated with adjustments made for smoking, body mass index, socioeconomic status, comorbid physical conditions, and medication use. Analyses revealed a 47% lower risk for respiratory cancer in schizophrenia patients relative to patients without schizophrenia. In contrast, risk rates associated with bipolar illness in this cohort were comparable to those reported in nonpsychiatric samples, suggesting a relatively specific effect in schizophrenia. These findings are consistent with a recent meta-analysis of cancer incidence in schizophrenia patients and their first-degree relatives, which reported lower rates for a number of non-smoking-related cancers in schizophrenia patients as well as a lower risk for lung cancer after controlling for smoking prevalence (1). Taken together, these studies suggest that the discrepancy between cancer risk exposure and cancer incidence in schizophrenia is consistent with a protective effect; however, the nature of the proposed protective effect is unknown.
It has been hypothesized that decreased cancer susceptibility in schizophrenia is either related to medications used to treat the illness (3) or genetically influenced, linked with tumor suppressor genes (4) or enhanced natural killer cell activity (5). To date, results are inconsistent with regard to the potentially protective effects of antipsychotic medication, with some studies reporting decreased cancer rates in patients treated with antipsychotics (6) while others have reported an increased incidence of certain cancers (i.e., colon) in patients treated with antipsychotics compared with those not treated with these agents (2). A genetic influence on the paradoxical cancer-schizophrenia relationship is supported by family studies, in which decreased cancer rates have been reported in unaffected family members of schizophrenia patients never exposed to antipsychotic medications (1, 7–9), as well as case-control studies describing associations between schizophrenia and the tumor suppressor genes adenomatous polyposis coli (APC) (10) and p53 (11, 12). Tumor suppressor genes may act to increase risk for schizophrenia by disruption of cell growth or through abnormal apoptosis during neurodevelopment while simultaneously acting to decrease risk for cancer due to the same—in this case advantageous—apoptotic mechanism (4).
While previous studies have focused on tumor suppressor genes and their potential role in disorders such as schizophrenia, one widely studied cancer-related gene is the MET proto-oncogene, whose primary functions are related to the metastasis of several forms of cancer and peripheral organ development and repair (13–16). MET spans 125 kb on chromosome 7q31, consists of 21 exons, and codes for the MET receptor tyrosine kinase. While activation of the MET signaling pathway can result in the abnormal growth and spread of cancerous tumors, MET also plays a critical role in cortical and cerebellar development (17–20), making it a candidate gene of interest for a broad range of neuropsychiatric disorders with a neurodevelopmental etiology, including autism. Campbell et al. (21) reported that the C allele at a single nucleotide polymorphism (SNP; rs1858830) in the promoter region of MET was significantly overtransmitted to affected family members in two independent family cohorts ascertained for autism spectrum disorders (21) and subsequently replicated this finding in a third independent sample (22).
Although DSM-IV-TR criteria exclude the presence of schizophrenia and autism in the same individual (23), both disorders may be related to abnormal neurodevelopment. Although the two illnesses are clearly distinct, they have a number of clinical features in common. Autism spectrum disorders are characterized by social deficits, abnormal language development, restricted interests, and repetitive behaviors. In addition, approximately 30% of individuals with autism are significantly cognitively impaired, although the degree of impairment varies by disorder subtype (24). Similarly, schizophrenia patients also commonly display social deficits (asociality), anhedonia (23), and significant neurocognitive impairment (25). Finally, data from family and molecular genetic studies suggest an overlap between these illnesses (26).
To date, there have been no studies examining the relationship between MET and susceptibility to schizophrenia or other phenotypes common to autism and schizophrenia, such as neurocognitive impairment. Thus, we initially conducted a case-control study comprising 173 Caucasian schizophrenia patients and 137 Caucasian healthy comparison subjects. We genotyped 21 SNPs within MET and tested for an association with schizophrenia. We next carried out a replication study of the same SNPs in a second sample of 107 patients with schizophrenia and 112 healthy comparison subjects. Finally, we explored the effect of MET variation on neurocognition in a combined sample of 191 schizophrenia patients and 188 healthy comparison subjects.
Method
Participants
The initial study group included 173 Caucasian schizophrenia patients; 110 of them were male; the group's mean age was 37.7 years (SD=10.7); their mean age at illness onset was 21.4 years (SD=5.9); their mean Global Assessment of Functioning Scale (GAF) score was 39.4 (SD=17); and their mean estimated IQ, based on the Wide-Range Achievement Test, 3rd ed. (WRAT-3), was 96.5 (SD=12.4). The second sample comprised 107 schizophrenia patients; 70 of them were male; their mean age was 37.4 years (SD=11.6); their mean age at illness onset was 24.4 years (SD=8.4); their mean GAF score was 42.1 (SD=16.6); and their mean estimated IQ was 96.6 (SD=12.8). Patients for both the initial and the replication samples were recruited from the Zucker Hillside Hospital in Glen Oaks, New York. All patients provided written informed consent to the study protocol, which was approved by the hospital's institutional review board.
Caucasian healthy comparison subjects were recruited from the general population; potential participants were excluded if they had a DSM-IV axis I diagnosis or a first-degree relative with a known or suspected axis I disorder or if they had a history of CNS trauma, neurological disorder, or previously diagnosed learning disability. In the initial sample of comparison subjects (N=137), 76 were male, the mean age was 42.9 years (SD=13.0), and the mean estimated IQ was 104.2 (SD=9.4). In the replication sample of comparison subjects (N=112), 43 were male, the mean age was 54.8 years (SD=21.9), and the mean estimated IQ was 105.9 (SD=7.2).
Race was self-identified as Caucasian, and population structure was assessed using a principal components analysis approach applied to the full data set (N=365,721 SNPs passing quality control filters [27], implemented in SVS7 software [GoldenHelix, Inc., Bozeman, Mont.], using default settings derived from EIGENSTRAT [28]). The first two principal components had eigenvalues >1 (2.04 and 1.95), and the remaining eight ranged from 0.83 to 0.63. Case and comparison subjects did not differ on the first principal component (p=0.99); however, between-group differences were noted on both the second and third principal components (p values ≤0.001). These two variables were included as covariates in the association analyses described below. There were no significant differences between case and comparison subjects on any of the remaining principal components.
Diagnostic Measures
Patients' diagnoses were established with the Structured Clinical Interview for DSM-IV (SCID) (29) and confirmed by diagnostic consensus conference, which utilizes expert clinical opinion alongside SCID and corroborating medical record information. Comparison subjects were assessed with the SCID–Non-Patient Edition to rule out axis I diagnoses.
Cognitive Measures
Patients were clinically stable and were assessed for estimated intellectual functioning (WRAT-3), auditory attention and verbal working memory (WAIS-R, digit span subtest), visual attention (Continuous Performance Test–Identical Pairs Version), rapid visual search (Trail Making Test, part A), verbal learning (California Verbal Learning Test), executive functioning (letter/category fluency), and set-shifting (Trail Making Test, part B) (30). A measure of "general cognitive ability," or g, was calculated with an unrotated principal components analysis, as in our prior work (31). A single-factor model resulted, including extracted variables with eigenvalues >1.0. This single factor explained 49.5% of the variance and represented g. Cognitive analysis focused on g since global cognitive impairment is characteristic of both autism and schizophrenia, making this a phenotype of particular interest with regard to MET.
DNA Analysis
We genotyped 21 SNPs in MET on 7q31 (B36 positions 116057424–116253319) using the Affymetrix 500K chip (SA1). The Tagger program (r2=1.0) (http://www.broad.mit.edu/mpg/tagger/) was used to reduce the redundancy of the included SNPs prior to analyses; 16 SNPs were retained with frequencies >0.05. Using pairwise tagging (r2≥0.80) and downloaded data from the HapMap project CEU sample, we calculated that these 16 SNPs were able to capture 71% (70/98 alleles) of the common allelic variation in the MET region. Linkage disequilibrium structure was examined using Haploview 3.32 (32) with solid spine D′>0.80 (Figure 1). In the replication sample, identical genotyping methods were used (SA1). Phase and diplotype assignments were estimated using PHASE 2.1.1 (33) for each of the MET haplotype blocks individually and in each sample separately.

aLinkage disequilibrium (D′) for the region was computed using Haploview 3.32 (32).
Statistical Analyses
MET disease association
Haploview was used to test for the significance of each haplotype (with frequency ≥10%) within the defined blocks, and global chi-square values were calculated using the VassarStats web site (http://faculty.vassar.edu/lowry/VassarStats.html). Phased individual haplotypes were tested in SPSS, version 11.5 (SPSS, Inc., Chicago), to determine the best model for significant associations, and neurocognitive analyses used the best-fit genetic model. Correction for multiple testing was carried out using permutation testing in Haploview, including 10,000 permutations. For all association analyses, odds ratios were calculated as a measure of effect size with 95% confidence intervals.
MET association with cognition
To optimize statistical power, we tested for MET's effect on cognition using a univariate analysis of covariance first in the combined comparison sample (N=188). Secondary analyses in the schizophrenia cohort included 191 patients with complete neurocognitive data. MET diplotype status was entered as a fixed factor, and age was used as a covariate. Eta2 was calculated as an estimate of effect size.
Results
We first tested for association between MET variation and schizophrenia in the initial sample. Four haplotype blocks were identified, each consisting of three major haplotypes. The strongest association was observed in block 3, with the most common haplotype (GCAATACA) overrepresented in the comparison group (47% frequency) compared with the schizophrenia group (33% frequency) (Table 1). Post hoc analyses indicated that the best fitting model was a dominant model of inheritance, with individuals carrying at least one copy of GCAATACA being significantly less likely to develop schizophrenia as compared with those carrying no copies (χ2=13.4, p=2.5×10–4; odds ratio=0.40; 95% CI=0.24–0.65). Results remained significant after correction with permutation (corrected permutation p=0.0019). Individual SNP associations for the initial sample are presented in Table 2. Three of the SNPs survived correction, all residing in block 3; rs2237717 is located in intron 11, rs41735 is located in intron 19, and rs42336 is just downstream from the MET coding region. At each of these individual SNPs, the associated "protective" allele is the ancestral allele.
 |
For replication, we repeated the block 3 haplotype association analyses in our second independent sample and tested the effect of MET GCAATACA on disease susceptibility. The results were highly consistent with the initial analyses, at both the haplotype and SNP levels. The most common haplotype in block 3 (GCAATACA) was overrepresented in the comparison group (frequency, 46%) relative to the schizophrenia group (frequency, 36%) (Table 1). The dominant model was also significant, with individuals carrying at least one copy of GCAATACA being significantly less likely to develop schizophrenia relative to those carrying no copies (χ2=4.0, p=0.05; odds ratio=0.55, 95% CI=0.30–0.99). SNP associations for the replication sample are consistent with results from the initial sample (Table 2). To address the possible influence of population stratification, we carried out a backward stepwise logistic regression with subject type as the dependent variable and GCAATACA haplotype status, as well as the second and third principal components from the population structure analysis, as independent factors. We found that although both principal components remained in the model (p≤0.001), GCAATACA haplotype remained significant (p=0.001) (SA1).
 |
MET Association With Cognition
We next tested for an effect of MET on neurocognition. To maximize power, we merged the initial and replication data sets for all participants for whom complete neurocognitive data were available (191 schizophrenia patients and 188 comparison subjects) and tested for effects in each diagnostic group separately.
The sample characteristics are presented in Table 3 by MET GCAATACA haplotype group. The haplotype groups did not differ significantly in age in the comparison group or in the schizophrenia group, nor did they differ in estimated premorbid IQ. Significant differences were noted for sex distribution in the schizophrenia group (χ2=6.8, df=1, p=0.01) but not in the comparison group. In the schizophrenia group, illness characteristics (GAF score, age at illness onset, and duration of illness) did not differ by genotype.
 |
Univariate analyses of covariance in the combined comparison group revealed a significant effect of MET GCAATACA (F=3.99, df=1, 187, p=0.05) and age (F=16.99, df=1, 187, p<0.001) on general cognitive ability (g) (Figure 2). MET GCAATACA carriers had significantly better cognitive performance than noncarriers, with MET haplotype explaining approximately 2.1% of the variance in g. In the schizophrenia group, a similar pattern of performance was revealed, with carriers outperforming noncarriers (Figure 2), although the results did not reach statistical significance (MET GCAATACA, F=1.48, df=1, 190, p=0.23; ŋ2=0.01).
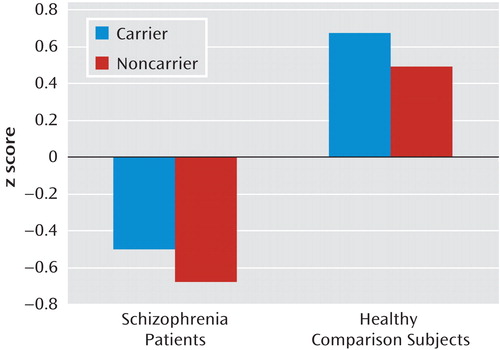
aThe x-axis labels the subject groups by MET genotype. The y-axis represents the composite g using a z score scale with a mean of 0 and a standard deviation of 1. The effect of MET GCAATACA on neurocognition is significant in the comparison group (p=0.05) but not in the schizophrenia group (p=0.23).
Discussion
We report a significant influence of genetic variation within the MET proto-oncogene and susceptibility to schizophrenia in two independent cohorts. The strongest association we observed was with a haplotype spanning the majority of the coding region of MET, as participants carrying one or more copies of the most common haplotype (GCAATACA) were significantly less likely to develop schizophrenia than those carrying no copies (odds ratio=0.40; 95% CI=0.3–0.8). These data represent the first report of an association between MET and schizophrenia and the second of an association of MET with susceptibility to neuropsychiatric illness. Campbell et al. (21) reported an association between a SNP within the promoter region of MET (rs1858830) and autism and subsequently replicated this finding and expanded the association to include several other genes within the MET pathway (22).
We also report that the MET GCAATACA genotype had a significant impact on neurocognition, such that healthy MET GCAATACA carriers in the comparison group performed significantly better than noncarriers on an empirically derived factor of general cognitive ability (g). This preliminary evidence of a role for MET in neurocognitive function may shed light on the mechanism through which variation in MET might influence risk for both autism and schizophrenia, as the phenotype of neurocognitive impairment is common to both diseases. These data could be interpreted to indicate that the influence of MET on disease susceptibility might not be specific to risk for schizophrenia or autism. Rather, these findings may be reflective of the effects that MET may have on brain function in general. This potential explanation warrants follow-up in other clinical disorders characterized by brain morphological abnormalities, neurodevelopmental pathology, or cognitive impairment.
The role of MET in cancer has been definitively established. There are several mechanisms by which MET influences cancer development and progression: 1) overexpression of MET—the most common alteration of MET in human tumors; 2) autocrine/paracrine activation in which MET is activated by its ligand, hepatocyte growth factor, which may be abnormally produced by cancer cells; 3) hepatocyte growth factor-independent activation via transactivation by other membrane receptors; and 4) MET structural alterations, such as missense mutations, which result in hereditary forms of cancer. Regardless of the mechanism by which MET activity is altered, increased MET activation is associated with abnormal tissue growth related to tumor development and metastasis. In contrast, MET activation plays a beneficial role during normal physiological states and is critical to normal cortical and cerebellar development (17–20). Disrupted MET signaling in the cerebral cortex results in a lower number of interneurons and abnormal interneuron migration from the ganglionic eminence (17, 18). In the cerebellum, reduced MET signaling decreases proliferation of granule cells, resulting in cerebellar volume reductions, especially in the vermis (19). Abnormalities that result from perturbation of this system are consistent with known changes in the brains of schizophrenia patients, including dysfunction and a lower number of inhibitory interneurons in the prefrontal cortex (34) and volumetric reductions in the vermis, both of which are correlated with cognitive dysfunction (35). Taken together, these data suggest that MET may influence risk for disorders such as autism and schizophrenia via a decreased level of pathway activity during critical periods of neurodevelopment. This hypothesis is supported by data from Campbell et al. (21), who found that an autism risk allele (C) at rs1858830 was associated with a reduction in MET transcription. A subsequent study by the same group demonstrated that MET mRNA expression was decreased in the postmortem brain tissue of individuals with autism and that the level of MET expression was associated with rs1858830 in healthy comparison subjects (36). In the present study, minor alleles of multiple intragenic SNPs (such as rs42336) were associated with an elevated risk for schizophrenia; these alleles, or perfect proxies of them, have similarly been associated with reduced gene expression in prior studies (37, 38).
These data implicating the MET gene in schizophrenia, coupled with the autism data, are consistent with a recent study that used computational probabilistic modeling to investigate the relationship among multiple common disorders that might be likely to share genetic susceptibility, including cancer, autism, and schizophrenia. Rzhetsky et al. (39) proposed a disease network hypothesis based on an analysis of more than 1.5 million patient records from a clinical database and tested whether a genetic variant that predisposes an individual to a given disease may increase or decrease the risk for multiple other diseases. As in many Mendelian disorders, the tested hypothesis was that complex phenotypes are probably explained by genetic variation that is shared, in either a competitive or a cooperative manner, by multiple disease phenotypes. Among 161 disorders studied, a highly connected network of pairwise correlations emerged, including a strong positive correlation between autism and schizophrenia (p=5.78×10–11), suggesting significant genetic overlap, such that approximately 20%–75% of autism-predisposing variants were estimated also to predispose to schizophrenia. Of particular note, a strong inverse relationship between schizophrenia and breast cancer was also observed in these analyses (p=1.22×10–10) (39), providing support for the hypothesis that genetic variation at a single locus, such as MET, may have both shared and divergent effects across different phenotypes.
A specific example of the potential pleiotropic effects of MET is provided by a recent study (40) that investigated the effect of the MET promoter SNP rs1858830 on susceptibility to autism in 214 autism families that were characterized for gastrointestinal comorbid conditions. MET rs1858830 was associated with an increased risk for autism and increased susceptibility to gastrointestinal disease in 118 families with at least one child with co-occurring autism and a gastrointestinal condition; however, no association with autism was observed in families with children who did not have a comorbid gastrointestinal disease. These data provide preliminary evidence that decreased activity in the MET pathway may have both central and peripheral effects, contributing to abnormal brain development (autism) as well as dysfunctional organ repair (40).
Our study has a number of limitations. First, medications may confound analyses in samples of patients with chronic schizophrenia; however, we would not expect that medication type or dosage would differ significantly by genotype, as the severity of illness was comparable between groups in our sample. We did not have sufficient data on medication dosage to conduct a formal test of this question. Second, the genotyped markers were selected using an Affymetrix 500K chip based on genomic spacing; we were able to capture only 71% of the common allelic variation within the MET region using the 16 SNPs analyzed. The use of genome-wide association methods raises the issue as to which analyses are considered appropriate in the context of statistically nonsignificant genome-wide results. In the strictest statistical sense, analytic follow-up of results that do not meet the genome-wide threshold after correction may not be considered valid. However, we suggest that genes such as MET, with prior data suggestive of biological plausibility related to pathophysiology, also warrant follow-up, especially given the previous association with autism and the consistency of our results in two independent samples. Finally, we acknowledge that the magnitude of the effect of MET on both disease susceptibility and neurocognitive function is relatively small, which is consistent with the complexity of the genetic architecture of schizophrenia and its related phenotypes.
The data we present here highlight the importance of assessing molecular networks that may be implicated in the pathophysiology of multiple CNS disorders with overlapping phenotypes. Our results also provide evidence for at least one potential genetic locus that may explain both a cooperative polymorphism model (increasing risk for both schizophrenia and autism) and a competitive polymorphism model (increasing risk for schizophrenia and protecting against cancer) via a specific hypothesized biological mechanism.
1 : Cancer incidence in patients with schizophrenia and their first-degree relatives: a meta-analysis. Acta Psychiatr Scand 2008; 117:323–336 Crossref, Medline, Google Scholar
2 : Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Arch Gen Psychiatry 2007; 64:1368–1376 Crossref, Medline, Google Scholar
3 : Are antipsychotic drugs potentially chemopreventive agents for cancer? Eur J Clin Pharmacol 1999; 55:487–488 Crossref, Medline, Google Scholar
4 : Apoptosis and schizophrenia: is the tumour suppressor gene, p53, a candidate susceptibility gene? Schizophr Res 2000; 41:405–415 Crossref, Medline, Google Scholar
5 : Higher natural killer cell activity in schizophrenic patients: the impact of serum factors, medication, and smoking. Brain Behav Immun 2000; 14:153–169 Crossref, Medline, Google Scholar
6 : Cancer risk among users of neuroleptic medication: a population-based cohort study. Br J Cancer 2006; 95:934–939 Google Scholar
7 : Cancer risk among parents and siblings of patients with schizophrenia. Br J Psychiatry 2007; 190:156–161 Crossref, Medline, Google Scholar
8 : Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 2001; 58:573–578 Crossref, Medline, Google Scholar
9 : Risk for cancer in parents of patients with schizophrenia. Am J Psychiatry 2004; 161:903–908 Link, Google Scholar
10 : The tumor suppressor adenomatous polyposis coli gene is associated with susceptibility to schizophrenia. Mol Psychiatry 2005; 10:669–677 Crossref, Medline, Google Scholar
11 : Differences in p53 gene polymorphisms between Korean schizophrenia and lung cancer patients. Schizophr Res 2004; 67:71–74 Crossref, Medline, Google Scholar
12 : Tumor suppressor gene TP53 is genetically associated with schizophrenia in the Chinese population. Neurosci Lett 2004; 369:126–131 Crossref, Medline, Google Scholar
13 : Met, metastasis, motility, and more. Nat Rev Mol Cell Biol 2003; 4:915–925 Crossref, Medline, Google Scholar
14 : Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J Pharmacol Exp Ther 2003; 307:146–151 Crossref, Medline, Google Scholar
15 : HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem 2003; 88:408–417 Crossref, Medline, Google Scholar
16 : Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA 2004; 101:4477–4482 Crossref, Medline, Google Scholar
17 : Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron 2001; 30:79–89 Crossref, Medline, Google Scholar
18 : Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci 2003; 23:622–631 Crossref, Medline, Google Scholar
19 : Viable hypomorphic signaling mutant of the Met receptor reveals a role for hepatocyte growth factor in postnatal cerebellar development. Proc Natl Acad Sci USA 2002; 99:15200–15205 Crossref, Medline, Google Scholar
20 : Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci 2004; 27:400–406 Crossref, Medline, Google Scholar
21 : A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci USA 2006; 103:16834–16839 Crossref, Medline, Google Scholar
22 : Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Res 2008; 1:159–168 Crossref, Medline, Google Scholar
23 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Text Revision (DSM-IV-TR). Washington, DC, American Psychiatric Association, 2000 Google Scholar
24 : Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry 2005; 162:1133–1141 Link, Google Scholar
25 : How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull 2007; 33:912–920 Crossref, Medline, Google Scholar
26 : Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics 2008; 121:1357–1362 Crossref, Google Scholar
27 : Converging evidence for a pseudoautosomal cytokine receptor gene locus in schizophrenia. Mol Psychiatry 2007; 12:572–580 Crossref, Medline, Google Scholar
28 : Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 2006; 38:904–909 Crossref, Medline, Google Scholar
29 : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995 Google Scholar
30 : A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 2nd ed. New York, Oxford University Press, 1998 Google Scholar
31 : Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet 2006; 15:1563–1568 Crossref, Medline, Google Scholar
32 : Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21:263–265 Crossref, Medline, Google Scholar
33 : A comparison of Bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet 2003; 73:1162–1169 Crossref, Medline, Google Scholar
34 : Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6:312–324 Crossref, Medline, Google Scholar
35 : Morphological correlates to cognitive dysfunction in schizophrenia as studied with Bayesian regression. BMC Psychiatry 2006; 6:31 Crossref, Medline, Google Scholar
36 : Disruption of cerebral cortex MET signaling in autism spectrum disorder. Ann Neurol 2007; 62:243–250 Crossref, Medline, Google Scholar
37 : Allelic variation in gene expression is common in the human genome. Genome Res 2003; 13:1855–1862 Crossref, Medline, Google Scholar
38 : Allelic imbalance in gene expression as a guide to cis-acting regulatory single nucleotide polymorphisms in cancer cells. Nucleic Acids Res 2007; 35:34 Crossref, Google Scholar
39 : Probing genetic overlap among complex human phenotypes. Proc Natl Acad Sci USA 2007; 104:11694–11699 Crossref, Medline, Google Scholar
40 : Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics 2009; 123:1018–1024 Crossref, Medline, Google Scholar



