Sex Differences in the Functional Neuroanatomy of Working Memory in Adults With ADHD
Abstract
Objective
Although attention deficit hyperactivity disorder (ADHD) in adults is associated with significant morbidity and dysfunction and afflicts both sexes, relatively few imaging studies have examined female subjects and none have had sufficient power to adequately examine sex differences. The authors examined sex differences in the neural functioning of adults with ADHD during performance of a verbal working memory task.
Method
The participants were 44 adults with ADHD matched on age, sex, and estimated IQ to 49 comparison subjects. Accuracy and reaction time on an N-back task were measured to assess working memory. The blood-oxygen-level-dependent functional MRI response was used as a measure of neural activity.
Results
A group-by-sex analysis of variance showed no between-group differences in either reaction time or percent correct for the working memory task. For both sexes combined, the adults with ADHD showed less activity than comparison subjects in prefrontal regions. However, sex-by-group analyses revealed an interaction, such that male ADHD subjects showed significantly less activity in right frontal, temporal, and subcortical regions and left occipital and cerebellar regions relative to male comparison subjects, whereas female ADHD subjects showed no differences from female comparison subjects. Exploratory correlation analyses revealed negative associations between working-memory-related activation and number of hyperactive symptoms for men and number of inattentive symptoms for women.
Conclusions
Male but not female adults with ADHD showed significantly altered patterns of neural activity during a verbal working memory task. Men and women showed different associations between neural activity and ADHD symptoms.
Attention deficit hyperactivity disorder (ADHD) is characterized by age-inappropriate symptoms of inattention and/or hyperactivity or impulsivity, and it is estimated to affect approximately 5% of adults (1, 2). Adults with ADHD show more psychiatric comorbidities and impairments in psychosocial, educational, neurocognitive, and occupational functioning than healthy comparison subjects (3).
Most ADHD research, including structural and functional neuroimaging, has used males. A recent meta-analysis (4) of structural imaging studies of ADHD showed that over 80% of the subjects were male and approximately 50% of the studies used 100% male study groups. A review of the literature on functional imaging showed that most studies included either all male (5, 6) or mostly male (7) study groups. In a meta-analysis of 16 functional imaging studies (8), 10 ADHD study groups were 100% male and four were mostly male. In contrast to the large number of all-male imaging studies, we found only one structural imaging study (9) and two functional imaging studies (10, 11) whose participants were solely females. Clinically, males with ADHD are more likely to have comorbid learning disabilities, disruptive behavior, social dysfunction, and depression, whereas females have a higher rate of substance use disorders and are twice as likely to have the inattentive subtype of the disorder (12). Given these differences in clinical phenomenology, the limited neuroimaging literature on females is problematic, in part because we do not know whether what has been discovered for males will also apply to females.
Findings based on males do not necessarily generalize to females, possibly because of sexual dimorphisms observed in the "normal" brain (13, 14). For example, it has been suggested that the greater bilateral functioning of the female brain may be protective for certain neurological insults (15). Thus, understanding sex differences in psychiatric disorders could have significant implications, e.g., for treatment (15). With increasing recognition that ADHD occurs in females across the lifespan (16) and that the rates in males and females are more comparable among adults, i.e., 1.5:1 (17), than among children, it is critical that female study groups be well represented and that the effect of sex be carefully examined. To help address this issue, we conducted a functional imaging study with adult ADHD subjects of both sexes.
In the ADHD functional imaging literature, there are only a few studies that examine potential effects of sex and/or use enough females to sufficiently analyze data on females. A positron emission tomography (PET) study of adolescents with ADHD (18) suggested sex differences in the cerebral metabolic rate of glucose when it showed that the female subjects with ADHD had lower glucose metabolism rates than female comparison subjects and male adolescents with ADHD. However, this result from a small number of subjects (five girls with ADHD, six comparison girls) was not replicated in an expanded group with nearly twice as many girls (10). Sheridan and colleagues (11) used functional magnetic resonance imaging (fMRI) to study prefrontal cortex functioning during working memory tasks in adolescent girls with and without ADHD and found no between-group differences in neural activation collapsed across working memory load. In contrast, a meta-analysis (8) of ADHD functional imaging studies, which included mostly male study groups, found activation differences between ADHD and comparison subjects in several brain regions, with some of the most robust differences in frontal areas. This suggests that the effects of ADHD in prefrontal and possibly other brain regions may be different for females and males.
In a previous investigation, we used an N-back task with fMRI to examine the neural underpinnings of working memory in ADHD (19). We found that, relative to the comparison subjects, adults with ADHD showed less activation in cerebellar and occipital regions, with nonsignificantly lower activation in the right prefrontal cortex. We have since expanded that study group substantially to examine sex effects.
Theories of ADHD pathophysiology postulate abnormalities in the right prefrontal cortex (20), including frontal hypofunction (8) and right frontal-striatal-cerebellar abnormalities (21). On the basis of such theories and our previous findings (19), we predicted hypoactivity for adults with ADHD relative to comparison subjects in right frontal and (contralaterally connected) left cerebellar regions for the entire ADHD group. We also predicted that these functional differences would be smaller for the women with ADHD than for the men based on the absence of significant functional hypoactivity in females with ADHD, relative to female comparison subjects (10, 11), whereas hypoactivity has been observed in male or mostly male ADHD groups, relative to male comparison subjects, in studies with nearly equivalent or smaller study groups (5, 22). We also conducted exploratory analyses to assess the correlation between ADHD symptoms and neural activation associated with performance on the working memory task for men and women separately.
Method
Participants
The subjects were 44 adults with ADHD and 49 comparison subjects similar in age, sex, handedness, and estimated IQ. Of these 93 subjects, 19 with ADHD and 13 comparison subjects (34%) were included in a preliminary report (19). These subjects are part of a larger fMRI study that recruited subjects from studies of ADHD conducted at Massachusetts General Hospital and from advertisements posted in the Boston area. Written informed consent was obtained for all subjects. The study was approved by the Partners Human Research Committee.
We excluded subjects if they 1) were younger than 18 or older than 55, 2) had an estimated full-scale IQ below 80, 3) had a current psychotic disorder, 4) had current alcohol or substance abuse or dependence or a chronic history of abuse or dependence as defined by clinician review of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (23), 5) had an inadequate command of English, 6) had sensorimotor handicaps or neurological disorders, 7) had contraindications to MRI, or 8) were currently taking psychotropic medications. Of the 44 participants with ADHD, 27 had taken psychostimulants in their lifetime. Nine had received such treatment only in the past, and the 18 who were currently being treated with stimulants underwent a 24-hour washout period before scanning.
The ADHD and comparison subjects received identical assessments (consistent with numerous previous studies from this laboratory, e.g., 19). To assess psychopathology we administered the SCID (23). To assess ADHD, we used a module derived from the Schedule of Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E) (24). This module systematically acquires information on all DSM-IV ADHD symptoms, measures, and domains of impairment and on age at onset. Previous work has shown that retrospective childhood diagnoses of ADHD can be made in a reliable and valid manner by using this method (25, 26). We considered a subject positive for ADHD if the DSM-IV diagnostic criteria were met in childhood and persisted into adulthood. At the time of the clinical interview, the distribution of ADHD subtypes was as follows: combined, N=13 (46% male); inattentive, N=17 (65% male); hyperactive/impulsive, N=1 (female); and not otherwise specified, N=13 (46% male). The subjects with ADHD not otherwise specified missed the DSM-IV symptom threshold at the time of the interview but otherwise met the criteria.
To assess current depression and anxiety we administered the Profile of Mood States (POMS) (27) on the day of the scan (see online supplemental text for details). Cognitive testing included subtests from the WAIS-III (28).
Working Memory and Control Tasks for fMRI
We used a block design with a 2-back variant of a visual N-back task with sequential letters (29), which has been used and described by us in previous neuroimaging studies (e.g., 19); a description appears in the online supplemental text. Briefly, in the vigilance control "X-task," the letter "X" was the target. In the working memory 2-back task, a letter was a target if it was the same as the letter that was presented two trials (i.e., letters) previously, or "2 back."
Demographic and Behavioral Data Analysis
We used 2×2 analyses of variance (ANOVAs) with group (ADHD versus comparison) and sex as factors when examining differences in demographic variables and task performance.
fMRI Data Acquisition and Analysis
Imaging was performed on a Siemens Sonata 1.5-T full-body scanner. fMRI was performed by using a gradient-echo echo-planar imaging pulse sequence (21 axial slices, TR=2000 msec, 5-mm thickness, 1-mm interslice interval, TE=40 msec, flip angle=90°, 168 images/run).
The fMRI data were analyzed by using Statistical Parametric Mapping (SPM2, Wellcome Department of Cognitive Neurology, London). Preprocessing included correction for head motion, spatial normalization, and spatial smoothing with a Gaussian filter (8-mm full width at half maximum [FWHM]). Runs exhibiting a spike of more than 3 mm of scan-to-scan head motion and/or stimulus-correlated motion (r>0.5) were dropped. Consequently, eight runs were dropped from each of the comparison and ADHD groups.
After preprocessing, statistical analysis was performed at the single-subject level. Each epoch of trials was modeled by using a boxcar function convolved with a canonical hemodynamic response function (30). Low-frequency components of the blood-oxygen-level-dependent (BOLD) fMRI signal were modeled as confounding covariates (30). Our contrast of interest was the activation associated with the 2-back task minus the activation associated with the X-task, as in previous studies (19, 29). All contrast values were saved for use in group analyses and submitted to a second-level analysis in which subjects were treated as a random effect. A 2×2 ANOVA (using a four-group one-way ANOVA as described in reference 31) with group and sex as factors was conducted to assess for effects in the BOLD response, which was used as a measure of neural activity. (We view fMRI-related findings as reflecting neural activation [32]). Whole-brain analyses were conducted to assess for the effects of group (comparison>ADHD and ADHD>comparison) and sex (men>women and women>men) and the group-by-sex interaction ([comparison men – ADHD men] > [comparison women – ADHD women] and [comparison women – ADHD women] > [comparison men – ADHD men]). We had an a priori hypothesis that, relative to the comparison group, the adults with ADHD would have lower levels of activation in the right dorsolateral prefrontal cortex because 1) this type of N-back task has been shown to rely on the dorsolateral prefrontal cortex (29), 2) ADHD theories postulate right frontal cortex abnormalities (20, 21), 3) relative to comparison subjects, men with ADHD have been shown to have less activation in prefrontal regions (8), and 4) we observed a nearly significant difference in this area previously (19). Therefore, we conducted region-of-interest analyses for the right dorsolateral prefrontal cortex using the same center coordinates (x=33, y=39, z=21) used previously (19), which were based on the original N-back study of Cohen et al. (29). According to anatomical and MRI studies, our region of interest, approximating Brodmann's areas 46 and 9, extends for approximately 40 mm in both the anterior/posterior and vertical dimensions (33, 34). We therefore used a 20-mm radius.
Analyses of covariance (ANCOVAs) were conducted to assess confounding effects of depression, tension or anxiety, and age on the interaction effects. For all group analyses, statistical maps were thresholded for cluster-based analyses by using a height threshold of p=0.005 (uncorrected) and an extent threshold determined by Gaussian random field theory; this conjoint thresholding provides p values that are corrected for the entire volume (30). Clusters were reported as significant for p<0.05 (corrected, on the basis of Gaussian random field theory [30]). We conducted simple regressions to explore relationships between neural activation and ADHD symptoms.
Results
Demographic Characteristics
There were no significant differences in the group-by-sex ANOVAs for age or estimated IQ (Table 1), and the adults with ADHD and comparison subjects did not significantly differ in sex ratio (χ2=0.26, df=1, p>0.05). Group-by-sex ANOVAs for current levels of depression and anxiety showed group effects on the POMS scores for depression (F=14.1, df=1, 89, p<0.001) and for tension or anxiety (F=12.6, df=1, 89, p<0.005) and a sex effect for depression (F=13.0, df=1, 89, p<0.005) but no interactions. The subjects with ADHD had higher levels of depression and anxiety than the comparison subjects, and the men had higher levels of depression than the women.
 |
Behavioral Data Analyses
No significant differences were found in the group-by-sex ANOVAs for any of the task performance measures (Table S1 in online data supplement).
fMRI Contrast of 2-Back Task and X-Task
Effect of group (ADHD versus comparison subjects). Whole-brain analyses showed that, relative to the comparison subjects, the adults with ADHD had less activation in one left prefrontal cluster. The analysis of the right prefrontal region of interest showed that the ADHD subjects had less activation than the comparison subjects within the right prefrontal region (Table 2, Figure 1). There were no regions for which the ADHD subjects showed greater activation than the comparison subjects.
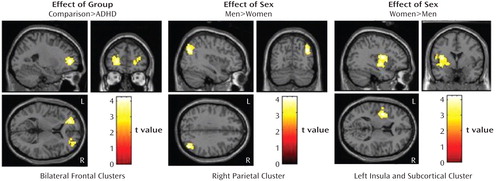
aBased on the 2×2 ANOVA of the difference in activation between a 2-back working memory task and a control task (X-task). There were no regions for which the ADHD subjects showed greater working-memory-related activation than the comparison subjects. In the coronal view, the left side of the image is the left hemisphere. The clusters shown were significant at p<0.05 (corrected). Comparison subjects, N=49; ADHD subjects, N=44; men, N=46; women, N=47.
 |
Effect of sex (men versus women). The men had significantly greater activation than the women in the right inferior parietal lobule; the women showed significantly greater activation than the men in the left insula, putamen, and pallidum (Table 2, Figure 1).
Group-by-sex interaction (comparison–ADHD difference in men versus comparison–ADHD difference in women). A significant group-by-sex interaction indicated that the comparison-versus-ADHD difference was greater for the men than for the women in frontal, temporal, cerebellar, occipital, and subcortical regions (Table 3, Figure 2).
 |
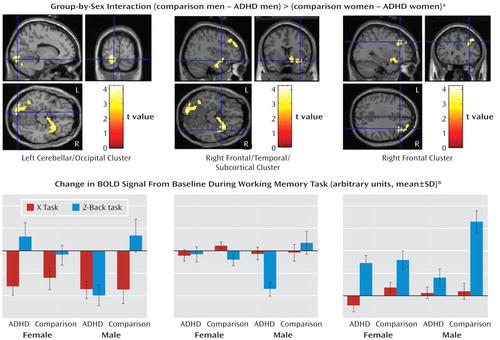
aBased on the 2×2 ANOVA of the difference in activation between a 2-back working memory task and a control task (X-task). The regions depicted are where the group difference (comparison minus ADHD) for working-memory-related activation (2-back task minus X-task) for men was significantly greater than the group difference for women (comparison minus ADHD). The right subcortical/prefrontal and left cerebellar/occipital pattern of differences can be seen in the axial images. The crosshairs indicate the location of the clusters labeled beneath each image. There were no regions for which the difference between the female groups was larger than the difference between the male groups. In the coronal view, the left side of the image is the left hemisphere. The clusters shown were significant at p<0.05 (corrected). Comparison men, N=23; ADHD men, N=23; comparison women, N=26; ADHD women, N=21.
bEach bar represents the beta value for all voxels averaged within each cluster. The primary differences between diagnostic groups are in levels of activation for the men with ADHD while performing the 2-back task. Across all three regions, the men with ADHD have less activation or more deactivation than any of the other three groups. Also, all four groups show relatively similar patterns of activation for the X-task. The groups do not differ much, if at all, from zero on this task in either the frontal or the frontal/subcortical cluster. However, all four groups show deactivations for the X-task in the occipital/cerebellar cluster. There were no significant group-by-sex interactions for the X-task.
To determine whether these interaction effects were due to the male comparison subjects showing greater activation than the men with ADHD, we masked the simple effect of diagnosis in men (comparison men > ADHD men) with the interaction map (using analyses of the whole brain and the frontal region of interest). These results indicated that for nearly all the regions significant in the interaction, including the right frontal and subcortical as well as left cerebellar and occipital regions (Table 3), the men with ADHD had less activation than the male comparison subjects. There were no regions for which the female between-group differences in the sex-by-group interaction were greater than the male between-group differences, nor were there significant differences between the female groups (see online supplemental text).
ANCOVAs for depression, tension or anxiety, and age revealed that these variables were not accounting for the interaction effects. Comparisons of groups with different psychostimulant histories also revealed no differences between groups. (See online supplemental text for details on these and other analyses.)
Exploratory Symptom Correlation Analyses
Analyses of the correlation of the contrast in BOLD signal between the 2-back and X-tasks with the number of hyperactive symptoms for the men with ADHD showed a negative correlation in one cluster encompassing cerebellar and occipital regions (maximum voxel correlation, r=–0.74); see Table S2 and Figure S1 in the online data supplement. There were no correlations with inattentive symptoms.
In contrast, the women with ADHD showed a negative correlation between inattentive symptoms and neural activation in a cluster spanning numerous cortical and subcortical brain regions (maximum voxel correlation, r=–0.84); see Table S2 and Figure S1 in the online data supplement. There were no correlations with hyperactive symptoms.
Discussion
We used fMRI and a working memory task to examine sex differences in neural activation in adults with ADHD. Interaction analyses revealed greater differences in neural activation between male groups (ADHD versus comparison) than between female groups. Whereas the men with ADHD showed less activation in various brain regions than the comparison men, the women with ADHD showed no significant differences from the comparison women. To our knowledge, this is the first fMRI study to demonstrate significant sex differences in persons with ADHD.
These findings expand working memory findings in ADHD adults and highlight the importance of obtaining large enough study groups to assess sex differences. When our male and female subjects were combined, only regions in the bilateral prefrontal cortices differed significantly between the adults with ADHD and the comparison subjects. However, examination of the sexes separately revealed that compared to matched comparison subjects of the same sex, men and women with ADHD had different patterns of neural activation for this N-back task. In relation to the comparison subjects, the men with ADHD showed less activation in a network of brain regions that was lateralized to right frontal and subcortical regions and left occipital and cerebellar regions. This pattern of neural activation differences is consistent with hypotheses and other work suggesting right frontal abnormalities in ADHD (19, 20), as well as a pre-established hypothesis regarding abnormal cerebellar-prefrontal-striatal networks in ADHD (21), at least in males.
Relative to the female comparison subjects, the women with ADHD did not show neuroimaging differences while performing the working memory task. Nonetheless, they had levels of ADHD symptoms and behavioral performance similar to those in the men with ADHD. These findings suggest several possibilities. For example, it could be that the functional neuroanatomical abnormalities associated with certain cognitive functions in females with ADHD are not as severe as they are in males or that the neural network associated with the clinical phenomenology of ADHD is not also associated with performance or brain activation related to this working memory task in females. It could also be that normal sexual dimorphisms protect females on this particular task.
These negative findings for women with ADHD are largely consistent with the only two functional imaging studies of which we are aware that used all-female study groups with ADHD. In one study, there were no differences between adolescent girls with ADHD and comparison girls in cerebral glucose metabolism while performing an attention task (10). In another study (11), there were no differences in degree of functional activation across working memory load between adolescent girls with ADHD and comparison girls. Thus, these data indicate a lack of differences between ADHD and comparison females in regions shown to have relatively robust differences in studies using mostly or all male subjects (8). Nonetheless, these previous studies of females examined adolescents, and thus the studies' effects could be weaker because of varying developmental stages.
As in previous research (11, 19, 35, 37), there were no significant differences in behavioral task performance between the ADHD subjects and the comparison subjects. The lack of behavioral differences in our study was by design. First, we matched on IQ, which also partially matched on working memory since these two variables are often significantly correlated (38); in this study group there was a significant correlation (r=0.51, N=90, p<0.001) between IQ and the percent of correct responses on the 2-back task. Second, the mean IQs of our ADHD and comparison groups were above average (118 and 114, respectively), increasing the likelihood that the subjects would perform the task well given the significant correlation between working memory and IQ. Finally, as has been recommended (39), we chose a task on which we expected our subjects to perform well, so that we could interpret the neuroimaging findings without the confound of additional error-related activation for the ADHD group. Despite the absence of behavioral differences, we found differences in brain functioning between the male, but not female, ADHD and comparison groups in regions that comprise a network predicted to be abnormal in ADHD. This finding supports the hypothesis that ADHD affects males and females differently. It is possible that the imaging data are a more sensitive indicator of an alteration in functioning than are the behavioral data, as our findings are consistent with those from several other ADHD imaging studies in which functional differences were found despite no significant differences in behavioral performance (19, 22, 37). It is also possible that neural reorganization and/or different functional connectivity patterns, which may be idiosyncratic or diffuse, could have developed to compensate for the lower activation observed in the men with ADHD relative to their comparison subjects. Future research employing parametric versions of this task with greater difficulty may help to clarify the complex relationship between working memory demand, performance deficits, and neural processing in adults with ADHD.
Exploratory analyses demonstrated that neural activity during the working memory task showed negative correlations between brain regions and the number of hyperactive symptoms in men and inattentive symptoms in women. Consistent with these findings are results from previous studies with mostly or all male subjects that showed negative correlations between the BOLD signal and hyperactive symptoms and no correlations with inattentive symptoms (if examined) (6, 22). Overall, this suggests a negative relationship between task-related neural activity and severity of ADHD that may differ by sex and symptom subtype. Understanding more about these correlations could lend insight into why females are more likely than males to have the inattentive subtype of ADHD (12).
Our findings need to be considered in light of methodological limitations. Although no participants in this study were taking psychotropic medications at the time of the scan and the numbers of stimulant-naive subjects were approximately equal across sexes, there were variable histories of past psychotropic medication use. However, analyses of the imaging data in groups with different psychostimulant histories (i.e., past, never, or current use) revealed no differences. Also, a portion of our subjects no longer met the DSM-IV symptom threshold criterion at the time of the interview. These subjects were fairly evenly distributed across sexes, and a subthreshold number of symptoms is common in adult ADHD (40). If anything, we would predict that inclusion of subthreshold subjects would result in weaker study effects, rather than creating spurious significant effects. Furthermore, the diagnoses of adult ADHD relied on self-report based on an interview derived from the K-SADS-E. Nonetheless, we are confident in our diagnoses as there is substantial evidence for the validity of diagnoses made in this manner (26). Additionally, there were variable rates of lifetime comorbidities for depression and anxiety. Using current POMS levels of depression and anxiety as covariates in the analyses had no significant effect on our results, indicating that these variables were not accounting for the observed differences. Even so, we used the POMS, and other popular scales that would have assessed mood differently, such as the Hamilton scale, could have been used. Also, given our subjects' higher than average mean IQs, it is unclear how these results would generalize to adults with ADHD who have average mean IQs. However, a substantial portion of our subjects had IQs in the "normal" range. Future work should examine the effects of IQ on neural functioning. Finally, we reiterate that we interpreted changes in the BOLD response as neural activation. Also, spatial resolution was limited by the 8-mm FWHM smoothing filter.
In sum, relative to comparison subjects, we found less neural activity in cortical, subcortical, and cerebellar regions for male but not female adults with ADHD during performance on a working memory task. These data suggest that it is important to take sex into account when studying the functional neuroanatomy of ADHD, as failure to do so may mask important neural differences observed solely in males or females. These findings also indicate that neuroimaging findings from studies using mostly or all males, which account for most studies, may not necessarily apply to females. Thus, these results may be important with regard to treatment issues for females. Follow-up studies using frontal, cerebellar, and striatal regions of interest could be fruitful.
1 : The scientific foundation for understanding attention-deficit/hyperactivity disorder as a valid psychiatric disorder. Eur Child Adolesc Psychiatry 2005; 14:1–10 Crossref, Medline, Google Scholar
2 : The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006; 163:716–723 Link, Google Scholar
3 : Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry 2005; 57:1215–1220 Crossref, Medline, Google Scholar
4 : Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 61:1361–1369 Crossref, Medline, Google Scholar
5 : Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiatry 2006; 60:1062–1070 Crossref, Medline, Google Scholar
6 : Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry 2005; 162:1067–1075 Link, Google Scholar
7 : Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry 2006; 163:1052–1060 Link, Google Scholar
8 : The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry 2006; 47:1051–1062 Crossref, Medline, Google Scholar
9 : Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001; 58:289–295 Crossref, Medline, Google Scholar
10 : Cerebral glucose metabolism in adolescent girls with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:1399–1406 Crossref, Medline, Google Scholar
11 : Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2007; 46:1357–1366 Crossref, Medline, Google Scholar
12 : Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry 2002; 159:36–42 Link, Google Scholar
13 : Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Res 1994; 1:175–183 Crossref, Google Scholar
14 : Sex differences in brain-behavior relationships between verbal episodic memory and resting regional cerebral blood flow. Neuropsychologia 2000; 38:451–461 Crossref, Medline, Google Scholar
15 : Sex and the suffering brain. Science 2005; 308:1574 Crossref, Medline, Google Scholar
16 : Attention deficit hyperactivity disorder in adults: an overview. Biol Psychiatry 2000; 48:9–20 Crossref, Medline, Google Scholar
17 : Gender effects on attention-deficit/hyperactivity disorder in adults, revisited. Biol Psychiatry 2004; 55:692–700 Crossref, Medline, Google Scholar
18 : Reduced brain metabolism in hyperactive girls. J Am Acad Child Adolesc Psychiatry 1994; 33:858–868 Crossref, Medline, Google Scholar
19 : Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 2005; 57:439–447 Crossref, Medline, Google Scholar
20 : Association between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology? Biol Psychiatry 2007; 61:1395–1401 Crossref, Medline, Google Scholar
21 : Brain imaging of attention deficit/hyperactivity disorder. Ann NY Acad Sci 2001; 931:33–49 Crossref, Medline, Google Scholar
22 : Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry 2007; 61:720–724 Crossref, Medline, Google Scholar
23 : Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington, DC, American Psychiatric Press, 1997 Google Scholar
24 : Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E), 4th revision. Fort Lauderdale, Fla, Nova University, Center for Psychological Studies, 1987 Google Scholar
25 : Retrospective assessment of DSM-III attention deficit disorder in non-referred individuals. J Clin Psychiatry 1990; 51:102–106 Medline, Google Scholar
26 : Assessing symptoms of attention deficit hyperactivity disorder in children and adults: which is more valid? J Consult Clin Psychol 2000; 68:830–842 Crossref, Medline, Google Scholar
27 : EdITS Manual for the Profile of Mood States (POMS). San Diego, EdITS/Education and Industrial Testing Service, 1992 Google Scholar
28 : Wechsler Adult Intelligence Scale, 3rd ed. San Antonio, Tex, Psychological Corp, 1997 Google Scholar
29 : Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Hum Brain Mapp 1994; 1:293–304 Crossref, Medline, Google Scholar
30 Friston KJAshburner JTKiebel SJNichols TEPenny WD(eds): Statistical Parametric Mapping: The Analysis of Functional Brain Images. London, Academic Press, 2007 Crossref, Google Scholar
31 : ANOVAs and SPM. London, University College London, Wellcome Department of Imaging Neuroscience, 2003 Google Scholar
32 Buxton RB(ed): Introduction to Functional Magnetic Resonance Imaging Principles and Techniques. Cambridge, UK, Cambridge University Press, 2002 Crossref, Google Scholar
33 : Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988 Google Scholar
34 : Cytoarchitectonic definition of prefrontal areas in the normal human cortex, II: variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cereb Cortex 1995; 5:323–337 Crossref, Medline, Google Scholar
35 : Depression and anxiety disorders in parents and children: results from the Yale family study. Arch Gen Psychiatry 1984; 41:845–852 Crossref, Medline, Google Scholar
36 : Towards defining a meaningful anxiety phenotype for research in ADHD children. J Atten Disord 2000; 3:192–199 Crossref, Google Scholar
37 : Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry 2006; 59:48–56 Crossref, Medline, Google Scholar
38 : Working memory is (almost) perfectly predicted by "g." Intelligence 2004; 32:277–296 Crossref, Google Scholar
39 : Scanning patients with tasks they can perform. Hum Brain Mapp 1999; 8:102–108 Crossref, Medline, Google Scholar
40 : Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry 2006; 163:1720–1729 Link, Google Scholar



