Higher Rates of Cigarette Smoking in Male Adolescents Before the Onset of Schizophrenia: A Historical-Prospective Cohort Study
Abstract
OBJECTIVE: The prevalence of cigarette smoking among schizophrenia patients is significantly higher than in the general population; this may reflect self-medication of symptoms and/or adverse effects of neuroleptics. The authors examined the prevalence of cigarette smoking in apparently healthy adolescents later hospitalized for schizophrenia. METHOD: Each year, a random sample of male Israeli military recruits, who have been screened and found not to be suffering from major psychopathology, complete a smoking questionnaire. Through the Israeli National Psychiatric Hospitalization Case Registry, 14,248 of these adolescents were followed to determine later psychiatric hospitalization. RESULTS: Of the 14,248 adolescents assessed, 4,052 (28.4%) reported smoking at least one cigarette a day. Over a 4–16-year follow-up, the prevalence of schizophrenia in the entire cohort was 0.3% (N=44). Smokers were at greater risk for later schizophrenia; the adjusted relative risk was 1.94, and the 95% confidence interval (CI) was 1.05–3.58. The number of cigarettes smoked was significantly associated with the risk for schizophrenia. Compared to nonsmokers, adolescents who smoked 1–9 cigarettes/day were 1.38 times (95% CI=0.48–4.00) as likely to be hospitalized later for schizophrenia, and adolescents who smoked 10 cigarettes/day or more were 2.28 times (95% CI=1.19–4.34) as likely; the latter difference was statistically significant. CONCLUSIONS: Taken together with the existing data on abnormalities in nicotinic transmission in patients and their relatives, this higher prevalence of smoking in future schizophrenia patients, before the onset of their illness, might indicate that impaired nicotinic neurotransmission is involved in the pathophysiology of schizophrenia.
Although there is universal agreement among researchers and clinicians that smoking is more prevalent among persons with schizophrenia than in the general population (1–6), the reasons for this high prevalence remain debatable (7). Some authors have suggested that nicotine might benefit positive (1, 3) or negative (8) symptoms and/or the adverse effects of neuroleptics, such as extrapyramidal symptoms or cognitive deficits, perhaps by reducing the blood levels of antipsychotic medications (3, 9–11).
All of the aforementioned studies involved smoking in patients who were already diagnosed with schizophrenia, most of them inpatients and/or receiving active treatment. In one study (5), patients already diagnosed with schizophrenia were interviewed about their smoking habits before the assignment of the diagnosis, and it was found that 90% of them had started smoking before the first episode of psychosis, a number significantly higher than the prevalence of smoking in the nonpatient population of the same age. This finding, of a higher rate of smoking in schizophrenia patients before the overt manifestation of symptoms, raises the possibility that smoking is an intrinsic, illness-related phenomenon. However, this finding and a similar report (12) were based on retrospective assessments of patients’ recollections, which might be affected by recall bias and thus might not be reliable (13).
The purpose of this study was to ascertain if indeed, before the manifestation of psychosis and diagnosis of the illness, the rate of later hospitalization for schizophrenia is higher in smokers than in nonsmokers. Using a historical-prospective method, we examined routinely collected, standardized data on a large cohort to test the hypothesis that future schizophrenia patients had higher rates of smoking before the manifestation of psychosis and the diagnosis and treatment of schizophrenia. This was accomplished by using data from a survey of smoking habits that is administered to a random sample of Israeli military recruits every year and by following them to ascertain hospitalization for schizophrenia by using the National Psychiatric Hospitalization Case Registry, which contains data on all psychiatric hospitalizations in the country.
Method
Assessment of Smoking
The Israel Defense Forces Medical Corps conducts an ongoing survey designed to provide prevalence estimates of health indices. As part of the survey, recruits are asked, “Do you smoke?” and, if the answer is yes, “How many cigarettes do you smoke in a day?” The survey is administered to a random sample of recruits at age 18 upon induction into military service. The sample is drawn by taking every 20th recruit from a list based on a predetermined combination of digits of the subjects’ military serial number; the method was described in detail by Kark and Laor (14). The survey and the questionnaire are administered by trained nurses from the Israel Defense Forces Health Surveillance Section. Of the recruits who were asked to participate in the study, 91% agreed and provided signed informed consent, which was approved by the Israel Defense Forces Medical Corps Internal Review Board. We identified the questionnaires of 17,262 male adolescents who completed the questionnaire.
Israeli Draft Board Registry
All male adolescents in the country undergo a mandatory predraft screening at age 16–17 that is designed to ascertain their eligibility to serve in the military. The screening includes medical, including psychiatric histories obtained by a physician, intelligence testing, and a semistructured screening interview assessing personality and behavioral traits. On the basis of the screening interview and findings from the physician’s examination, adolescents who are suspected of having a behavioral disturbance or mental illness are referred for an in-depth assessment by a mental health professional, and if the adolescent warrants a psychiatric diagnosis, he is then referred to a board-certified psychiatrist. On the basis of this screening procedure, male adolescents who are found to have ICD 9, ICD-10, or DSM axis I or axis II pathology with significant functional impairment are released from military service; thus, the sample of adolescents who filled out the smoking questionnaires does not include very poorly functioning individuals.
National Psychiatric Hospitalization Case Registry
This national registry is a complete listing of all psychiatric hospitalizations in Israel, including the diagnosis assigned and coded on admission and discharge by a board-certified psychiatrist at the facility. During the time periods covered by this study, ICD-9 and ICD-10 diagnoses were used by the registry. All inpatient psychiatric facilities in the country, including psychiatric hospitals, day hospitals, and psychiatric units in general hospitals, are required by law to report all admissions and discharges to the registry.
After obtaining permission from the local institutional review board, the file containing the data from the health survey was linked with the National Psychiatric Hospitalization Case Registry. Following the merger, all identifiers were deleted from the merged file by the managers of the registry, and the analyses were performed on the unidentified data.
Study Sample
Out of the 17,262 randomly selected male adolescents who completed the health questionnaire, we removed 3,014 subjects because of missing data from the smoking questionnaire or from the draft board data. Of the 14,248 subjects remaining, 28.4% reported smoking at least one cigarette a day. This rate of smoking is in line with data on the rates of smoking in Israel (15) and among adolescents and young adults in the Western world (16). Through the National Psychiatric Hospitalization Case Registry, these subjects were followed up for 4–16 years; the mean follow-up duration was 10.2 years (SD=3.6). Of the 14,248, 44 (0.3%) had been hospitalized during follow-up with an ICD-10 diagnosis of schizophrenia (F20.0–F20.9) at last discharge; the mean age at case ascertainment was 28.2 years (SD=3.5). This prevalence is affected by the fact that not all of these adolescents had lived through the age of risk. In an attempt to exclude individuals who were already in the prodromal phase of schizophrenia when the smoking questionnaire was administered, we did not include two adolescents who were admitted to psychiatric hospitals within a year from the time that they completed the smoking questionnaire. Neither of them smoked.
We examined the prevalence of schizophrenia in the 3,014 persons with missing data. The prevalence of schizophrenia in the smokers in that group was 0.5%, which is identical to the prevalence of schizophrenia in the smokers included in the analysis. This probably means that the adolescents with missing data did not cause a sampling bias.
Data Analysis
As the individuals assessed by the draft board were followed up to different ages, Cox regression analysis was used. Time intervals were rounded to the nearest year. Age at first psychiatric admission was used to estimate the onset of illness. Data on individuals with no psychiatric hospitalization were censored on the last day of follow-up, which was the date when the smoking data were merged with the case registry.
Because cognitive performance, social adjustment, the presence of nonpsychotic mental illness, and socioeconomic status have been reported to affect both the risk for schizophrenia and the risk for smoking (17–24), these factors were entered into the Cox regression model in the first step, and smoking status was entered in the second step.
In order to assess the specificity of the association between cigarette smoking and risk for schizophrenia, the Cox regression was repeated to assess the association between smoking and later hospitalization for affective disorder.
The association between the number of cigarettes smoked and time to first hospitalization was assessed by using Pearson’s correlation. Population attributable risk was calculated according to the method of Goldbaum et al. (25).
Results
Out of 10,196 nonsmokers, 24 (0.2%) were later hospitalized for schizophrenia, compared with 20 (0.5%) out of 4,052 smokers; the relative risk was 2.08, with a 95% confidence interval (CI) of 1.15–3.77 (Wald χ2=5.85, df=1, p=0.02). After adjustment for the presence or absence of a nonpsychotic psychiatric disorder, below-normal social or intellectual functioning in adolescence, and socioeconomic status, the risk for schizophrenia remained higher in adolescents who reported smoking at least one cigarette a day (adjusted relative risk=1.94, 95% CI=1.05–3.58; Wald χ2=4.53, df=1, p=0.04). There was a significant linear association between the number of cigarettes smoked and the risk of schizophrenia (Wald χ2=6.22, df=1, p=0.02) (Figure 1). Compared to nonsmokers, adolescents who smoked 1–9 cigarettes/day were 1.38 times (95% CI=0.48–4.00; Wald χ2=0.36, df=1, p=0.55) as likely to be hospitalized later for schizophrenia, and adolescents who smoked 10 cigarettes/day or more were 2.28 times (95% CI=1.19–4.34; Wald χ2=6.22, df=1, p=0.02) as likely to be hospitalized later for schizophrenia.
No significant association between cigarette smoking and hospitalization for affective disorder (N=22) was observed (adjusted relative risk=1.28, 95% CI=0.47–3.49; Wald χ2=0.23, df=1, p=0.64). This result must be interpreted with caution, however, as many persons with affective disorder are not hospitalized.
The association between the number of cigarettes smoked and time to first hospitalization for schizophrenia was not statistically significant (r=0.20, N=44, p=0.15).
The population attributable risk was calculated by using a prevalence of schizophrenia of 0.3%, a prevalence of smoking of 30%, and a relative risk of schizophrenia in smokers of 2.00. This yielded a population attributable risk of 23%.
Discussion
Among the adolescents in this study, who had been screened and found not to be suffering from major psychopathology, cigarette smoking was associated with greater risk for later hospitalization for schizophrenia. This higher prevalence remained significant after we controlled for possible confounders associated with smoking behavior. There was a significant association between the number of cigarettes smoked and the risk for schizophrenia, with heavier smoking being associated with greater risk for schizophrenia.
Several interpretations could be offered to explain these results. Nicotine normalizes a sensory gating abnormality, the P50 inhibitory defect, found in most patients with schizophrenia and in 50% of their first-degree relatives (26–28). This in turn has been related to the impaired attention commonly present in patients with schizophrenia and linked to the 15q14 locus of the alpha-7 nicotinic receptor (29–31). Indeed, many but not all patients with schizophrenia have been shown to have inherent, illness-related cognitive deficits that are not necessarily related to active symptoms or medication and are present before the onset of psychosis (32, 33).
Perhaps the most parsimonious explanation of these findings is that the familial (probably genetically mediated) nicotinic receptor dysfunction that is present in some patients with schizophrenia is partially responsible for the premorbid cognitive deficits. Hence, just as schizophrenia patients might smoke in order to self-medicate cognitive deficits present after the onset of psychosis (34–36), future schizophrenia patients might smoke more in an attempt to use nicotine to self-medicate their premorbid cognitive dysfunction. Similarly, nicotine, like cocaine and amphetamine, activates mesolimbic dopamine neurotransmission (37), which is of critical importance in reinforcing and reward (38), and future schizophrenia patients often suffer from nonpsychotic psychiatric symptoms (23) and/or depression (39). Hence, perhaps future schizophrenia patients smoke cigarettes to improve premorbid anxiety and depression. It is conceivable that some future patients smoke to self-medicate premorbid cognitive dysfunction, others smoke to improve premorbid anxiety and depression, and yet for other future patients, neither of these possibilities is relevant. If either of these possibilities is correct, then our finding of a higher rate of cigarette smoking in future schizophrenia patients could be considered to be an epiphenomenon of the nicotinic receptor dysfunction in future schizophrenia patients and not related causally to the later appearance of schizophrenia.
One might further speculate, with extreme caution, that the nicotine in cigarettes causes chronic activation of mesolimbic dopamine neurotransmission (37), which in predisposed individuals might increase the risk of the appearance of psychosis, thus giving cigarettes a causative role in the pathway toward the later appearance of schizophrenia. This is supported by the temporal sequence between cigarette smoking and later schizophrenia, the positive association between the number of cigarettes smoked and the risk for schizophrenia, the finding that smoking was associated specifically with later schizophrenia but not later affective disorder, and the biological plausibility just described. If this association is causative, the population attributable risk contributed by smoking to later schizophrenia is high, at 23%. However, the fact that the vast majority of adolescent smokers do not later suffer from schizophrenia should make one treat this hypothesis very cautiously.
This higher rate of smoking before the onset of illness might imply that smoking is an intrinsic, disease-related phenomenon, which might explain the particularly low rates of smoking cessation among patients with schizophrenia (40, 41). If so, this might be considered before smoking is barred in inpatient facilities and indicates that schizophrenia patients might need extra help in smoking cessation programs.
A study similar to the present one (42) used a similar design, following Swedish army conscripts who smoked to determine the rate of later schizophrenia according to a psychiatric hospitalization registry. Over a 27-year follow-up, smoking had a protective effect on the risk of later hospitalization for schizophrenia. However, the authors found that for conscripts followed for up to 5 years, before adjustment for confounders, smokers were 1.7 times as likely to be hospitalized later for schizophrenia. This effect became nonsignificant when possible confounders were controlled for. Possible explanations for the differences between those findings and ours include a much higher rate of smoking in the Swedish population (59% versus 28% in the present study), a longer follow-up, as well as the availability of information about important confounders or mediating factors in their cohort, including drug use, behavioral disturbances, history of psychiatric illnesses in the family, and alcohol problems, which were not available to us.
A potential limitation of this study is the fact that the case registry diagnoses are clinical, not research, diagnoses. However, these diagnoses were assigned by board-certified psychiatrists who had the benefit of observing the patients throughout one or more hospitalizations and had been trained and retrained in the use of the diagnostic criteria of the ICD and DSM. Moreover, studies that have compared clinical diagnoses of schizophrenia assigned in state hospitals (43) with research diagnoses have shown a high degree of concordance. In an attempt to assess the reliability of the diagnoses of schizophrenia in the National Psychiatric Hospitalization Case Registry, we compared those diagnoses of schizophrenia with Research Diagnostic Criteria best-estimate diagnoses based on clinical research interviews (44). We found a sensitivity of 0.89, which is consistent with the reliability of diagnoses from other registries (45, 46).
Another limitation is the fact that we did not have data on the rates of cannabis use in this population. This is relevant because cannabis use, which has been shown to be associated with later schizophrenia (47–50), is more common in cigarette smokers (51). In addition, as smoking status is assessed at age 18, we do not know who stopped or began smoking after age 18. Also, as male adolescents with significant functional impairment are screened out at age 16 and not drafted, the sample of adolescents studied does not include very poorly functioning individuals.
In summary, the association between smoking and future schizophrenia might imply that smoking reflects an intrinsic, disease-related disorder of nicotinic transmission and/or that smoking reflects self-medication of premorbid symptoms. The results of this study indicate the need for more intense research on the nicotinic system in schizophrenia and the need to give special consideration to patients’ particular difficulties in quitting smoking.
Received June 2, 2003; revision received Oct. 7, 2003; accepted Oct. 9, 2003. From the Department of Psychiatry, Sheba Medical Center; the Division of Mental Health and the Public Health Branch, Israel Defense Forces, Tel Hashomer, Israel; the Department of Psychiatry, Sackler Medical School, Tel Aviv University, Ramat Aviv, Israel; the Department of Psychiatry, Mount Sinai School of Medicine, New York; the Department of Social Work, Bar Ilan University, Ramat Gan, Israel; and the Department of Mental Health, Ministry of Health, Jerusalem. Address reprint requests to Dr. Davidson, Department of Psychiatry, Sheba Medical Center, Tel Hashomer 52621, Israel; [email protected] (e-mail). Supported by a Distinguished Investigator Award (Dr. Davidson) and Young Investigator Award (Dr. Weiser) from the National Alliance for Research on Schizophrenia and Depression, the Stanley Medical Research Foundation (Dr. Davidson and Dr. Weiser), and the German Federal Ministry of Education and Research within the framework of German-Israeli Project Cooperation (Dr. Rabinowitz and Dr. Davidson).
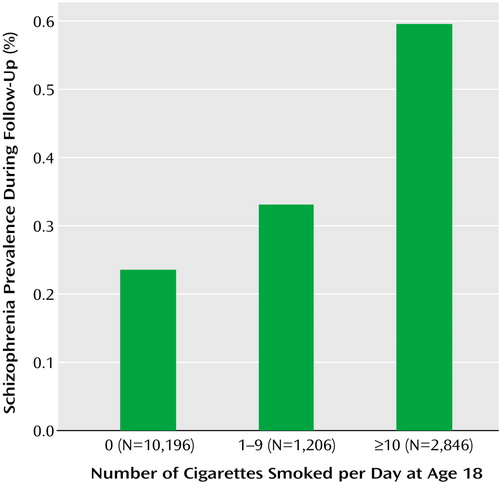
Figure 1. Association Between Number of Cigarettes Smoked at Age 18 and Risk for Schizophrenia During 4–16-Year Follow-Up Among Male Israeli Military Recruits
1. Ziedonis DM, Kosten TR, Glazer WM, Frances RJ: Nicotine dependence and schizophrenia. Hosp Community Psychiatry 1994; 45:204–206Abstract, Google Scholar
2. Masterson E, O’Shea B: Smoking and malignancy in schizophrenia. Br J Psychiatry 1984; 145:429–432Crossref, Medline, Google Scholar
3. Goff DC, Henderson DC, Amico E: Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry 1992; 149:1189–1194Link, Google Scholar
4. Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA: Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 1986; 143:993–997Link, Google Scholar
5. Kelly C, McCreadie RG: Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry 1999; 156:1751–1757Link, Google Scholar
6. Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW: Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr Res 1998; 33:113–118Crossref, Medline, Google Scholar
7. Lohr JB, Flynn K: Smoking and schizophrenia. Schizophr Res 1992; 8:93–102Crossref, Medline, Google Scholar
8. Hall RG, Duhamel M, McClanahan R, Miles G, Nason C, Rosen S, Schiller P, Tao-Yonenaga L, Hall SM: Level of functioning, severity of illness, and smoking status among chronic psychiatric patients. J Nerv Ment Dis 1995; 183:468–471Crossref, Medline, Google Scholar
9. Decina P, Caracci G, Sandik R, Berman W, Mukherjee S, Scapicchio P: Cigarette smoking and neuroleptic-induced parkinsonism. Biol Psychiatry 1990; 28:502–508Crossref, Medline, Google Scholar
10. Menza MA, Grossman N, Van Horn M, Cody R, Forman N: Smoking and movement disorders in psychiatric patients. Biol Psychiatry 1991; 30:109–115Crossref, Medline, Google Scholar
11. Sandyk R: Cigarette smoking: effects on cognitive functions and drug-induced parkinsonism in chronic schizophrenia. Int J Neurosci 1993; 70:193–197Crossref, Medline, Google Scholar
12. Beratis S, Katrivanou A, Gourzis P: Factors affecting smoking in schizophrenia. Compr Psychiatry 2001; 42:393–402Crossref, Medline, Google Scholar
13. Riutort M, Cuervo C, Danion JM, Peretti CS, Salame P: Reduced levels of specific autobiographical memories in schizophrenia. Psychiatry Res 2003; 117:35–45Crossref, Medline, Google Scholar
14. Kark JD, Laor A: Cigarette smoking and educational level among young Israelis upon release from military service in 1988—a public health challenge. Isr J Med Sci 1992; 28:33–37Medline, Google Scholar
15. Brook U, Feigin R, Sherer M, Geva D: Prevalence, attitudes and knowledge of high school pupils towards drugs and other addictions: implications for school health education in Israel. Patient Educ Couns 2001; 43:199–204Crossref, Medline, Google Scholar
16. Kann L, Kinchen SA, Williams BI, Ross JG, Lowry R, Grunbaum JA, Kolbe LJ (State and Local YRBSS Coordinators, Youth Risk Behavior Surveillance System): Youth risk behavior surveillance—United States, 1999. MMWR CDC Surveill Summ 2000; 49:1–32Medline, Google Scholar
17. Croudace TJ, Kayne R, Jones PB, Harrison GL: Non-linear relationship between an index of social deprivation, psychiatric admission prevalence and the incidence of psychosis. Psychol Med 2000; 30:177–185Crossref, Medline, Google Scholar
18. Stanton WR, Oei TP, Silva PA: Sociodemographic characteristics of adolescent smokers. Int J Addict 1994; 29:913–925Crossref, Medline, Google Scholar
19. Hover SJ, Gaffney LR: Factors associated with smoking behavior in adolescent girls. Addict Behav 1988; 13:139–145Crossref, Medline, Google Scholar
20. Kubicka L, Matejcek Z, Dytrych Z, Roth Z: IQ and personality traits assessed in childhood as predictors of drinking and smoking behaviour in middle-aged adults: a 24-year follow-up study. Addiction 2001; 96:1615–1628Crossref, Medline, Google Scholar
21. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH: Smoking and mental illness: a population-based prevalence study. JAMA 2000; 284:2606–2610Crossref, Medline, Google Scholar
22. Pederson LL, Koval JJ, O’Connor K: Are psychosocial factors related to smoking in grade-6 students? Addict Behav 1997; 22:169–181Crossref, Medline, Google Scholar
23. Weiser M, Reichenberg A, Rabinowitz J, Kaplan Z, Mark M, Bodner E, Nahon D, Davidson M: Association between nonpsychotic psychiatric diagnoses in adolescent males and subsequent onset of schizophrenia. Arch Gen Psychiatry 2001; 58:959–964Crossref, Medline, Google Scholar
24. Lewis G, David AS, Malmberg A, Allebeck P: Non-psychotic psychiatric disorder and subsequent risk of schizophrenia: cohort study. Br J Psychiatry 2000; 177:416–420Crossref, Medline, Google Scholar
25. Goldbaum GM, Kendrick JS, Hogelin GC, Gentry EM: The relative impact of smoking and oral contraceptive use on women in the United States. JAMA 1987; 258:1339–1342Crossref, Medline, Google Scholar
26. Adler LE, Hoffer LD, Wiser A, Freedman R: Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 1993; 150:1856–1861Link, Google Scholar
27. Adler LE, Hoffer LD, Griffith J, Waldo MC, Freedman R: Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry 1992; 32:607–616Crossref, Medline, Google Scholar
28. Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, Freedman R: Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 1998; 24:189–202Crossref, Medline, Google Scholar
29. Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R: Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry 2002; 59:1085–1096Crossref, Medline, Google Scholar
30. Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W: Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci USA 1997; 94:587–592Crossref, Medline, Google Scholar
31. Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H: Elementary neuronal dysfunctions in schizophrenia. Schizophr Res 1991; 4:233–243Crossref, Medline, Google Scholar
32. Jones P, Rodgers B, Murray R, Marmot M: Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 1994; 344:1398–1402Crossref, Medline, Google Scholar
33. Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M: Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry 1999; 156:1328–1335Abstract, Google Scholar
34. Levin ED, Wilson W, Rose JE, McEvoy J: Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology 1996; 15:429–436Crossref, Medline, Google Scholar
35. George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE: Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology 2002; 26:75–85Crossref, Medline, Google Scholar
36. Depatie L, O’Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, Lal S: Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology 2002; 27:1056–1070Crossref, Medline, Google Scholar
37. Pontieri FE, Tanda G, Orzi F, Di Chiara G: Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996; 382:255–257Crossref, Medline, Google Scholar
38. Iversen LL: Smoking…harmful to the brain. Nature 1996; 382:206–207Crossref, Medline, Google Scholar
39. Hafner H, Loffler W, Maurer K, Hambrecht M, an der Heiden W: Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand 1999; 100:105–118Crossref, Medline, Google Scholar
40. Dalack GW, Becks L, Hill E, Pomerleau OF, Meador-Woodruff JH: Nicotine withdrawal and psychiatric symptoms in cigarette smokers with schizophrenia. Neuropsychopharmacology 1999; 21:195–202Crossref, Medline, Google Scholar
41. Leonard S, Giordano L: Are differential behavioral responses to smoking and smoking cessation in schizophrenia related to nicotinic receptor levels? (letter). Neuropsychopharmacology 2002; 27:1082–1083; author reply 1084–1085Crossref, Medline, Google Scholar
42. Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingsson T, Lewis G: Investigating the association between cigarette smoking and schizophrenia in a cohort study. Am J Psychiatry 2003; 160:2216–2221Link, Google Scholar
43. Pulver AE, Carpenter WT, Adler L, McGrath J: Accuracy of the diagnoses of affective disorders and schizophrenia in public hospitals. Am J Psychiatry 1988; 145:218–220Link, Google Scholar
44. Weiser M, Kanyas K, Malaspina D, Harvey PD, Glick I, Goetz D, Karni O, Yakir A, Turetsky N, Fennig S, Nahon D, Lerer B, Davidson M: Sensitivity of ICD-10 diagnosis of psychotic disorders in the Israeli national hospitalization registry. Compr Psychiatry (in press)Google Scholar
45. Dalman C, Broms J, Cullberg J, Allebeck P: Young cases of schizophrenia identified in a national inpatient register—are the diagnoses valid? Soc Psychiatry Psychiatr Epidemiol 2002; 37:527–531Crossref, Medline, Google Scholar
46. Kirkby KC, Hay DA, Daniels BA, Jones IH, Mowry BJ: Comparison between register and structured interview diagnoses of schizophrenia: a case for longitudinal diagnostic profiles. Aust NZ J Psychiatry 1998; 32:410–414Crossref, Medline, Google Scholar
47. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE: Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. Br Med J 2002; 325:1212–1213Crossref, Medline, Google Scholar
48. Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G: Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ 2002; 325:1199Crossref, Medline, Google Scholar
49. Weiser M, Reichenberg A, Rabinowitz J, Kaplan Z, Caspi A, Yasvizky R, Mark M, Knobler HY, Nahon D, Davidson M: Self-reported drug abuse in male adolescents with behavioral disturbances, and follow-up for future schizophrenia. Biol Psychiatry 2003; 54:655–660Crossref, Medline, Google Scholar
50. van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H: Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol 2002; 156:319–327Crossref, Medline, Google Scholar
51. Lewinsohn PM, Rohde P, Brown RA: Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction 1999; 94:913–921Crossref, Medline, Google Scholar



