Interacting Effects of Genetic Predisposition and Depression on Adolescent Smoking Progression
Abstract
OBJECTIVE: The goal of the present study was to identify specific genetic associations with smoking progression in adolescents and to determine whether these genetic effects on smoking practices are potentiated by depression symptoms. METHOD: Effects of dopamine transporter (SLC6A3) and dopamine receptor (DRD2) genetic variants on smoking progression were evaluated in a cohort of 615 adolescents, including those who had never smoked, and in a subgroup including only adolescents who had been exposed to nicotine (i.e., smoked at least a puff of a cigarette) (N=292). These adolescents were followed from 9th to 11th grade. Depression symptoms were also assessed. RESULTS: In the model of adolescents with a previous smoking experience, the likelihood of progressing to a higher level of smoking by the 11th grade increased almost twofold with each additional DRD2A1 allele. The likelihood of smoking progression with each additional A1 allele was more pronounced among adolescents with substantial depression symptoms. The model including never-smokers revealed no significant genetic effects. Neither model revealed effects of SLC6A3. CONCLUSIONS: These results provide the first evidence, to the authors’ knowledge, for an association of the DRD2A1 allele with smoking progression among adolescents. This effect is potentiated by depression symptoms. These effects appear to be specific to adolescents who have had at least some nicotine exposure (i.e., at least a puff of a cigarette).
Adolescent smoking is a major public health problem, yet much remains to be learned about why some adolescents progress from smoking experimentation to regular smoking while others do not. Individual differences in genetic susceptibility may account, in part, for the variability in rates of adolescent smoking and progression. There is abundant evidence for the heritability of smoking initiation, age at smoking onset, and smoking persistence (1–4). Heritable predisposition to smoking may be mediated, in part, by genetic variation in the dopamine pathway (5). Support for this hypothesis has emerged from studies linking smoking behavior with the more rare A1 and B1 alleles of the gene for the dopamine 2 receptor (DRD2) (6–8) and the 10-repeat allele of the dopamine transporter gene (SLC6A3) (9, 10) in adults. However, these findings have not been replicated in all cases (11–13). The 9-repeat allele of SLC6A3 has also been associated with a 22% reduction in dopamine transporter protein, resulting in less clearance and greater bioavailability of dopamine (14). Thus, it is possible that individuals who carry the other common allele of SLC6A3, the 10-repeat allele, may achieve greater reward from nicotine’s effects on dopamine activity.
Although these genetic association studies have provided preliminary evidence for specific genetic effects on smoking practices, they have several limitations. For example, case-control designs have been criticized for not accounting for the potential biasing effects of ethnic admixture (i.e., cases and control subjects may have been drawn from populations with different ethnic ancestries) (5, 15). A second limitation of previous research has been small subject groups, which may have affected the ability to detect associations with genes having small effects (16). In addition, previous research has relied on general assessments of smoking status (e.g., smokers versus nonsmokers). Given the complex nature of smoking behavior and its etiology, it is critical that the phenotypes for genetic studies of smoking be well defined (17). Finally, there has been a lack of attention to adolescent populations in research evaluating genetic contributions to smoking behavior. The genes involved in smoking experimentation and acquisition of a habit during adolescence may be different from those that maintain a smoking habit or explain nicotine dependence in adulthood.
Another gap in our understanding relates to the interactions of genes with other factors that have been shown to increase adolescent vulnerability to smoking. For example, depression appears to be an important factor in adolescent smoking (18–20). Evidence exists to suggest that there may be common influences, possibly genetic, on both depression and smoking (21, 22). Depression symptoms have also been shown to moderate the effects of dopaminergic genes on smoking practices (23).
To generate new knowledge about the biobehavioral basis of adolescent smoking, the present study evaluated the effects of polymorphisms in SLC6A3 and DRD2 on smoking practices and smoking progression in a large cohort of 615 high school adolescents of European ancestry who were followed from 9th grade to 11th grade. We hypothesized that adolescents with DRD2A1 and/or SLC6A3 10-repeat alleles who had an initial biological exposure to nicotine (i.e., had smoked at least a puff) would be more likely to progress to higher levels of smoking over the 2 years of longitudinal follow-up. We further hypothesized that genetic effects on smoking practices would be potentiated by depression symptoms, as measured by the Center for Epidemiological Studies Depression Scale (CES-D Scale) (24). Finally, we did not expect to find significant effects of DRD2 and SLC6A3 on smoking initiation (i.e., progression from never to ever) because students who had never smoked would not have had the opportunity for the genetic predisposition to enhanced nicotine reward to be expressed (13, 25, 26).
Method
Study Group
The participants were 615 9th grade high school students of European ancestry, each of whom was enrolled in one of five public high schools in northern Virginia. These adolescents were participating in a longitudinal cohort study of biobehavioral determinants of adolescent health habits. Of these 615 adolescents, 293 (48%) were male and 322 (52%) were female.
This study group is a subset of a larger cohort that was drawn from 2,393 students identified through class rosters at the beginning of 9th grade. Students were ineligible to participate in the cohort study of adolescent health habits if they had a special classroom placement (i.e., severe learning disability and/or English as a second language). The cohort was formed in the 9th grade and is being followed until the end of the 12th grade.
On the basis of the cohort selection criteria, a total of 2,120 students (89%) were eligible to participate. Of the 2,120 eligible students, the parents of 1,533 (72%) provided a response. Of these 1,533, the parents of 1,151 (75%) consented to their teen’s participation in the study, yielding an overall consent rate of 54%. An analysis of differences between students whose parents consented and those whose parents did not consent to participation in the study revealed a race-by-education interaction. The interaction indicated that the likelihood of consent was over twice as great for Caucasian parents with more than a high school education than for those with a high school education or less (27).
Participation in the study required student assent as well as parental consent. Fifteen students declined participation. An additional 13 students were absent for both the baseline administration and the makeup session. The final baseline subject group contained 1,123 of the 2,120 eligible students. The university’s institutional review board approved the study protocol.
The students were resurveyed in the fall and spring of the 10th grade and in the spring of 11th grade, for a total of four data collection waves. The rates of participation at the three follow-ups were 95% (1,070 students surveyed) for the fall of 10th grade, 96% (1,081 students surveyed) for the spring of 10th grade, and 93% (1,043 students surveyed) for the spring of 11th grade.
To limit potential bias due to ethnic admixture, the analyses were limited to adolescents of European ancestry (N=685). Sixteen adolescents with rare SLC6A3 alleles (i.e., other than 9- or 10-repeat alleles) were excluded from the analyses. The primary variables of interest were smoking, depression, SLC6A3 and DRD2 genotypes, academic performance, and gender. The data presented herein are based on 615 adolescents of European ancestry who had complete responses for the predictor and outcome variables at the 9th and 11th grade time points (54 adolescents had missing data on one or more of these variables, including 22 who were lost to follow-up).
We compared the eligible adolescents lost to follow-up (N=22) to the adolescents of European ancestry who entered the study and were retained. Compared to the adolescents who were retained, this group displayed higher school performance (mean estimated grade point average, 2.5 [SD=0.7] versus 1.9 [SD=0.7]) (rank sum test, z=–3.76, p<0.0002), were more likely to have a genotype containing the DRD2A1 allele (60% [12 of 20] versus 33% [203 of 615]) (χ2=6.30, df=1, p<0.02), and were more likely to have smoked at least a puff of a cigarette by or in the 9th grade (59% [13 of 22] versus 35% [213 of 615]) (rank sum test, z=–2.38, p<0.02). No other differences were observed.
Procedures
Data were collected on-site, during a class common to all students (e.g., health, science, history). A member of the research team (J.A.-M.) distributed the survey. Each student received a survey with a subject identification number. The survey contained a front page with the student’s name. The front page was removed when a survey was given to the student. The completed survey contained only an identification number. A member of the research team (J.A.-M.) read aloud a set of instructions, emphasizing confidentiality to promote honest responding, and encouraged questions if survey items were not clear. The survey took about 30 minutes to complete. Make-up sessions were held in the library for students who were absent during administration of the survey.
Biological samples were collected by using buccal swabs as previously described (28), and DNA was extracted with standard phenol-chloroform techniques. Genotyping was done as described in prior reports (9, 10). The assays were validated by confirming a polymorphic inheritance pattern in seven human family lines that encompassed three generations (data not shown; National Institute of General Medical Sciences Human Genetic Mutant Cell Repository, Coriell Institute, Camden, N.J.). Quality control procedures included positive and negative controls with each assay and independent repeat genotyping for 20% of the results. The rate of discordance was less than 5%, and ambiguous results were not reported.
Measures
Smoking status and progression
An ordered categorical variable was generated from responses to a series of standard epidemiological smoking questions derived from the Youth Risk Behavior Survey (29, 30). These included, “Have you ever tried or experimented with cigarette smoking, even a few puffs?” “Have you smoked at least one whole cigarette?” “Have you smoked a cigarette in the past 30 days?” and “How many cigarettes have you smoked in your lifetime?” Research supports the interrater reliability of the items of the Youth Risk Behavior Survey that assess smoking practices; the kappa coefficients are in the substantial or higher range (kappa ≥61%) (31, 32). Research also supports the validity of self-report measures of smoking behavior in adolescents, particularly in nontreatment contexts where confidentiality is emphasized (33–35).
On the basis of the participants’ responses to these items, the adolescents were categorized as 1) never-smoker—never having smoked a cigarette, not even a puff, 2) puffer—not ever having smoked a whole cigarette, 3) experimenter—smoked at least one whole cigarette but fewer than 100 cigarettes total in a lifetime, or 4) current smoker—smoked in the last 30 days and 100 or more cigarettes in a lifetime. Adolescents who reported not smoking in the past 30 days but having smoked more than 100 cigarettes in a lifetime were classified as experimenters (N=2). The smoking progression variable was designed to capture any progression from never to current smoker and from puffer to current smoker, by assessing smoking level at each wave. These categories for smoking status have been used in previous studies (36, 37).
Genotype
Genotyping was done as in prior studies (9, 10). The SLC6A3 genotype was classified as the number of 10-repeat alleles (zero, one, or two), and the DRD2 genotype was classified as the number of A1 alleles (zero, one, or two) (6, 9).
Depression symptoms
Depression symptoms were assessed with the CES-D Scale at baseline. This scale is a 20-item self-report measure of depression symptoms (24). Each item is rated along a 4-point Likert scale to indicate how frequently in the past week the symptom occurred (0=rarely or none of the time, 3=most of the time). Scores range from 0 to 60, and higher scores indicate a greater degree of depression symptoms. Previous adolescent research indicates that gender- and age-appropriate dichotomous cutoff scores (>24 for females, >22 for males) can be used to distinguish subjects with and those without clinically significant levels of depression symptoms (38). We used a score of 22 or greater as suggestive of clinical depression symptoms. The results did not differ when a cutoff score of 24 or greater was used.
Covariates
School performance was measured by one item similar to that used and shown in previous research to be related to adolescent smoking (39, 40). This item asked, “How do you do in school? Would you say mostly A’s, mostly B’s, mostly C’s, or mostly D’s and F’s?” The responses were scored from 4 (mostly A’s) to 1 (mostly D’s and F’s). Finally, gender was assessed by self-report.
Results
Of the 615 participants, 412 (67%) had no DRD2A1 alleles (A2/A2), 184 (30%) had one DRD2A1 allele (A1/A2), and 19 (3%) had two DRD2A1 alleles (A1/A1). For SLC6A3, 54 (9%) had no 10-repeat alleles (9/9), 226 (37%) had one 10-repeat allele (9/10), and 335 (54%) had two 10-repeat alleles (10/10). Neither SLC6A3 nor DRD2 alleles departed significantly from Hardy-Weinberg equilibrium (p<0.08 and p<0.90, respectively). The average CES-D Scale score in the 9th grade was 13.3 (SD=8.9). One hundred adolescents (16%) scored in the depressed range on the CES-D Scale. Approximately 26% of the subjects progressed in their smoking over the 2-year period.
Baseline Smoking Status, Depression Symptoms, and Genotype
At the 9th grade baseline assessment, participants were classified as never-smokers (65%), puffers (12%), experimenters (19%), and current smokers (4%). Smoking status was not significantly associated with DRD2 genotype (χ2=3.19, df=6, p=0.78) or SLC6A3 genotype (χ2=5.27, df=6, p=0.51). There was, however, a highly significant dose-response effect of DRD2A1 alleles on depression symptoms (F=8.32, df=2, 612, p=0.0003): the mean CES-D Scale scores were 12.3 (SD=8.1) for adolescents with no A1 alleles (A2/A2), 15.1 (SD=10.1) for those with one A1 allele (A1/A2), and 16.7 (SD=8.4) for those with two A1 alleles (A1/A1). The SLC6A3 genotype was unrelated to depression symptoms (F=0.86, df=2, 612, p=0.43).
There was also a highly significant association of depression symptoms with smoking status in the 9th grade (F=7.91, df=3, 610, p<0.0001). The mean CES-D Scale scores were 12.5 (SD=8.4) for never-smokers, 14.6 (SD=9.5) for puffers, 13.7 (SD=7.7) for experimenters, and 20.8 (SD=14.4) for current smokers. Sex was unrelated to smoking status in the 9th grade (χ2=3.4, df=3, p=0.34).
Relation of Smoking Progression to DRD2 and SLC6A3
Subjects with exposure to nicotine
Ordinal logit modeling was used to estimate the effect of the number of DRD2A1 and SLC6A3 10-repeat alleles on progression in smoking status by the second year of follow-up of the cohort. The model controlled for sex, baseline smoking status, school performance, and level of depression symptoms. Participants who had never smoked any cigarettes by 11th grade (i.e., never-smokers) were excluded (N=323). Thus, the study group included the 292 participants who had at least some biological exposure to nicotine (puffers, experimenters, or current smokers), which is considered necessary for a genetic predisposition to be expressed (13, 25, 26).
There are three different varieties of ordinal logit models, each differing in construction of the odds. We chose the proportional odds model, which models the three-level ordinal outcome by splitting the probability space (sum=1) at two different dichotomies or cut points to create the three ordered categories. The model then predicts the odds of being above each of the cut points with a linear model. Although a separate model could be used for each dichotomy, proportional odds uses a common set of parameters across all the dichotomies, except for the intercept value. The reported odds ratio is the factor by which the odds of being in a higher category increase (across all the dichotomies) for a unit change in the explanatory variable, corresponding roughly to that of conventional logistic regression.
The final model of smoking progression, shown in Table 1, was highly significant (R2=0.18, p<0.00005). These results revealed an effect of the number of DRD2A1 alleles. Specifically, each A1 allele nearly doubled the odds of smoking progression. In addition, school performance significantly decreased the odds of smoking progression. The number of SLC6A3 10-repeat alleles was not associated with progression. The results further revealed a significant DRD2-by-depression interaction (Table 1). The interaction indicated that an increase in depression symptoms of one standard deviation nearly doubled the effect of the A1 alleles on smoking progression. For illustrative purposes, depression was dichotomized as shown in Figure 1. Among adolescents carrying at least one A1 allele, 25% (40 of 161) of nondepressed versus 33% (17 of 52) of depressed adolescents progressed in their smoking within 2 years.
The proportional odds model depends on the assumption that the separate models of the dichotomies differ only in their intercepts. A generalized ordinal logit model relaxes the assumption of proportionality (41), evaluating separate logit models for each of the cutoff points (effectively doubling the number of parameters estimated). We used a likelihood ratio test to determine whether the generalized model offered a significant improvement over the proportional odds model and found the improvement nonsignificant (p=0.08).
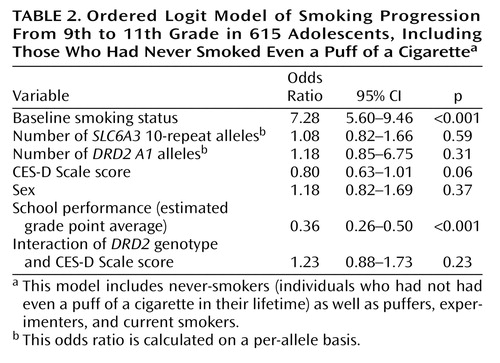
All subjects
The four-level ordinal data (i.e., never-smoker, puffer, experimenter, current smoker) were modeled by using the proportional odds model, which begins by splitting the data at three different dichotomies or cut points to create four ordered categories. The model then predicts the odds of being above each of the cut points with a linear model. As predicted, with the never-smokers in the analysis (Table 2) the effect of the DRD2 genotype was no longer significant. Similarly, SLC6A3 genotype was not significantly associated with smoking progression. Only baseline smoking status and school performance had significant effects in predicting progression by grade 11.
Ethnic Admixture
Although our study group included only adolescents of European ancestry, there is some concern that ethnic admixture within this group could lead to false positive results. We used the confounding odds ratio technique described by Wacholder and colleagues (42) for analyzing the effects of ethnic admixture. The Caucasian group was split into three blocks of predominantly northwestern, southwestern, and eastern European heritage. Those who could not trace at least three-fourths of their ancestry to one subgroup were removed from the analyses. The adjusted odds ratio for the effect of the presence of an A1 allele on smoking progression differed by less than 2% when ancestry was accounted for in the model.
Discussion
These results provide the first evidence, to our knowledge, for the association of specific genetic variants with smoking progression in adolescents. Among adolescents who had smoked at least one puff of a cigarette, the likelihood of a 9th grade student progressing to a higher level of smoking by the 11th grade increased almost twofold with each additional DRD2A1 allele. This effect was significantly more pronounced among adolescents with more severe depression symptoms. The SLC6A3 polymorphism was not associated with smoking progression in this model. The same effects were not observed when adolescents who had never smoked a cigarette (even a puff) were included in the model.
The observed association of the DRD2 polymorphism and smoking progression among adolescents who have ever smoked can be interpreted in the context of previous neurobiological and epidemiological research. Correlative data suggest that individuals who carry DRD2A1 alleles exhibit altered receptor density and binding characteristics and therefore may have lower levels of endogenous dopamine activity (43–45). Thus, adolescents who carry this variant allele may experience greater reward from nicotine’s dopamine-stimulating effects. Consistent with this line of thought is the association Noble and colleagues (46) found between novelty seeking, a trait characterized by sensitivity to reward, and DRD2 in adolescents. It is possible that the effects of DRD2 on smoking progression are mediated by novelty seeking, which may also affect peer smoking influences. The association between DRD2 and smoking progression is consistent with findings from studies of adult smokers (6, 7). However, these findings have not been replicated in other studies (10, 11). Further investigation of the genetic contributions to adolescent smoking is needed to replicate these findings.
When never-smokers were considered in the model, the associations with DRD2 and the interaction between DRD2 and depression were no longer significant. There are several possible explanations for these findings. First, adolescent smoking progression may result from a complex interplay of genetic, psychological, and socioenvironmental factors, and key determinants may vary at different points in the smoking acquisition trajectory (30, 47). For example, socioenvironmental factors may play a larger role in smoking initiation than do psychological factors, and genetic factors may play a larger role in progression. Support for this premise comes from studies showing that peer and parental smoking contribute more to youth smoking initiation than to escalation and that psychological mechanisms (e.g., smoking intentions, outcome expectancies) are more influential in escalation (48). In addition, heritability studies have shown that the genetic and environmental factors that influence the initiation of regular smoking differ from those that influence maintenance (4). Second, if the DRD2 polymorphism is associated with a differential response to nicotine, then biological exposure is necessary for the genetic effects to be expressed (13, 25, 26). If never-smokers were to smoke a cigarette, one would likely find wide variation in their biological responses (26). In fact, previous research has shown that the 10-repeat allele of the SLC6A3 polymorphism is associated with smoking (9). However, a recent study had the opposite result when never-smokers were distinguished from nonsmokers; the 10-repeat allele was more common in the never-smokers (13). Never-smokers may differ in a number of ways from those who have been exposed to nicotine through smoking. Separating people who have never smoked from those who have has been considered a logical first step in refining smoking phenotypes (49).
Of particular significance for adolescent smoking prevention, our findings provide strong evidence that adolescents who carry at least one DRD2A1 allele may be especially vulnerable to depression symptoms and that genetic predisposition and depression symptoms may act synergistically in progression of adolescent smoking. Previous studies have indicated that depression symptoms may modify the effects of genetic predisposition on smoking practices among adults (23). However, to our knowledge the present study is the first to document this in an adolescent population. This finding is consistent with a growing body of evidence suggesting that depression symptoms increase the risk of adolescent smoking (19, 50) and may increase susceptibility to other factors, such as tobacco advertising (51). It is possible that adolescents with elevated levels of depression have fewer alternative sources of reward, and smoking may be a quick and easy way to increase overall pleasure and reward (52). The findings of recent studies provide some support for this notion in that clinically depressed adult smokers found smoking more appealing than other sources of reward (53). In addition, brain reward systems involving dopamine appear to be dysfunctional in individuals with depression, such that they are more responsive to substances that activate these reward systems (54), such as nicotine.
Research indicates that over 20% of high school students suffer from clinically significant depression symptoms (55), supporting the importance of targeting smoking prevention efforts to this high-risk group. The observed association and interaction with DRD2 genotype provide additional mechanistic data that may help to define such efforts. Successful interventions for adolescent depression (55) and research aimed at informing smoking prevention efforts (36, 52) have focused on increasing the quantity and quality of rewarding activities. Such interventions may improve mood and reduce the likelihood of smoking progression in adolescents.
We believe ours to be the first investigation of specific genetic effects on adolescent smoking progression. As such, the present study has both strengths and weaknesses. The strengths include the collection of DNA samples and psychological data from a large group of adolescents and the use of more refined longitudinal smoking phenotypes. One limitation, however, is that there were insufficient numbers of adolescents in other racial groups (e.g., African American, Asian American, Hispanic) to conduct meaningful analyses stratified by race. Another potential limitation of this study is that the results could be biased by ethnic admixture within the Caucasian population. However, this is less likely, since all adolescents were drawn from the same population, and our analyses show that adjusting ancestry does not alter the effect of genotype on smoking progression. Moreover, reports have challenged the notion that ethnic admixture leads to significant bias in genetic epidemiological studies (42).
A common limitation of protocols requiring active consent is the rate of nonresponse (56–58). Although the parents of 72% of the eligible participants returned responses regarding study participation, we are unable to ascertain the characteristics of the 28% who did not respond. Seventy-five percent of the parents who responded did provide consent, and the differences between those who provided consent and those who declined were relatively small and few (27). However, some caution is warranted in generalizing the results of this study, especially in light of the study’s consent rate. Although our study group may not be representative of all adolescents nationwide, it is representative of the population of high school students in the county from which it was drawn. Further, our smoking rates are representative according to the rates in a random sample of more than 4,000 grade-matched students in the county in which our study group was drawn; for example, the rates of lifetime smoking in our subjects and the random sample were 41% and 43%, respectively, and the rates of current smoking were 12% and 15%, respectively. Although we lost only 22 adolescents (4%) from our study group by the 11th grade, this group differed with respect to genotype and baseline smoking status. Although their progression by 11th grade is unknown, their omission from the group may have served to understate the effect of DRD2 on progression.
Another limitation of this work is that the adolescents’ self-reports of their depression symptoms were not confirmed by a formal clinical interview. Thus, the full extent of their depression cannot be determined, especially in light of the 1-week time frame used in the CES-D Scale measure. In addition, conduct disorder and antisocial problems were not measured in the current study, and evidence suggests comorbidity with smoking behavior (59).
Despite these potential limitations, the present study provides a first step toward identifying genetic determinants of smoking progression among adolescents, may provide a starting point for understanding the biology of smoking behavior and comorbid conditions in adolescents, and may inform smoking prevention and intervention efforts (16, 60).
While replication of these findings in other groups is necessary, these results provide directions for future investigations. Such studies could include animal and human laboratory investigations to elucidate the mechanisms of genetic and psychological influences on nicotine self-administration. Additional prospective studies that are amply powered to detect complex polygenic, psychological, and socioenvironmental interactions in different racial groups are also warranted. Given the substantial adolescent smoking problem in this country, the public health implications of such research could be significant.
 |
 |
Received April 14, 2003; revision received Aug. 19, 2003; accepted Nov. 21, 2003. From the Department of Psychiatry, the Abramson Cancer Center, and the Annenberg Public Policy Center, University of Pennsylvania; and the Department of Oncology, Lombardi Cancer Center, Georgetown University Medical Center, Washington, D.C. Address reprint requests to Dr. Audrain-McGovern, Department of Psychiatry, University of Pennsylvania, Suite 4100, 3535 Market St., Philadelphia, PA 19104; [email protected] (e-mail). Supported by Transdisciplinary Tobacco Use Research Center grant P50 84718 from the National Cancer Institute and the National Institute on Drug Abuse.
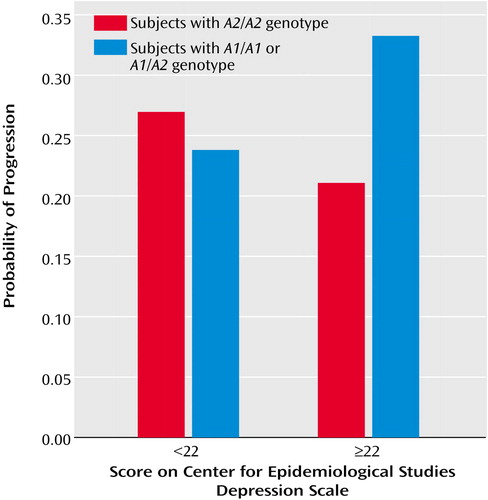
Figure 1. Interacting Effects of DRD2 Genotype and Depression Symptoms on Smoking Progression From 9th to 11th Grade in 292 Adolescentsa
aIncludes adolescents who had smoked at least one puff of a cigarette.
1. Heath A, Martin N: Genetic models for the natural history of smoking: evidence for a genetic influence on smoking persistence. Addict Behav 1993; 18:19–34Crossref, Medline, Google Scholar
2. Heath A, Kirk K, Meyer J, Martin N: Genetic and social determinants of initiation and age at onset of smoking in Australian twins. Behav Genet 1999; 29:395–407Crossref, Medline, Google Scholar
3. Kendler K, Neale M, Sullivan P, Corey L, Gardner C, Prescott C: A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med 1999; 29:299–308Crossref, Medline, Google Scholar
4. Madden P, Heath A, Pedersen N, Kaprio J, Koskenvuo M, Martin N: The genetics of smoking persistence in men and women: a multicultural study. Behav Genet 1999; 29:423–431Crossref, Medline, Google Scholar
5. Lerman C, Berrettini W: Elucidating the role of genetic factors in smoking behavior and nicotine dependence. Am J Med Genet 2003; 118B:48–54Google Scholar
6. Comings D, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D: The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics 1996; 6:73–79Crossref, Medline, Google Scholar
7. Noble EP, St Jeor ST, Ritchie T, Syndulko K, St Jeor SC, Fitch RJ, Brunner RL, Sparkes RS: D2 dopamine receptor gene and cigarette smoking: a reward gene? Med Hypotheses 1994; 42:257–260Crossref, Medline, Google Scholar
8. Spitz M, Shi H, Yang F, Hudmon K, Jiang H, Chamberlain R: Case-control study of the D2 dopamine receptor gene and smoking status in lung cancer patients. J Natl Cancer Inst 1998; 90:358–363Crossref, Medline, Google Scholar
9. Lerman C, Audrain J, Main D, Boyd N, Caporaso N, Bowman E, Lockshin B, Shields P: Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol 1999; 18:14–20Crossref, Medline, Google Scholar
10. Sabol S, Nelson M, Fisher C, Gunzerath L, Brody C, Hu S: A genetic association for cigarette smoking behavior. Health Psychol 1999; 18:7–13Crossref, Medline, Google Scholar
11. Bierut L, Rice J, Edenberg H, Goate A, Foroud T, Cloninger C: Family-based study of the association of the dopamine D2 receptor gene (DRD2) with habitual smoking. Am J Med Genet 2000; 90:299–302Crossref, Medline, Google Scholar
12. Jorm AF, Henderson AS, Jacomb PA, Christensen H, Korten AE, Rodgers B, Tan X, Easteal S: Association of smoking and personality with a polymorphism of the dopamine transporter gene: results from a community survey. Am J Med Genet 2000; 96:331–334Crossref, Medline, Google Scholar
13. Vandenbergh DJ, Bennett CJ, Grant MD, Strasser AA, O’Connor R, Stauffer RL, Vogler GP, Kozlowski LT: Smoking status and the human dopamine transporter variable number of tandem repeats (VNTR) polymorphism: failure to replicate and finding that never-smokers may be different. Nicotine Tob Res 2002; 4:333–340Crossref, Medline, Google Scholar
14. Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR: Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 2000; 22:133–139Crossref, Medline, Google Scholar
15. Sullivan P, Eaves L, Kendler K, Neale M: Genetic case-control association studies in neuropsychiatry. Arch Gen Psychiatry 2001; 58:1015–1024Crossref, Medline, Google Scholar
16. Merikangas KR, Risch N: Will the genomics revolution revolutionize psychiatry? Am J Psychiatry 2003; 160:625–635Link, Google Scholar
17. Lerman C, Swan GE: Non-replication of genetic association studies: is DAT all, folks? Nicotine Tob Res 2002; 4:247–249Crossref, Medline, Google Scholar
18. Covey LS, Tam D: Depressive mood, the single-parent home, and adolescent cigarette smoking. Am J Public Health 1990; 80:1330–1333Crossref, Medline, Google Scholar
19. Escobedo LG, Kirch DG, Anda RF: Depression and smoking initiation among US Latinos. Addiction 1996; 91:113–119Crossref, Medline, Google Scholar
20. Choi WS, Patten CA, Gillin JC, Kaplan RM, Pierce JP: Cigarette smoking predicts development of depressive symptoms among US adolescents. Ann Behav Med 1997; 19:42–50Crossref, Medline, Google Scholar
21. Breslau N, Kilbey MM, Andreski P: Nicotine dependence and major depression: new evidence from a prospective investigation. Arch Gen Psychiatry 1993; 50:31–35Crossref, Medline, Google Scholar
22. Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC: Smoking and major depression: a causal analysis. Arch Gen Psychiatry 1993; 50:36–43Crossref, Medline, Google Scholar
23. Lerman C, Main D, Audrain J, Caporaso N, Boyd N, Bowman E, Shields P: Depression and self-medication with nicotine: the modifying influence of the dopamine D4 receptor gene. Health Psychol 1998; 17:56–62Crossref, Medline, Google Scholar
24. Radloff LS: The CES-D Scale: a self-report depression scale for research in the general population. J Applied Psychol Measurement 1977; 1:385–401Crossref, Google Scholar
25. Kozlowski L: Rehabilitating a genetic perspective in the study of tobacco and alcohol use. Br J Addict 1991; 86:517–520Crossref, Medline, Google Scholar
26. Kozlowski LT, Harford MR: On the significance of never using a drug: an example from cigarette smoking. J Abnorm Psychol 1976; 85:433–434Crossref, Medline, Google Scholar
27. Audrain J, Tercyak KP, Goldman P, Bush A: Recruiting adolescents into genetic studies of smoking behavior. Cancer Epidemiol Biomarkers Prev 2002; 11:249–252Medline, Google Scholar
28. Harty LC, Caporaso NE, Hayes RB, Winn DM, Bravo-Otero E, Blot WJ, Kleinman DV, Brown LM, Armenian HK, Fraumeni JF Jr, Shields PG: Alcohol dehydrogenase 3 genotype and risk of oral cavity and pharyngeal cancers. J Natl Cancer Inst 1997; 89:1698–1705Crossref, Medline, Google Scholar
29. Kann L, Kinchen SA, Williams BI, Ross JG, Lowry R, Hill CV, Grunbaum JA, Blumson PS, Collins JL, Kolbe LJ: Youth risk behavior surveillance—United States, 1997. MMWR CDC Surveill Summ 1998; 47:1–89Google Scholar
30. Mayhew KP, Flay BR, Mott JA: Stages in the development of adolescent smoking. Drug Alcohol Depend 2000; 59(suppl 1):S61-S81Google Scholar
31. Brener ND, Collins JL, Kann L, Warren CW, Williams BI: Reliability of the Youth Risk Behavior Survey Questionnaire. Am J Epidemiol 1995; 141:575–580Crossref, Medline, Google Scholar
32. Brener ND, Kann L, McManus T, Kinchen SA, Sundberg EC, Ross JG: Reliability of the 1999 Youth Risk Behavior Survey Questionnaire. J Adolesc Health 2002; 31:336–342Crossref, Medline, Google Scholar
33. Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S: The validity of self-reported smoking: a review and meta-analysis. Am J Public Health 1994; 84:1086–1093Crossref, Medline, Google Scholar
34. Whalen CK, Jamner LD, Henker B, Delfino RJ: Smoking and moods in adolescents with depressive and aggressive dispositions: evidence from surveys and electronic diaries. Health Psychol 2001; 20:99–111Crossref, Medline, Google Scholar
35. Botvin GJ, Botvin EM: Adolescent tobacco, alcohol, and drug abuse: prevention strategies, empirical findings, and assessment issues. J Dev Behav Pediatr 1992; 13:290–301Crossref, Medline, Google Scholar
36. Audrain-McGovern J, Rodriguez DR, Moss HB: Smoking progression and physical activity. Cancer Epidemiol Biomarkers Prev 2003; 12:1121–1129Medline, Google Scholar
37. Rodriguez D, Audrain-McGovern J: Team sport participation and smoking: a growth mixture model. J Pediatr Psychol (in press)Google Scholar
38. Roberts RE, Lewinsohn PM, Seeley JR: Screening for adolescent depression: a comparison of depression scales. J Am Acad Child Adolesc Psychiatry 1991; 30:58–66Crossref, Medline, Google Scholar
39. Gritz ER, Prokhorov AV, Hudmon KS, Chamberlain RM, Taylor WC, DiClemente CC, Johnston DA, Hu S, Jones LA, Jones MM, Rosenblum CK, Ayars CL, Amos CI: Cigarette smoking in a multiethnic population of youth: methods and baseline findings. Prev Med 1998; 27:365–384Crossref, Medline, Google Scholar
40. Pierce JP, Choi WS, Gilpin EA, Farkas AJ, Merritt RK: Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol 1996; 15:355–361Crossref, Medline, Google Scholar
41. McCullagh P, Nelder JA: Generalized Linear Models. London, Chapman & Hall, 1998Google Scholar
42. Wacholder S, Rothman N, Caporaso N: Population stratification in epidemiologic studies of common genetic variants and cancer: quantification of bias. J Natl Can Inst 2000; 92:1151–1158Crossref, Medline, Google Scholar
43. Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, Morris CM, Perry RH, Ferrier IN, Court JA: D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 1997; 7:479–484Crossref, Medline, Google Scholar
44. Ritchie T, Noble EP: [3H]Naloxone binding in the human brain: alcoholism and the TaqI A D2 dopamine receptor polymorphism. Brain Res 1996; 718:193–197Crossref, Medline, Google Scholar
45. Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ: Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 1991; 48:648–654Crossref, Medline, Google Scholar
46. Noble E, Ozkaragoz T, Ritchie T, Zhang X, Belin T, Sparkes R: D2 and D4 dopamine receptor polymorphisms and personality. Am J Med Genet 1998; 81:257–267Crossref, Medline, Google Scholar
47. Clayton RR, Scutchfield FD, Wyatt SW: Hutchinson Smoking Prevention Project: a new gold standard in prevention science requires new transdisciplinary thinking. J Natl Cancer Inst 2000; 92:1964–1965Crossref, Medline, Google Scholar
48. Flay BR, Hu FB, Siddiqui O, Day LE, Hedeker D, Petraitis J, Richardson J, Sussman S: Differential influence of parental smoking and friends’ smoking on adolescent initiation and escalation of smoking. J Health Soc Behav 1994; 35:248–265Crossref, Medline, Google Scholar
49. Vandenbergh DJ, Kozlowski LT, Bennett CJ, Grant MD, Strasser AA, O’Connor R, Stauffer RL, Vogler GP: DAT’s not all, but it may be more than we realize. Nicotine Tob Res 2002; 4:251–252Crossref, Medline, Google Scholar
50. Wang MQ, Fitzhugh EC, Green BL, Turner LW, Eddy JM, Westerfield RC: Prospective social-psychological factors of adolescent smoking progression. J Adolesc Health 1999; 24:2–9Crossref, Medline, Google Scholar
51. Tercyak KP, Goldman P, Smith A, Audrain J: Interacting effects of depression and tobacco advertising receptivity on adolescent smoking. J Pediatr Psychol 2002; 27:145–154Crossref, Medline, Google Scholar
52. Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, Wileyto EP: Applying a behavioral economic framework to understanding adolescent smoking. Psychol Addict Behav 2004; 18:64–73Crossref, Medline, Google Scholar
53. Spring B, Pingitore R, McChargue DE: Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. Am J Psychiatry 2003; 160:316–322Link, Google Scholar
54. Cardenas L, Tremblay LK, Naranjo CA, Herrmann N, Zack M, Busto UE: Brain reward system activity in major depression and comorbid nicotine dependence. J Pharmacol Exp Ther 2002; 302:1265–1271Crossref, Medline, Google Scholar
55. Lewinsohn P, Rohde P, Seeley J: Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clin Psychol Rev 1998; 18:765–794Crossref, Medline, Google Scholar
56. Dent CW, Galaif J, Sussman S, Stacy A, Burton D, Flay BR: Demographic, psychosocial and behavioral differences in samples of actively and passively consented adolescents. Addict Behav 1993; 18:51–56Crossref, Medline, Google Scholar
57. Harrington KF, Binkley D, Reynolds KD, Duvall RC, Copeland JR, Franklin F, Raczynski J: Recruitment issues in school-based research: lessons learned from the High 5 Alabama Project. J Sch Health 1997; 67:415–421Crossref, Medline, Google Scholar
58. O’Donnell LN, Duran RH, San Doval A, Breslin MJ, Juhn GM, Stueve A: Obtaining written parent permission for school-based health surveys of urban young adolescents. J Adolesc Health 1997; 21:376–383Crossref, Medline, Google Scholar
59. Upadhyaya HP, Deas D, Brady KT, Kruesi M: Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiatry 2002; 41:1294–1305Crossref, Medline, Google Scholar
60. Insel TR, Collins FS: Psychiatry in the genomics era. Am J Psychiatry 2003; 160:616–620Link, Google Scholar



