Behavioral and Intellectual Markers for Schizophrenia in Apparently Healthy Male Adolescents
Abstract
OBJECTIVE: Subtle behavioral and intellectual abnormalities are often present in apparently healthy adolescents who later develop schizophrenia. The authors investigated whether these abnormalities can predict vulnerability for schizophrenia before the first psychotic manifestation. METHOD: The study consisted of linking the Israeli Draft Board Registry with the National Psychiatric Hospitalization Case Registry. The draft board tests measure intelligence, social functioning, organizational ability, interest in physical activity, and individual autonomy. Patients (N=509) were compared to nonpatients, i.e., adolescents not appearing in the National Psychiatric Registry (N=9,215), matched to patients by age, gender, and school attended at time of testing. RESULTS: Healthy male adolescents who were later hospitalized for schizophrenia had significantly lower test scores on all measures than adolescents not reported to the National Psychiatric Registry. The strongest predictors for schizophrenia were deficits in social functioning, organizational ability, and intellectual functioning. When patients were compared to matched nonpatients, the prediction model had a 75% sensitivity, a 100% specificity, a positive predictive value of 72%, and an overall rate of correct classification of 87.5%. Applied to the Israeli Draft Board Registry, the model yielded a sensitivity of 74.7%, a validated specificity of 99.7%, and a positive predictive value of 42.7%. CONCLUSIONS: This study demonstrated that simple assessment tools can predict predisposition to schizophrenia in healthy male adolescents. The model’s predictive ability does not change as a function of the time elapsed between testing and first hospitalization. This suggests that the model identifies apparently healthy individuals who will manifest the disease later who are not prodromal to psychosis. Easily applied tools allowing early identification of schizophrenia or vulnerability to it may enable early intervention.
Frequently, a diagnosis of schizophrenia is assigned when florid psychosis first manifests itself. However, converging evidence indicates that subtle behavioral and intellectual abnormalities often precede the first psychotic episode. Apparently healthy children and adolescents destined to develop schizophrenia manifest lower intelligence, withdrawn social behavior, conduct and adjustment abnormalities, and very mild neurological deficits (in comparison to classmates, siblings, matched comparison subjects, or population norms) (1–30).
Although the notion that some of the children and adolescents predestined to develop schizophrenia present subtle behavioral and intellectual abnormalities has been described long ago (29, 30), their prevalence, phenomenology, and pathophysiological significance are far from clear. Studies attempting to clarify the antecedents of schizophrenia have employed one of the following strategies: high-risk studies, birth cohort studies, retrospective studies, and follow-back studies. However, all strategies have inevitable methodological limitations because schizophrenia is a relatively low-incidence disease, and no powerful risk factors for schizophrenia have been identified; a high number of at-risk individuals has to be followed to obtain a small number of patients. As a result, high-risk studies are very expensive, and the results are not suitable to test multiple hypotheses with sufficient statistical power. Birth cohort studies, which draw patients from an epidemiological group, have the advantage of examining the early antecedents of a representative group of individuals who are later diagnosed with schizophrenia. However, like high-risk studies, they are very expensive and, in the end, yield a relatively small number of patients. Also, because these studies are generally not intended to specifically study schizophrenia but all aspects of development (31), they do not include measurements presumed to be relevant to schizophrenia like the high-risk studies. Studies based on a retrospective assessment of the developmental history of ill individuals are limited by selective recall and by the high prevalence of subtle behavioral and intellectual deviations in the general adolescent population that are retrospectively attributed to the illness. The present study is a follow-back or historical, prospective study. It has the advantage of including an entire national population; hence, it can examine markers predicting vulnerability to the illness with high statistical power. The study was done by merging the Israeli National Psychiatric Hospitalization Case Registry with the Israeli Draft Board Registry, which contains the scores of behavioral and intellectual assessments of male adolescents obtained at ages 16 to 17 years. The specific purpose of the study was to determine if future psychiatric hospitalization caused by schizophrenia could be predicted from the results of the Israeli Draft Board Registry preinduction assessment tests. If sensitive, specific, and reliable tests predictive of psychosis and hospitalization can be developed, it may be possible to treat at-risk individuals very early, perhaps improving the outcome of the illness (32–34).
METHOD
Draft Board Assessment
Israeli law requires that all adolescents between the ages of 16 and 17 undergo preinduction assessment to determine their intellectual, medical, and psychiatric eligibility for military service. This assessment is compulsory and is administered to the entire unselected population of Israeli adolescents. It includes individuals who are eligible for military service, as well as those who will be excused from service on the basis of medical, psychiatric, or social reasons.
The draft board assessment consists of 1) a physical examination, a review of systems, and a psychiatric history—all conducted by a physician; 2) a cognitive test battery; and 3) an interview assessing personality and behavioral traits, conducted by a psychometrician. The cognitive and behavior test battery and its validation are described in detail elsewhere (35).
The cognitive test battery yields a total score that is a highly valid measure of general intelligence equivalent to a normally distributed IQ (35). All tests are in pen-and-paper format and are administered by a trained psychometrician. The cognitive assessment is composed of four subtests: 1) Arithmetic—R, a multiple-choice test assessing reasoning, concentration, and concept manipulation. This subtest is similar to the arithmetic subtest from the Wechsler Intelligence Scale but contains twice as many items, some of which are more difficult than the Wechsler items. 2) Similarities—R, a multiple-choice test assessing verbal abstraction and categorization (i.e., the ability to understand the relationship between words and the use of this relationship in several contexts). It is a revised version of the similarities subtest from the Wechsler Intelligence Scale. Unlike in the Wechsler test, subjects are requested not only to identify and report the semantic or causal relationships between the test items but also to apply these relations to target items. 3) Raven’s Progressive Matrices—R, a modified version of Raven’s Progressive Matrices, which is a multiple-choice measure of nonverbal abstract reasoning and problem-solving abilities. 4) Otis test of mental ability, a modified, Otis-type verbal intelligence test adapted from the U.S. Army Alpha Instructions Test. It measures the ability to understand and carry out verbal instructions (36).
The behavioral assessment is done by a trained psychometrician who administers a structured interview evaluating 1) social functioning, which assesses social potency (e.g., likes to take charge, likes to be noticed at social events), and social closeness (e.g., sociable, has close interpersonal ties); 2) individual autonomy, which assesses personal autonomy, maturity, and self-directed behavior (e.g., the ability to function and make decisions independently); 3) organizational ability, which assesses compliance to timetables, self-mastery, and self-care (e.g., the ability to adhere to a schedule and tidiness responsibility); and 4) physical activity, which assesses involvement in extracurricular activities, concentrating on health-related physical activities (e.g., interest in sports and hiking). Behaviors are rated on a 1 (lowest)-to-5 (highest) scale on the basis of predetermined reliable and validated instructions. Examples of questions in the interview are as follows: How many good friends do you have? Do you tend to be the center of attention at parties? How often are you late for school? Do you consider yourself organized? Who cleans your room? (35). If the interviewer suspects any abnormal behavior or psychiatric illness, or if the scores achieved by the draftees are below predetermined standard norms, the draftees are referred for further assessment, which is conducted by a clinical psychologist or a psychiatrist.
National Psychiatric Hospitalization Case Registry
The National Psychiatric Hospitalization Case Registry is a complete listing of all psychiatric hospitalizations in Israel, including the ICD-9 diagnosis assigned and coded on discharge by a board-certified psychiatrist at the facility. All inpatient psychiatric facilities in the country, including day hospitals and psychiatric units in general hospitals, are required by law to report all admissions and discharges to the registry. Reporting is monitored by a special unit of the Ministry of Health that verifies reporting compliance and consistency of information, thus, ensuring the completeness and correctness of the registry.
Study Population
The National Psychiatric Hospitalization Case Registry file was merged with the Israeli Draft Board Registry file by the managers of the registry by using an algorithm to preserve medical record confidentiality (37) and in compliance with local institutional review board approval. The linking variable was the national identification number (equivalent to the U.S. Social Security number). The merged file included all adolescents assessed by the draft board between 1985 and 1991 who were reported to the National Psychiatric Hospitalization Case Registry between 1970 and 1995 and given a diagnosis of schizophrenia. This allowed for a follow-up of between 4 and 10 years. Because the hypothesis tested was that both behavioral and intelligence variables predict future schizophrenia and because the draft board administered intelligence but not behavioral tests to adolescent girls, only adolescent boys were included in the current analysis.
The merger of the draft board file with the National Psychiatric Hospitalization Case Registry identified 994 adolescent boys with a diagnosis of schizophrenia, bringing the risk for schizophrenia in this population to 0.52%, similar to the risk reported by others (17, 38). To limit this analysis to apparently healthy individuals with no obvious signs of disease, 359 of the 994 draftees who presented clinically detectable signs or symptoms of any mental illness or of mental retardation for which they were exempted from the draft were excluded from further analysis. Also, in order to lessen the chance of including patients in the prodrome or initial stages of the disease, an additional 62 individuals who had a psychiatric hospitalization before the draft board assessment or within 1 year from the date of draft board assessment were excluded. An additional 64 of the remaining 573 individuals were excluded from further analysis because of incomplete test data. The proportion of missing data in the entire Israeli Draft Board Registry was similar to the proportion of missing data in the study population (11% versus 13%). The remaining group used for analysis included 509 draftees who had their first hospitalization for schizophrenia 1 year or more after testing (defined as patients).
The patients’ scores were compared to the mean scores of all individuals tested at the same age and attending the same high school who were found eligible for military service and did not appear in the case registry (defined as nonpatients) (N=9,215). Matching patients to nonpatients by high school attended at the time of testing was an attempt to control for educational and social opportunities. To avoid unforeseeable biases that might affect case comparison studies (39), in addition to comparisons to classmates, patients were compared to the entire population of draftees eligible for military service not identified in the case registry. At time of testing, the patients had moderately—but statistically significantly—fewer years of formal education than the entire draft-eligible population (mean=10.5 years, SD=2.1, versus mean=11.3, SD=1.5) (t=11.03, df=171,971, p=0.0001).
Data Analyses
Data analyses examined the extent to which test scores could be used to correctly classify individuals as patients or nonpatients. In the first stage of the analysis, the 509 patients were compared to their matched comparison subjects (i.e., nonpatient schoolmates) by using a paired-samples t test. The next stage of the analysis examined differences between patients and nonpatients on the four behavioral measures and on intellectual functioning by using the chi-square test. To adjust for multiple comparisons, the significance level was set at p<0.01.
To evaluate the independent predictive value of the preinduction test scores, multivariate logistic regression was carried out. The predictive model was further refined by using conditional logistic regression (40). The conditional logistic regression model was specifically developed for the analysis of matched comparison data, which are usually highly stratified (40). The conditional logistic regression model is highly effective in analyzing retrospective matched data (41). In this model, for each pair of predictor variables (e.g., scores of social functioning for patients and nonpatients), the difference between the corresponding values for the patient and the nonpatient is calculated, yielding the difference in social functioning—a delta score. Using this delta score in the conditional logistic regression yielded a model specificity of 100%. To further test the model’s specificity, the model was applied to the entire population, excluding individuals diagnosed by the draft board assessment as suffering from a psychiatric illness, subsyndromal psychiatric manifestations, severely abnormal behavior, or mental retardation. The results of this procedure are referred to as validated specificity.
To avoid the bias inherent in using the same data to test the predictive accuracy of the model, the jackknifing method was applied to the data. To account for the prevalence of schizophrenia in the general population (i.e., prior probability), Bayes’s theorem was applied to the model (42). The logistic regression was carried out in a step-forward fashion. All analyses were done by using PROC FREQ, PROC MEANS, PROC T-TEST, and PROC LOGISTIC from the SAS package.
RESULTS
As a group, the individuals destined to develop schizophrenia (N=509) obtained statistically significant lower (worse) scores on all measures than matched nonpatients (N=9,215) and the entire population (all p values were lower than 0.0001).
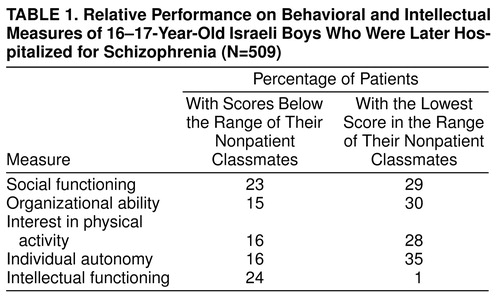
The differences between patients and their respective nonpatients are presented in Table 1. This table specifies the percentages of patients who performed below the normal range relative to their comparison group (i.e., a comparison to the poorest performance of a healthy individual of the same age attending the same high school) on each measure. As shown, a high proportion of the patients performed below the range of their nonpatient peers. This was most pronounced in social functioning, where 23.2% of the patients performed below the range of their nonpatient classmates and 29.1% performed at the lowest normal range. Thus, 52.3% of the patients had a social functioning score that was equal to or lower than that of their matched nonpatients. This analysis illustrates the power of comparing patients to matched subjects from the same school.
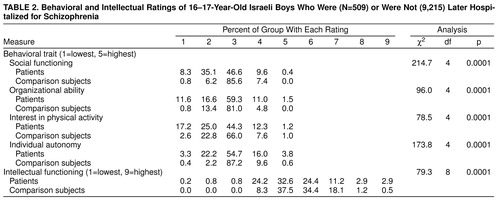
Table 2 presents a comparison of the distribution of all patients to all nonpatients. For this and subsequent analyses, the mean score of the nonpatient matched comparison subjects was used. As shown, there are large and statistically significant differences on all behavioral measures. Again, the most pronounced differences were in social functioning, where 8.3% of patients had the lowest score and 35.1% had the second-to-lowest score, whereas only 0.8% and 6.2% of nonpatients, respectively, had scores in these categories. By using, for example, the social functioning scale alone, with a cut-off point of the lowest two quintiles, one could accurately predict membership in the patient group in 43.4% of the patients (8.3% plus 35.1%) and membership in the nonpatient group in 93.0% (85.6% plus 7.4% plus 0.0%). On the measure of intellectual functioning, which was rescaled into a variable with nine categories, there was also a large and statistically significant difference between patients and nonpatients. Despite the differences, it should be noted that patients performed over the same range as nonpatients.
The combined predictive ability of the four behavioral measures and intellectual functioning were studied by using logistic regression. Using standard logistic regression, we found that the best predictors of future schizophrenia were poor social functioning (Wald χ2=87.9, df=1, p=0.0001; odds ratio=3.22, 95% confidence interval [CI]=2.54–4.13) and lower intellectual functioning (scaled) (Wald χ2=6.5, df=1, p=0.01; odds ratio=1.18, 95% CI=1.04–1.33). Using a cutoff point of 0.7, which best divided the groups, we obtained a sensitivity of 53.1% and a validated specificity of 95.0% (model χ2=129.6, df=2, p=0.0001), with an overall rate of correct classification of 92.8% and a positive predictive value of 36.9%. The positive predictive value was calculated by using the sensitivity and validated specificity values, assuming a prevalence rate of 1% for schizophrenia.
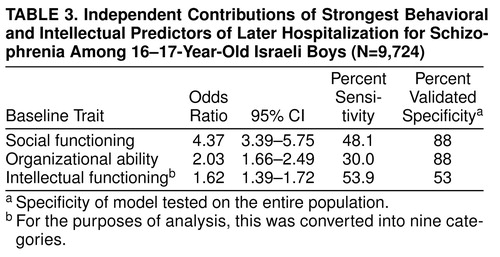
The predictive model was further refined by using conditional logistic regression, which found that the strongest predictors of future schizophrenia were poor social functioning (Wald χ2=69.2, df=1, p=0.0001; odds ratio=2.9, 95% CI=2.22–3.94), poor organizational ability (Wald χ2=42.7, df=1, p=0.0001; odds ratio=1.4, 95% CI=1.09–1.81), and low intellectual functioning (scaled) (Wald χ2=7.5, df=1, p=0.006; odds ratio=1.5, 95% CI=1.30–1.83). With a cutoff point of 0.5, the model had an overall rate of correct classification of 87.5% (sensitivity=75.0%, validated specificity=99.7%, model χ2=117.1, df=3, p=0.0001) and a positive predictive value of 71.6%. The positive predictive value was calculated by using the sensitivity and validated specificity values, assuming a prevalence rate of 1% for schizophrenia. The independent contribution of each predictor variable is presented in Table 3.
To further test the model, the study population was randomly divided into two halves, and the same logistic regression procedure was conducted on each half. The resultant regression coefficients were used to classify the other half of the group and vice versa (43). Both models identified social functioning, organizational ability, and intellectual functioning as the variables best predictive of future schizophrenia. The sensitivities ranged from 74.2% to 75.9%, and specificity was not affected.
Moreover, the model was applied to the entire healthy population, after excluding individuals diagnosed by the draft board preinduction assessment as suffering from a psychiatric illness, subsyndromal psychiatric manifestations, severely abnormal behavior, or mental retardation. This procedure yielded a sensitivity of 74.7%, a specificity of 99.7%, a correct classification of 99.6%, and a positive predictive value of 42.7% (calculated according to the cohort prevalence values).
To test for linearity of the relationship between risk for schizophrenia and test scores, an additional variable was added to the model aimed at assessing departure from linearity (40). The results indicated that there is a linear relationship between risk of schizophrenia and intellectual functioning (i.e., no significant departure from linearity, Wald χ2=2.6, df=1, p=0.11) but no linear relationship between risk of schizophrenia and behavioral variables (social functioning: Wald χ2=25.4, df=1, p=0.0001; organizational ability: Wald χ2=19.7, df=1, p=0.0001).
To distinguish whether the prediction model identifies future occurrence of schizophrenia in apparently healthy individuals or in individuals already experiencing prodromal symptoms, the data were reanalyzed as a function of the time that elapsed between the draft board testing and the first hospitalization. If the model was identifying prodromal or mildly symptomatic individuals, the model’s predictive ability should differ as a function of time elapsing between testing and hospitalization (i.e., the model’s predictive ability should be better for patients hospitalized closer to the time of testing). To assess this assumption, the group was divided into two on the basis of the median time difference between testing and first hospitalization. The two groups were therefore composed of patients hospitalized 1 to 4 years after testing and patients hospitalized 4 to 10 years after testing. A conditional logistic regression was conducted on each of the groups. There was only a 5% difference in sensitivity between the groups and no difference in specificity, indicating that the model has a similar predictive ability regardless of the time lag between testing and first hospitalization. These results, which suggest no difference between the groups, were further confirmed by comparing the means of the two groups on the predictor variables by using independent group t tests, which revealed no significant differences.
DISCUSSION
Main Findings
Results of this study strongly suggest that scores measuring social functioning, organizational ability, and intellectual functioning can be used to predict future hospitalization for schizophrenia in a population of apparently healthy male adolescents. The study also confirms and extends existing reports indicating that as a group, individuals destined to develop schizophrenia manifest subtle behavioral and intellectual abnormalities before the symptoms essential to diagnose schizophrenia become evident.
The model assessing risk for schizophrenia is based on differences in test scores between individuals who will develop schizophrenia in the future and their healthy schoolmates. The model is sensitive, specific, and appropriate for use with population-based groups, since it takes into account the prevalence of schizophrenia in the general population. Social functioning and intellectual performance scores reported here to predict risk for schizophrenia were also reported to predict risk for schizophrenia in other population-based studies (19, 20) and in case-control studies (17).
Schizophrenia did not occur exclusively within the population with the poorest intellectual performance, nor did it spare those who performed very well. Rather, a linear association was revealed between greater risk for schizophrenia and poor cognitive performance. These results indicate that risk for schizophrenia is a function of intellectual performance over the entire range of cognitive scores in the population and that intellectual impairment does not necessarily define a subgroup of individuals with schizophrenia (17, 19).
Poor performance of patients, relative to nonpatients, was also evident in variables assessing behavior. Poor social adjustment, characterized by few and tenuous social relationships, was the most powerful variable characterizing patients. Additional predictive behavioral attributes were the ability to function independently in everyday life, precise timing and organizational ability, and participation in physical activities, which were also lower in patients. These results support existing data indicating relatively poor premorbid behavioral and personality adjustment among those destined to be diagnosed with schizophrenia, especially manifested in impaired social relationships (16, 17).
Limitations of the Study
Since this is a case registry study in which the diagnosis was made by the treating physician on the basis of signs, symptoms, and history and not a research diagnosis, concerns regarding the validity and reliability of the diagnoses are pertinent (44). However, the wide acceptance of the DSM-III, DSM-IV, and ICD-9 criteria since the 1980s addresses this concern. A study examining the accuracy of the diagnosis of affective disorders and schizophrenia in public hospitals found convincing evidence that the tendency to overdiagnose schizophrenia has diminished and the agreement between chart diagnosis and research diagnosis is very good (45). This report does not address the specificity of the predictive model in terms of psychiatric diagnosis. Patients suffering from nonschizophrenic psychosis or from affective disorders also manifest psychological and behavioral antecedents that, as a group, distinguish them from normal comparison subjects (19, 46). In a preliminary analysis of our data, patients who later developed affective disorder and schizoaffective disorder obtained scores that were intermediate between scores of nonpatients and patients with schizophrenia on most, but not all, behavioral and intellectual variables.
The results are limited to male adolescents, since the draft board administers behavioral tests only to male adolescents (female adolescents only undergo cognitive assessment). Since male patients are more likely than female patients to be hospitalized for schizophrenia (47), and since male patients may suffer from a more severe form of illness (48), the more severely ill patients might be overrepresented in this study.
Despite the fact that this study is based on an entire country’s population of adolescents and on a national psychiatric registry, it is still possible that some patients have been missed. Although the overwhelming majority of individuals who receive a diagnosis of schizophrenia in the course of their lives will be hospitalized at their first psychotic episode or shortly thereafter (49), some individuals who suffer from schizophrenia are never hospitalized and others are hospitalized later in life. Hence, most, but not all, individuals affected by schizophrenia are included in the National Psychiatric Hospitalization Case Registry. Nevertheless, the calculated risk for schizophrenia by age 26 in the registry was 0.52%, which is close to the predicted risk of 0.61% by age 40 (16, 17) and compatible with the incidence of schizophrenia in other studies (38). Hence, our study population probably includes a good representation of lifelong incidence of schizophrenia. Nevertheless, it is conceivable that the predictive model would differ in less severely ill patients who never require hospitalization. Also, because the follow-up period does not cover the entire at-risk period for schizophrenia, some of the nonpatients who are not different from patients in performance might become patients later in life.
The draft board assessment is intended to assign recruits to training according to general intelligence and personality but not specifically to detect recruits who will manifest schizophrenia. Those who will eventually develop the illness, as a group, perform slightly, but not dramatically, worse than the entire population of recruits. Hence, there is considerable overlap between individual scores obtained by patients and the entire population. Furthermore, since the base rate of schizophrenia is less than 1%, for each abnormally low score, there will be more nonpatients than patients, despite the fact that the proportion of patients obtaining the low score will also be larger than the proportion of nonpatients obtaining the low score.
The predictive method presented here refers to the specific tests used by the Israel Defense Forces Draft Board; however, there is no reason to believe that the model is confined to just this country or is dependent on this test battery. The tests are administered in a standardized fashion, are continuously validated, and have available population norms (35). Parts of the tests resemble the U.S. Army Alpha Instructions Test, which is a well-known and extensively employed psychometric tool. Similar models can be developed by using standard psychological tests that assess behavioral and intellectual variables (e.g., the Wechsler Intelligence Scales, the Life Stressors and Social Resources Inventory [50], and the NEO Personality Inventory [51]), which are not dissimilar to the tests used in this investigation.
It could also be argued that the tests and the model identify individuals who are already ill but not yet hospitalized or individuals in an active prodromal phase. However, patients who were hospitalized before the assessment by the draft board or within 1 year from the date of assessment were not included in the analyses. Moreover, it is important to point out that the draft board assessment team maintains a low threshold for diagnosis of mental illness or abnormal behavior. For example, approximately one-third of the individuals who eventually appeared in the National Psychiatric Hospitalization Case Registry with a diagnosis of schizophrenia were identified by the draft board and excused from military service. Thus, despite this rigorous assessment process, no mental illness or grossly abnormal behavior was detected in these 509 male adolescents destined to develop schizophrenia and included as patients in this analysis. The debate as to whether the subtle behavioral and intellectual abnormalities present in many, but not all, of the draftees destined to develop schizophrenia but classified by the draft board assessment as healthy individuals could be called prodromal schizophrenia is not within the scope of this report. If, indeed, schizophrenia is the most severe manifestation of a group of hierarchical developmental disorders such as learning and language disabilities, attention deficit hyperactivity disorder, conduct disorder, and autism, or if these disorders are common comorbid conditions (52), it may be impossible to draw a clear line between comorbid conditions, early antecedents, premorbid adjustments, and prodromal stages of the disease.
Strengths of the Study
The main strength of this study was that it was a population-based, case registry study with no apparent delineating base population difficulties and the reidentification of individuals across different data sets. The use of such a cohort protects against selection and information biases (39) and enables analysis of a low-prevalence disease with high statistical power.
Results of this study are consistent with most published studies in terms of the magnitude of differences in social and cognitive performance between patients and nonpatients and in terms of odds ratios for individual test items. However, the ability of the predictive model to identify future schizophrenia patients seems to be higher. Unlike studies that use either cognitive (19) or behavioral (20) parameters to identify future schizophrenia patients, this study used both cognitive and behavioral measures. Since, for some patients, cognitive impairment might be the predominant abnormality in the context of almost normal social behavior and vice versa for others, the use of both parameters might improve sensitivity.
Using the logistic regression model presented here yields the best-fitted model reflecting risk for schizophrenia, but there are even simpler ways to predict risk for schizophrenia. For example, individuals in our study population whose scores were 1 standard deviation below the norm on social functioning, 0.5 standard deviation below the norm on intellectual functioning, and below the norm to any extent on at least one additional personality or behavioral measure had at least an 80% chance of developing schizophrenia. Using this rule of thumb yields a model with a 30% sensitivity and a 99.8% validated specificity.
Comment
The results reported here are consistent with the developmental hypothesis of schizophrenia. The early developmental hypothesis (53, 54) suggests that an infranatal event determines cortical pathology, which alone or in combination with genetic (55) and environmental (56) factors, manifests as schizophrenia. According to this hypothesis, in the course of normal brain maturation, an unfolding multistage pattern leads from early, clinically indiscernible manifestation such as the behavioral and intellectual abnormalities reported here to psychotic symptoms. A corollary hypothesis suggests that early brain pathology manifested as subtle behavioral and intellectual abnormalities acts as a risk factor rather than a sufficient factor, which, in combination with other risk factors or in the absence of protective factors, may or may not manifest as schizophrenia (52). The late neurodevelopmental formulation (57) suggests that excessive or insufficient synaptic elimination during adolescence is the main event responsible for schizophrenic manifestation, implying that the earlier childhood abnormalities are nonspecific risk factors. This formulation may explain why behavioral and intellectual markers collected in 16- and 17-year-olds, as in the study reported here, are much more specific and sensitive than markers collected in younger individuals (16, 17).
Easily applicable, reliable, and specific markers to predict schizophrenia, along with the availability of safer and better-tolerated antipsychotic drugs, may improve the risk-to-benefit ratio of early intervention and, it is hoped, the outcome of the illness (32, 58–62). However, before any prevention recommendations can be implemented, it is essential to consider the potential limitations and pitfalls of predicting schizophrenia on the basis of behavioral and intellectual assessments but in the absence of reliable biological markers. This dilemma must be addressed by using a true prospective design. This future study will follow up on individuals identified by the prediction equation as being at high risk for schizophrenia and an age-, gender-, and school-matched comparison group identified by the equations as being at low risk for schizophrenia. These individuals will receive a comprehensive neuropsychological and behavioral assessment and will be followed prospectively. This design will enable prospective testing of the sensitivity and specificity of the prediction model and its utility for standard clinical practice and will yield detailed, disease-specific neuropsychological and behavioral data characterizing future patients. Although the proposed study lacks a biological marker, its results will aid in the differential diagnosis of adolescents with behavioral or intellectual abnormalities for whom future schizophrenia is a part of the differential diagnosis.
Received Oct. 6, 1998; revision received Feb. 11, 1999; accepted Feb. 23, 1999. From Tel-Aviv University; Hebrew University, Jerusalem; Bar Ilan University, Ramat-Gan, Israel; and Ben-Gurion University, Beer-Sheva, Israel. Address reprint requests to Dr. Davidson, Chaim Sheba Medical Center, Tel-Hashomer 52621, Israel; [email protected] (e-mail)
 |
 |
 |
1. Bower EM, Shellhamer TA: School characteristics of male adolescents who later became schizophrenics. Am J Orthopsychiatry 1960; 30:712–729Crossref, Google Scholar
2. Albee GW, Lane EA, Reuter JM: Childhood intelligence of future schizophrenics and neighborhood peers. J Psychol 1964; 58:141–144Crossref, Medline, Google Scholar
3. Schwartzman AE, Douglas VI: Intellectual loss in schizophrenia, part I. Can J Psychol 1962; 16:1–10Crossref, Medline, Google Scholar
4. Lane EA, Albee GW: Childhood intellectual development of adult schizophrenics. J Abnorm Soc Psychol 1963; 67:186–189Crossref, Medline, Google Scholar
5. Offord DR: School performance of adult schizophrenics, their siblings and age mates. Br J Psychiatry 1974; 125:12–19Crossref, Medline, Google Scholar
6. Watt NF, Lubensky AW: Childhood roots of schizophrenia. J Consult Clin Psychol 1976; 44:363–375Crossref, Medline, Google Scholar
7. Fish C: Neurobiological antecedents of schizophrenia in children. Arch Gen Psychiatry 1977; 34:1297–1313Google Scholar
8. Griffith JJ, Mednick SA, Schulsinger F, Diderichsen B: Verbal associative disturbances in children at high risk for schizophrenia. J Abnorm Psychol 1980; 89:125–131Crossref, Medline, Google Scholar
9. Andreasen NC, Hoenk PR: The predictive value of adjustment disorders: a follow-up study. Am J Psychiatry 1982; 139:584–590Link, Google Scholar
10. Aylward E, Walker E, Bettes B: Intelligence in schizophrenia: a meta analysis of the research. Schizophr Bull 1984; 10:430–459Crossref, Medline, Google Scholar
11. Erlenmeyer-Kimling L, Cornblatt B: Behavioral risk factors in children of schizophrenic parents. J Autism Dev Disord 1984; 14:357–374Crossref, Medline, Google Scholar
12. Walker E, Lewine RJ: Prediction of adult-onset schizophrenia from childhood home movies of the patients. Am J Psychiatry 1990; 147:1052–1056Google Scholar
13. Ambelas A: Preschizophrenics: adding to the evidence, sharpening the focus. Br J Psychiatry 1992; 160:401–404Crossref, Medline, Google Scholar
14. Crawford JR, Besson JAO, Bremner M, Ebmeier KP, Cochrane RHB, Kirkwood K: Estimation of premorbid intelligence in schizophrenia. Br J Psychiatry 1992; 161:69–74Crossref, Medline, Google Scholar
15. Marcus J, Hans SL, Auerbach JG, Auerbach AG: Children at risk for schizophrenia: the Jerusalem infant development study, II: neurobehavioral deficits at school-age. Arch Gen Psychiatry 1993; 50:797–809Crossref, Medline, Google Scholar
16. Done DJ, Crow TJ, Johnstone EC, Sacker A: Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. BMJ 1994; 309:699–703Crossref, Medline, Google Scholar
17. Jones P, Rodgers B, Murray R, Marmot M: Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 1994; 344:1398–1402Google Scholar
18. Russell AJ, Munro JC, Jones PB, Hemsley DR, Murray RM: Schizophrenia and the myth of intellectual decline. Am J Psychiatry 1997; 154:635–639Link, Google Scholar
19. David AS, Malmberg A, Brandt L, Allebeck P, Lewis G: IQ and risk for schizophrenia: a population-based cohort study. Psychol Med 1998; 27:1311–1323Google Scholar
20. Malmberg A, David A, Allebeck P, Lewis G: Premorbid adjustment and personality in schizophrenia: a review and historical cohort study. Br J Psychiatry 1998; 172:308–312Crossref, Medline, Google Scholar
21. McGlashan TH: Early detection and intervention of schizophrenia: rationale and research. Br J Psychiatry 1998; 172:3–6Crossref, Google Scholar
22. Olin SCS, Mednick SA, Cannon T, Jacobsen B, Parnas J, Schulsinger F, Schulsinger H: School teacher ratings predictive of psychiatric outcome 25 years later. Br J Psychiatry 1998; 172:7–13Crossref, Medline, Google Scholar
23. Yung AR, Philips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S, Patton GC, Jackson HJ: Prediction of psychosis: a step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl 1998; 172:14–20Crossref, Medline, Google Scholar
24. Kremen WS, Buka SL, Seidman LJ, Goldstein JM, Koren D, Tsuang MT: IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study. Am J Psychiatry 1998; 155:672–677Link, Google Scholar
25. Nopoulos P, Flaum M, Arndt S, Andreasen N: Morphometry in schizophrenia revisited: height and its relationship to pre-morbid function. Psychol Med 1998; 28:655–663Crossref, Medline, Google Scholar
26. Carter JW, Parnas J, Cannon TD, Schulsinger F, Mednick SA: MMPI variables predictive of schizophrenia in the Copenhagen High-Risk Project: a 25-year follow-up. Acta Psychiatr Scand 1999; 99:432–440Crossref, Medline, Google Scholar
27. Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttunen T, Rabe-Hesketh S, Murray RM: School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry 1999; 56:457–463Crossref, Medline, Google Scholar
28. Parnas J: From predisposition to psychosis: progression of symptoms in schizophrenia. Acta Psychiatr Scand Suppl 1999; 395:20–29Crossref, Medline, Google Scholar
29. Kraepelin E: Dementia Praecox and Paraphrenia. Translated by Barclay RM; edited by Robertson GM. Edinburgh, E & S Livingstone, 1919Google Scholar
30. Kretschmer E: Physique and Character (1921). London, Kegan Paul, 1936Google Scholar
31. Wadsworth MEJ: The Imprint of Time: Childhood History and Adult Life. Oxford, UK, Clarendon Press, 1991Google Scholar
32. Johnstone EC, Crow TJ, Johnson AL, MacMillan JF: The Northwick Park study of first episode schizophrenia, 1: presentation of the illness and problems relating to admission. Br J Psychiatry 1986, 148:115–120Google Scholar
33. Loebel AD, Lieberman JA, Alvir JMJ, Meyerhoff DI, Geisler SH, Szymanski SR: Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry 1992; 149:1183–1188Google Scholar
34. McGorry P: Preventive strategies in early psychosis: verging on reality. Br J Psychiatry 1998; 172:7–13Crossref, Medline, Google Scholar
35. Gal R: The selection, classification and placement process, in A Portrait of the Israeli Soldier. Westport, Conn, Greenwood Press, 1986, pp 77–96Google Scholar
36. Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
37. Rabinowitz J: A method for preserving confidentiality when linking computerized registries (letter). Am J Public Health 1998; 88:836Crossref, Medline, Google Scholar
38. Bromet EJ, Dew MA, Eaton W: Epidemiology of psychosis with special reference to schizophrenia, in Psychiatric Epidemiology. Edited by Tsuang MT, Tohen M, Zahner GEP. New York, John Wiley & Sons, 1995, pp 283–300Google Scholar
39. Mortensen PB: The untapped potential of case registers and record-linkage studies in psychiatric epidemiology. Epidemiol Rev 1995; 17:205–209Crossref, Medline, Google Scholar
40. Hosmer D, Lemeshow S: Applied Logistic Regression. New York, Wiley-Interscience, 1989Google Scholar
41. Stokes EM, Davis CS, Koch GG: Categorical Data Analysis Using the SAS System. Cary, NC, SAS Institute, 1995Google Scholar
42. SAS/STAT User’s Guide, 4th ed. Cary, NC, SAS Institute, 1990Google Scholar
43. Tabachnick BG, Fidell LS: Using Multivariate Statistics, 3rd ed. New York, HarperCollins, 1996Google Scholar
44. Lipton AA, Simon FS: Psychiatric diagnosis in a state hospital: Manhattan State revisited. Hosp Community Psychiatry 1985; 36:368–373Abstract, Google Scholar
45. Pulver AE, Carpenter WT, Adler L, McGrath J: Accuracy of the diagnosis of affective disorders and schizophrenia in public hospitals. Am J Psychiatry 1988; 145:218–220Link, Google Scholar
46. Van Os J, Jones P, Lewis G, Wadsworth M, Murray R: Developmental precursors of affective illness in a general population birth cohort. Arch Gen Psychiatry 1997; 54:625–631Crossref, Medline, Google Scholar
47. Munk-Jorgensen P: The schizophrenia diagnosis in Denmark: a register-based investigation. Acta Psychiatr Scand 1985; 72:266–273Crossref, Medline, Google Scholar
48. Meltzer HY, Rabinowitz J, Lee MA, Cola P, Ranjan R, Findling RL, Thompson PA: Age at onset and gender of schizophrenic patients in relation to neuroleptic resistance. Am J Psychiatry 1997; 154:475–482Link, Google Scholar
49. Castle DJ, Phelan M, Wessely S, Murray RM: Why patients with non-affective functional psychosis are not admitted at first psychiatric contact. Br J Psychiatry 1994; 165:101–106Crossref, Medline, Google Scholar
50. Daniels D, Moos RH: Assessing life stressors and social resources among adolescents: applications to depressed youth. J Adolescent Res 1990; 5:268–289Crossref, Google Scholar
51. McCare RR, Costa PT: Comparison of EPI and psychoticism scales with measures of the five-factor model of personality. Personality and Individual Differences 1985; 6:587–597Crossref, Google Scholar
52. Hollins C, Taylor E: Schizophrenia: a critique from the developmental psychopathology perspective, in Neurodevelopment and Adult Psychopathology. Edited by Keshavan MS, Murray RM. New York, Cambridge University Press, 1997, pp 213–233Google Scholar
53. Murray RM, Lewis SW: Is schizophrenia a neurodevelopmental disorder? BMJ 1987; 295:681–682Google Scholar
54. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
55. Weickert CS, Weinberger D: A candidate molecule approach to defining developmental pathology in schizophrenia. Schizophr Bull 1998; 24:303–316Crossref, Medline, Google Scholar
56. Kendler KS: The genetics of schizophrenia, in Handbook of Schizophrenia, vol 3: Nosology, Epidemiology and Genetics. Edited by Tsuang MT, Simpson JC. Amsterdam, Elsevier Science, 1988, pp 437–462Google Scholar
57. Feinberg I: Schizophrenia as an emergent disorder of the late brain maturation, in Neurodevelopment and Adult Psychopathology. Edited by Keshavan MS, Murray RM. New York, Cambridge University Press, 1997, pp 237–252Google Scholar
58. Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM: Schizophrenia after prenatal famine: further evidence. Arch Gen Psychiatry 1995; 53:25–31Crossref, Google Scholar
59. Wyatt RJ: Neuroleptics and the natural course of schizophrenia. Schizophr Bull 1991; 17:325–351Crossref, Medline, Google Scholar
60. Falloon IRH, Coverdale JH, Laidlaw TM: Early interventions for schizophrenic disorders. Br J Psychiatry 1998; 172:33–38Crossref, Google Scholar
61. Jablenski A: High risk, low prediction: implication for early intervention. Br J Psychiatry 1998; 172:314–315Google Scholar
62. Tsuang MF, Faraone SV: The concept of target features in schizophrenia research. Acta Psychiatr Scand Suppl 1999; 395:2–11Crossref, Medline, Google Scholar



