Visual Event-Related Potentials in Subjects With Alexithymia: Modified Processing of Emotional Aversive Information?
Abstract
OBJECTIVE: A modified autonomous response (e.g., electrodermal activity) in subjects with alexithymia (a reduced ability to identify and communicate emotions) while processing emotional information is well known. However, the functional and neurobiological bases of this impairment are unclear. Do subjects with alexithymia suffer from a primary lack of perception (“emotional blindness”), or is alexithymia based on incomplete information processing due to immature undifferentiated cognitive schemes? The study investigates if subjects with alexithymia show a modified central response as a correlate of classifying emotional aversive stimuli. METHOD: Twenty subjects with high alexithymia and 20 with low alexithymia (selected by the 20-item Toronto Alexithymia Scale) were investigated within a modified odd-ball paradigm. Three different stimulus categories were presented: aversive (probes) and affective neutral pictures (nontargets and instructed targets). Visual event-related EEG potentials and subjective data were recorded. RESULTS: All subjects showed elevated positive amplitudes or mean activity after probe presentation in the latency range: 150–260, 280–450, and 600–1500 msec. Subjects with alexithymia displayed increased positive components (especially P2) of visual event-related potentials after probe presentation than subjects without alexithymia. Subjects without alexithymia more frequently verbalized the emotional impact of these aversive pictures than subjects with alexithymia. CONCLUSIONS: These findings do not support the assumption of a primary lack of perception in alexithymia. Subjects with alexithymia show central correlates of perception and classification of aversive pictures. They may need more effort and cognitive recourses to process emotional information. Nevertheless, spontaneous verbal reference to emotional stimulus aspects is reduced.
From the beginning of the 20th century, it has been reported that a subgroup of mentally ill patients have shown a reduced ability to identify and communicate feelings or emotional aspects of social interaction. This deficit, named alexithymia by Sifneos (1), is characterized by difficulty in identifying and describing subjective feelings, difficulty in distinguishing between feelings and bodily sensations of emotional arousal, a poor fantasy life, and an externally oriented cognitive style (2–4). In cross-sectional studies, a high prevalence of alexithymia was shown in clinical groups (e.g., with somatoform disorders [5, 6], depression [7], panic disorders [8, 9], or idiopathic hypertonia [10, 11]). In an epidemiological longitudinal study, even a reduced life expectancy was reported for alexithymic men (12). More recent studies have demonstrated the stability of alexithymia that is independent from the course of clinical symptoms (4, 13–16). However, it is still being debated whether alexithymia represents a vulnerability factor that promotes the appearance of psychic or physical symptoms, especially under emotional stress.
Perhaps as a result of a developmental deficit, subjects with alexithymia may have an incomplete set of cognitive-emotional schemes (17), which does not allow the integration of physical, motor-expressive, and linguistic aspects of emotion in a complex and adaptive way. The perception of emotional stimuli and the associated physical reactions may then be inadequate, leading to impairment in appraising and coping with emotionally stressful situations (18). According to this, many studies have shown modified autonomous stress arousal in subjects with alexithymia, but the results are still inconsistent (19–24). The directionality of autonomous arousal under emotional stimulation is especially contradictory.
Furthermore, it is not certain whether alexithymia is associated with a primary dysfunction of perception or an incomplete processing of perceived emotional information (25). Therefore, this study investigated two questions: 1) Are subjects with alexithymia able to perceive and classify emotional aversive stimuli? (2) If so, do subjects with alexithymia, compared to subjects without alexithymia, show a modified central processing of emotional information?
Central cortical activity was recorded by visual event-related EEG potentials. Palomba et al. (26) and Bradley and Lang (27) found elevated amplitudes of positive visual event-related potential components after the presentation of emotionally arousing pictures. Thus, stimulus-dependent differences between subjects with and without alexithymia should occur, especially in the positive components of visual event-related potentials, after the presentation of emotional pictures. Therefore, in this article, physiological data regarding amplitudes and mean activity of the positive visual event-related potential components (P2 and late positive complex: P3a, P3b, and slow wave) will be presented. If subjects with alexithymia are able to perceive the emotional aspects of affect-arousing pictures, we would expect a strong effect on both groups (subjects with and without alexithymia). Positive visual event-related potential components, in general, should be elevated after presenting affect-arousing pictures in comparison to neutral pictures. Additionally, if after presenting affect-arousing stimuli (26) subjects with alexithymia need more effort to process emotional information (maybe because of incomplete cognitive schemes), an elevated mean activity in the positive visual event-related potential components should occur in subjects with high alexithymia compared to those without alexithymia.
Method
Study Group
Twenty subjects with high alexithymia and 20 with low alexithymia took part in this study. The subjects (university students, former patients of the clinical facilities, and members of the university staff) were recruited by means of announcements. The identification of high and low alexithymia was based on the sum score of the Toronto Alexithymia Scale (German 20-item version) (2, 3, 6, 28). High and low alexithymia were defined by a random sample survey (N>2.000). The 33rd percentile (sum score <42) and the 66th percentile (sum score >52) were used as cutoffs (28) because no standardized threshold exists for the German version of the Toronto Alexithymia Scale. Subjects with low alexithymia had a mean sum score of 34.2 (SD=5.2), and subjects with high alexithymia had a mean score of 59.5 (SD=6.0). This is close to the international cutoff (>61). Both groups were parallel with regard to age (subjects with a high alexithymia mean=28.8, SD=6.8; subjects with a low alexithymia mean=27.3, SD=6.3), level of education and gender, and level of education (high school graduation). Scores on the Toronto Alexithymia Scale were as follows: 10 women (mean=59.1, SD=5.4) and 10 men (mean=59.9, SD=6.8) in the group with high alexithymia and 10 women (mean=31.8, SD=4.9) and 10 men (mean=36.3, SD=4.8) in the group with low alexithymia; mean differences in scores were not significant.
Exclusion criteria were left-handedness, lacking knowledge of German, disease of the central or peripheral nervous system, coronary heart disease, use of psychopharmaceuticals, drug or alcohol abuse, disorders of the visual system, age over 50 years, and age under 20. The exclusion criteria were checked with an anamnestic sheet and a medical interview after applying the Toronto Alexithymia Scale. After complete description of the study to the subjects, written informed consent was obtained. The local ethics committee approved the study.
Stimuli
Subjects were exposed to an odd-ball paradigm, according to Attias et al. (29). We presented three categories of visual stimuli, altogether 250 presentations of different pictures from the International Affective Picture System (24, 30, 31). The first category of pictures (“targets,” 20% of all stimuli, 50 presentations of 2×25 different pictures; relevant stimuli according to the instructions) were slides of animals, which were assessed as neutral with respect to arousal and valence. The second category of pictures (“nontargets,” 60% of all stimuli, 150 presentations of 3×50 different pictures) were also rated as neutral but showed common objects (e.g., chairs, tools, and landscapes).
The last category (“probes,” 20% of all stimuli, 50 presentations of different pictures) represented unpleasant and arousing motives (e.g., scenes with weapons, rape, and mutilations). Pictures were presented for 500 msec; interstimulus intervals varied randomly, between 12 and 15 seconds. All stimulus categories were presented in four different permutation blocks (250 pictures each). Subjects were assigned randomly to one block. The distribution of the blocks was counterbalanced across the subject groups.
Response Measurement
After presentation, the subjects were asked, “Was there anything referring to the presented pictures that attracted your attention?” The aim of this open question was to force the subjects to mention the emotional content of the probes. The answers were rated as positive if the subjects mentioned any emotional content of the probes; e.g., “Some pictures were violent,” etc. If the subjects negated this question (e.g., “No, nothing at all”), the answer was rated as negative. A second question followed, with a more open tone: “Have you noticed anything else during the investigation?” The rating of the answer to this question was assessed in the same manner.
EEGs were recorded by using silver/silver chloride cup electrodes (Ø=0.7 cm, Nihon Kohden Europe Inc., Rosbach, Germany; electrolyte paste, Elefix, Nihon Kohden Europe Inc., Rosbach, Germany). Electrodes were placed on the positions Fc, Cc, and Pc (10-20 system; reference 32). Reference electrodes on the left and right earlobe (A1 and A2) were switched together by the amplifying system (analogue inkjet polygraph, Nihon Kohden 4421; low-pass filter, 35 Hz, time constant=5 seconds, amplification=10 μV/mm). Skin resistance was reduced below 5 kΩ (SkinPure, Nihon Koden). A mass electrode was placed on the left mastoid. Vertical and horizontal electro-oculograms (EOGs) were recorded to control motor artefacts (low-pass filter, 35 Hz, time constant=5 seconds, amplification=20 μV/mm). Analogue signals were converted by an analog-digital converter card (ADAC 5801 MF, American Data Acquisition Corporation, COSYCO Inc., Germering, Germany; sampling rate=250s–1) and stored on a computer hard disk with Psylab software (J. Grabke, 1996, Department of Physiological Psychology, University of Wuppertal, Germany). The recording interval started 1 second before and stopped 2 seconds after each stimulus presentation. For each category of stimuli, 50 presentations were averaged after semiautomatic control for artefacts. The nontargets used for the averaging procedure were taken out of the 250 presented pictures and selected for those preceding a probe. EOG artefacts were corrected by the algorithm of Gratton et al. (33). Trials were rejected for averaging when different criteria, as follows, were violated:
| 1. | Gradient criterion: maximum allowed voltage step/sampling point=50 μV | ||||
| 2. | Maximum-minimum criterion: maximum allowed absolute difference/trial=100 μV | ||||
| 3. | Amplitude criterion: minimum allowed amplitude=–200 μV; maximum allowed amplitude=200 μV | ||||
| 4. | Low-activity criterion: lowest allowed activity/1000 msec=0.1 μV | ||||
For every trial, a baseline correction was performed. All offline analyses were performed with Brain Vision Analyser software (Brain Products Inc., Munich, Germany).
Procedure
The subjects sat in an armchair with a headrest, which was placed at a distance of 100 cm from a 21′′ computer monitor (the room was 4.10×2.40 m, 25°C). Then electrodes were placed. The subjects were filmed with a video camera, and the presentation was controlled by a computer. After baseline recording and adaptation (10 minutes), the subjects were instructed to count the number of slides with animals silently and to focus their attention on the stimuli. After this, the investigator left the room and the picture presentation was begun. During interstimulus intervals, the subjects focused on a cross on the computer screen to avoid EOG artefacts. After the presentation of 125 pictures, a break of 5 minutes was taken before the experiment continued. After the picture presentation, the investigator entered the room and asked how many pictures showed animals. He also asked questions concerning the emotional content of the probes. Then the electrodes were removed, and the subjects were disbanded.
Data Reduction and Statistical Analysis
Event-related potentials were averaged separately for each recording site (Fc, Pc, and Cc) and the three types of stimuli. Different peaks of the individual event-related EEG signal were identified within specific latency intervals. Figure 1 displays the grand averages for the visual event-related potentials (all subjects under the three stimulus conditions) that were exemplarily for electrode Pc. We defined the latency intervals by visual inspection of the grand average, according to Picton et al. (34), for all subjects in each stimulus category. Peak-to-baseline amplitudes (μV) and latencies (msec) were determined automatically for P2 (150–260 msec). The late positive complex (P3a, P3b, and slow wave) varied in grand averages depending upon the stimulus category. Therefore, we detected the P3a automatically as peak-to-baseline amplitude in the latency range of 280–450 msec. Because of the instruction to identify and count only the targets, the grand average of this stimulus category displayed a prominent additional positive component (P3b, as a correlate of this instructed classification process) after the peak of P3a. Because the presentation of nontargets and probes was not linked with any instruction, visual event-related potentials of these stimulus categories did not show this second peak. Therefore, we determined the mean activity instead of peak-to-baseline amplitude for the second positive component of the late positive complex (P3b) in the interval between 350 and 600 msec and for the following slow wave between 600 and 1500 msec for all stimulus categories.
The latency measurement of the late positive complex was based upon the peak detection of P3a. Latency was defined as the interval between stimulus onset and the maximum of positive activity in the latency range of P3a. An analogue procedure was chosen for P2.
Statistical evaluation of the amplitudes of the positive visual event-related potential components (P2, P3a, P3b, and slow wave) in a first step was performed as a multivariate analysis of variance. Alexithymia (high versus low) was used as a between-subjects factor. As within-subjects factors, 1) stimulus category (nontargets, targets, and probes), 2) electrode position (Fc, Cc, and Pc), and 3) components (P2, P3a, P3b, and slow wave) were taken into account. All components were treated simultaneously as dependent variables. This procedure was used to test a possible overall main effect and interaction of stimulus category and alexithymia and to avoid an accumulation of alpha error, which is present when single components are tested separately. Positive components of visual event-related potentials were used exclusively because they were the best at differentiating between affective arousing and neutral pictures (26, 27). Subsequently, additional analyses of variance were performed for each single component by using the same model as displayed here but without the last subject internal factor component. F ratios were tested by using degrees of freedom adjusted with the Greenhouse-Geisser procedure. Statistical analyses were performed with SPSS, version 9.0. Frequencies were analyzed with the chi-square procedure.
Results
Visual Event-Related Potentials
All effects of interest are presented (stimulus category, level of alexithymia, stimulus category by components, stimulus category by alexithymia). Figure 2 shows the grand averages of visual event-related potentials of subjects with high and low alexithymia for stimulus conditions and electrode positions. In contrast to the nontargets, the subjects of both groups showed a strong positivation in the latency range between 200 and 1000 msec after the presentation of targets and probes. Furthermore, the subjects with high alexithymia displayed more pronounced positive activity after the presentation of targets and particularly after the presentation of probes, compared to the subjects with low alexithymia.
Figure 3 displays the mean amplitudes (P2 and P3a) and the mean activity (P3b and slow wave) of all positive visual event-related potential components for all stimulus conditions. The simultaneous testing within the multivariate variance analytic model, including all positive visual event-related potential components, revealed a significant main effect for the factor stimulus category (F=53.92, df=2, 76, p<0.001). In general, the instructed targets evoked the highest positive activity in P2, P3a, and P3b, followed by probes and nontargets. The highest slow wave component (the later part of the late positive complex, 600–1500 msec) occurred after the presentation of probes, compared to targets and nontargets.
The main effect of the factor electrode position was significant (F=114.5, df=2, 76, p<0.001). For each component and all stimulus conditions, mean amplitudes/activity were highest under the EEG electrode position Pc, followed by Cc and Fc.
A significant main effect of the factor components was also found (F=14.15, df=3, 114, p<0.001). The P3a component showed the highest and the P2 the lowest amplitudes. The mean activity of P3b and slow wave did not differ substantially.
The interaction effect of the factors alexithymia by stimulus category reached statistical significance (F=3.57, df=3, 114, p<0.05). The analysis of contrasts (which allows for comparison of different factor steps of a statistically significant main or interaction effect) revealed a significantly higher mean amplitude/activity of the subjects with high alexithymia than those with low alexithymia after the presentation of probes compared to nontargets (F=7.06, df=1, 38, p<0.05). Both groups did not differ after target presentation compared to nontargets (F=2.15, df=1, 38, n.s.) This result indicates that the significance of the interaction of alexithymia by stimulus category is based mainly on the differences between subjects with high and low alexithymia after probe presentation. The interaction effect of stimulus category by components (F=30.65, df=6, 228, p<0.001) was also statistically significant (Figure 3).
Statistical data for the subsequent (single) variance analytical testing of each positive component for visual event-related potentials are presented in Table 1. P2 amplitude was highest after the presentation of targets and lowest for nontargets. Differences between EEG electrode positions were also significant (Fc had the lowest and Pc had the highest amplitudes). Interaction of the factors of stimulus category by alexithymia was also statistically significant. Analysis of contrasts revealed an elevated P2 amplitude after the presentation of targets (F=6.08, df=1, 38, p<0.05) and probes (F=7.78, df=1, 38, p<0.01) compared to nontargets in the subjects with alexithymia. Other interactions were not statistically significant.
In P3a, targets and probes caused higher amplitudes than nontargets. Targets induced the highest amplitudes. For all stimuli, amplitudes were the highest at Pc and the lowest at Fc. All other effects did not reach statistical significance.
In P3b, targets also induced the highest mean activity, followed by probes and nontargets. Although the alexithymia-by-stimulus category interaction was not significant (Table 1), contrast analysis showed a significant difference of mean activity between the subjects with high and low alexithymia, which were dependent on stimulus category (F=5.63, df=1, 38, p<0.05). The subjects with high alexithymia displayed increased positive P3b activity after presentation of probes compared to nontargets. All other effects were not significant.
Concerning slow wave, the probes caused the highest mean activity, followed by the targets and nontargets. All other effects did not reach the level of statistical significance. Peak latencies of P2 and P3a did not show any significant main or interaction effects.
Subjective Data
In contrast to the subjects with high alexithymia (nine of 20), the subjects with low alexithymia confirmed significantly more frequently (16 of 20) the first question by a positive response, mentioning the aversive impact of the probes (χ2=5.23, df=1, p<0.05). After the second question, no differences between the answers of the subjects with high alexithymia and the subjects with low alexithymia persisted (negation of both questions: one in 20 in the subjects with low alexithymia and two in 20 of the subjects with high alexithymia).
Discussion
By recording visual event-related potentials, we wanted to investigate if the subjects with alexithymia showed different central processing compared to the subjects without alexithymia after the presentation of emotionally aversive visual stimuli. This would show evidence of an impaired or modified ability of the subjects with alexithymia to perceive and classify the emotional impact or quality of aversive affect-inductive pictures.
The validity of the odd-ball paradigm (35) could be demonstrated. Both groups of subjects showed significantly higher positive components of visual event-related potentials in response to the instructed targets compared to the nontargets and the probes. Especially in the range between 280 msec and 600 msec (P3a and P3b), instruction-related cortical activation representing stimulus evaluation could be found (36, 37). This is an indication of a valid implementation of the experimental setting.
Additionally, the subjects with high alexithymia showed generally elevated positive components compared to the subjects with low alexithymia after the presentation of probes compared to nontargets between 150 msec and 1500 msec, which suggests the following two implications:
| 1. | Contradictory to the common view of alexithymia, subjects with alexithymia perceive, process, and differentiate visual stimuli with emotional information from emotionally neutral pictures. | ||||
| 2. | Subjects with alexithymia seem to show a more intense processing of probes accompanied by a stronger central arousal. This can be inferred by the studies of Palomba et al. (26) and Bradley and Lang (27). They suppose a deeper processing of emotional stimuli because of a higher relevance and priority of emotion-arousing information, e.g., for the induction of motivational states and adequate adaptive behavior. | ||||
Particularly, P2 and P3b show significant differences between subjects with high and low alexithymia after the presentation of emotion-arousing pictures. P2 may be linked to the early processes of categorizing different stimuli, and as a part of vertex potential, it could represent the initiation of orienting response (35). Although it is predominantly associated with the registration of aberrant physical stimulus characteristics (35), it also probably reflects an early scanning of emotional aspects and significance of the stimulus (38).
P3b mean activity was also higher in subjects with alexithymia, although the interaction of alexithymia and stimulus category did not reach the level of statistical significance. Therefore, the result of the contrast analysis (the subjects with high alexithymia showed an increased P3b after the presentation of probes compared to nontargets) should be interpreted with caution. P3b typically appears after the presentation of target stimuli within odd-ball paradigms and reflects an instruction-associated deeper stimulus evaluation (39, 40). In contrast to nontargets, the probes as well as the targets produced a P3b response in both groups. However, according to Donchin (37), unexpected stimuli have to be adapted to cognitive schemes in a consistent way (“context updating”). Thus, P3 is not exclusively associated with task-related instructions but with memory-dependent assimilation of aberrant environmental configurations. Under this view, a more complex and extensive adaptation process leads to an elevated P3. The higher mean activity of P3b in the alexithymic group may indicate that this process in subjects with alexithymia needs more effort and resources when they are confronted with emotional stimuli. This supports the hypothesis that alexithymia is associated with a modified central processing of emotional information.
According to Martin and Pihl (18) and Papciak et al. (19), we suggest an explanation for this more intensive context updating. Subjects with alexithymia, while they evaluate emotional stimuli, could refer only to incomplete or inconsistent cognitive schemes. This may lead to uncertainty about the meaning and appraisal of emotional information, which perhaps results from a preventive avoidance of emotional stimulation, e.g., based on adverse emotional childhood experiences (41–43).
Therefore, the characteristic attributes of alexithymic behavior (impaired identification and communication of emotional aspects of interaction, difficulty distinguishing between own feelings and correlates of somatic affect, and externally oriented thinking) are particularly evident in social relationships with high emotional relevance. A persistent affect-avoiding interpersonal behavior may be maladaptive and can cause disturbances and conflicts in such important relationships, finally contributing to an increased risk of symptoms such as depression or anxiety. Under this view, psychotherapeutic methods, which primarily focus on the verbalization of inner emotional states are probably not the first choice for alexithymic patients. Subjects with alexithymia could undermine these therapeutic strategies in a formal and superficial manner, which leads to pseudotherapeutic effects, based in fact on social desirability. Subjects with alexithymia are not blind to emotional information, but they probably avoid the processing and expression of their own affective states. This could suggest, particularly in the beginning of a psychotherapeutic treatment, that one should be cautious about the demonstrative expression of emotional signals or focusing on emotional conflicts to ensure the therapeutic attachment of high alexithymic patients.
The hesitant spontaneous verbal response of the subjects with alexithymia to our stimulus-related questions supports this view. Despite a higher evaluation effort and in contrast to the comparison subjects, the subjects with alexithymia, when asked in an open way, did not initially refer verbally to the probes. Other studies (21, 24, 31) did not find differences in the assessment of emotional stimulus valence between subjects with high and low alexithymia, but this could be a normative effect of forced-choice questionnaires (e.g., semantic differentials).
However, our findings do not support a deficit theory of a primarily blocked perception of emotional information in subjects with alexithymia. Subjects with alexithymia are obviously not blind to emotional stimuli, but they are probably doubtful about what they may mean and how to deal with them.
Some limitations of our study should be mentioned. The process of context updating, as described by Donchin (37), is based on the assumption of “stimulus novelty.” New environmental information has to be integrated into cognitive schemes, and as a correlate of this process, an elevated positive activity of P3 can be obtained. Typically, this component can be found predominantly in the early latency range of late positive complex (between 200 and 300 msec, P3a [35]).
Descriptively, the subjects with alexithymia also had a higher mean amplitude in this latency range compared to the subjects with low alexithymia, but this difference was not statistically significant. If a “context updating” would have been exclusively based on “stimulus novelty,” we would expect significant differences between the subjects with high and low alexithymia in this early latency range of late positive complex. This is probably caused by a habituation effect. While the first presentations of probes surely had novelty character, the subjects could establish an expectation about the presentation of probes in later trials. This expectation could cause a change in the process of “context updating,” shifting away from integrating novel stimulus material into cognitive schemes, toward a process of categorizing different classes of stimuli. Despite that, the higher mean positive activity of visual event-related potentials after the presentation of probes in subjects with alexithymia could be explained by the need for more effort and cognitive resources during context updating. Also, the relation between P2 and emotional processing is not assured (38); more specific investigations are needed.
For future research on the neurobiological aspects of alexithymia, electrophysiological studies should be endorsed with methods such as functional magnetic resonance imaging (fMRI) and positron emission tomography. First steps were recently made by Berthoz et al. (31). They could demonstrate by fMRI that alexithymic men show altered cortical activation (e.g., in the left mediofrontal paracingulate cortex or the anterior cingulate mediofrontal cortex) in response to emotion-arousing pictures.
 |
Presented in part at the 53rd annual meeting of the German Council for Psychosomatic Medicine, Ulm, Germany, March 6–9, 2002. From the Medical Department, Clinical Institute of Psychosomatic Medicine and Psychotherapy. Address reprint requests to Dr. Franz, Medical Department, Clinical Institute of Psychosomatic Medicine and Psychotherapy, University of Duesseldorf, P.O. Box 101007, D-40001 Duesseldorf, Germany; [email protected] (e-mail).
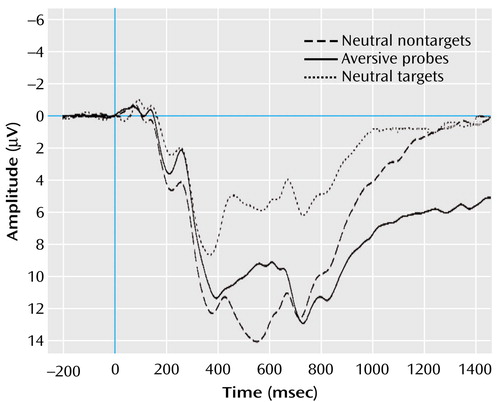
Figure 1. Grand Averages of Visual Event-Related Potentials at Electrode Position Pc Under Three Stimulus Conditions for Subjects With High (N=20) or Low (N=20) Alexithymia Scoresa
aAlexithymia was measured with the summed score on the Toronto Alexithymia Scale (20-item German version) (2, 3, 6, 28). Low score<33rd percentile, high score>66th percentile.
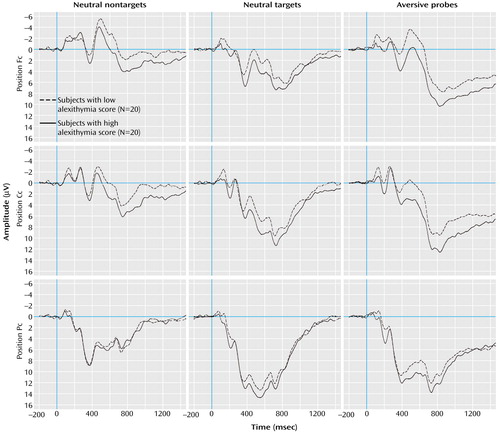
Figure 2. Grand Averages of Visual Event-Related Potentials at Three Electrode Positions Under Three Stimulus Conditions for 20 Subjects With High Alexithymia Scores and 20 Subjects With Low Alexithymia Scoresa
aAlexithymia was measured with the summed score on the Toronto Alexithymia Scale (20-item German version) (2, 3, 6, 28). Low score<33rd percentile, high score>66th percentile.
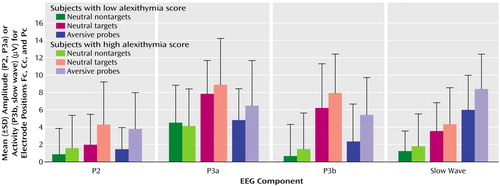
Figure 3. Mean EEG Amplitude or Activity of All Positive EEG Components of Visual Event-Related Potentials Under Three Stimulus Conditions for 20 Subjects With Low Alexithymia Scores and 20 Subjects With High Alexithymia Scoresa
aAlexithymia was measured with the summed score on the Toronto Alexithymia Scale (20-item German version) (2, 3, 6, 28). Low score<33rd percentile, high score>66th percentile.
1. Sifneos PE: The prevalence of “alexithymic” characteristics in psychosomatic patients. Psychother Psychosom 1973; 22:255–262Crossref, Medline, Google Scholar
2. Bagby RM, Parker JDA, Taylor GJ: The Twenty-Item Toronto Alexithymia Scale, I: item selection and cross-validation of the factor structure. J Psychosom Res 1994; 38:23–32Crossref, Medline, Google Scholar
3. Bagby RM, Taylor GJ, Parker JDA: The Twenty-Item Toronto Alexithymia Scale, II: convergent, discriminant, and concurrent validity. J Psychosom Res 1994; 38:33–40Crossref, Medline, Google Scholar
4. Taylor GJ: Recent developments in alexithymia theory and research. Can J Psychiatry 2000; 44:134–142Google Scholar
5. Cox BJ, Kuch K, Parker JDA, Shulman ID, Evans RJ: Alexithymia in somatoform disorder patients with chronic pain. J Psychosom Res 1994; 38:523–527Crossref, Medline, Google Scholar
6. Bach M, Bach D, de Zwaan M, Serim M, Boehmer F: Validierung der deutschen Version der 20-Item Toronto-Alexithymie-Skala bei Normalpersonen und psychiatrischen Patienten. Psychother Psych Med 1996; 46:23–28Google Scholar
7. Honkalampi K, Hintikka J, Tanskanen A, Lehtonen J, Viinamaki H: Depression is strongly associated with alexithymia in the general population. J Psychosom Res 2000; 48:99–104Crossref, Medline, Google Scholar
8. Zeitlin SB, McNally RJ: Alexithymia and anxiety sensitivity in panic disorder and obsessive-compulsive disorder. Am J Psychiatry 1993; 150:658–660Link, Google Scholar
9. Parker JDA, Bagby MR, Taylor GJ, Endler NS, Schmitz P: Factorial validity of the 20-Item Toronto Alexithymia Scale. Eur J Personality 1993; 7:221–232Crossref, Google Scholar
10. Todarello O, Taylor GJ, Parker JD, Fanelli M: Alexithymia in essential hypertensive and psychiatric outpatients: a comparative study. J Psychosom Res 1995; 39:987–994Crossref, Medline, Google Scholar
11. Jula A, Salminen JK, Saarijarvi S: Alexithymia: a facet of essential hypertension. Hypertension 1999; 33:1057–1061Crossref, Medline, Google Scholar
12. Kauhanen J, Kaplan GH, Cohen RD, Julkunen J, Salonen JT: Alexithymia and risk of death in middle-aged men. J Psychosom Res 1996; 41:541–549Crossref, Medline, Google Scholar
13. Porcelli P, Leoci C, Guerra V, Taylor GJ, Bagby RM: A longitudinal study of alexithymia and psychological distress in inflammatory bowel disease. J Psychosom Res 1996; 41:569–573Crossref, Medline, Google Scholar
14. Salminen J, Saarijärvi S, Äärelä E, Toikka T, Kauhanen J: Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. J Psychosom Res 1999; 46:75–82Crossref, Medline, Google Scholar
15. Martinez-Sanchez F, Ato-Garcia M, Adam EC: Stability in alexithymia levels: a longitudinal analysis on various emotional answers. Pers Individ Dif 1998; 24:767–772Crossref, Google Scholar
16. Luminet O, Bagby MR, Taylor GJ: An evaluation of the absolute and relative stability of alexithymia in patients with major depression. Psychother Psychosom 2001; 70:254–260Crossref, Medline, Google Scholar
17. Lane RD, Schwartz GE: Levels of emotional awareness: a cognitive-developmental theory and its application to psychopathology. Am J Psychiatry 1987; 144:133–143; correction, 144:542Link, Google Scholar
18. Martin JB, Pihl R: The stress-alexithymia-hypothesis: theoretical and empirical considerations. Psychother Psychosom 1985; 43:169–176Crossref, Medline, Google Scholar
19. Papciak AS, Feuerstein M, Spiegel JA: Stress reactivity in alexithymia: decoupling of physiological and cognitive responses. J Hum Stress 1985; 11:135–142Crossref, Medline, Google Scholar
20. Wehmer F, Brenjank C, Lumley M, Stettner L: Alexithymia and physiological reactivity to emotion-provoking visual scenes. J Nerv Ment Dis 1995; 183:351–357Crossref, Medline, Google Scholar
21. Rabavilas AD: Electrodermal activity in low and high alexithymic neurotic patients. Psychother Psychosom 1987; 47:101–104Crossref, Medline, Google Scholar
22. Friedlander L, Lumley MA, Farchione T, Doyal G: Testing the alexithymia hypothesis: physiological and subjective responses during relaxation and stress. J Nerv Ment Dis 1997; 185:233–239Crossref, Medline, Google Scholar
23. Franz M, Olbrich R, Croissant B, Kirsch P, Schmitz N, Schneider C: Gefühl ohne Sprache oder Sprache ohne Gefühl? Weitere Hinweise auf die Validitat der Entkopplungshypothese der Alexithymie. Nervenarzt 1999, 70:216–224Google Scholar
24. Roedema TM, Simons RF: Emotion-processing deficit in alexithymia. Psychophysiology 1999; 36:379–387Crossref, Medline, Google Scholar
25. Lane RD, Ahern GL, Schwartz GE, Kaszniak AW: Is alexithymia the emotional equivalent of blindsight? Biol Psychiatry 1997; 42:834–844Crossref, Medline, Google Scholar
26. Palomba D, Angrilli A, Mini A: Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. Int J Psychophysiol 1997; 27:55–67Crossref, Medline, Google Scholar
27. Bradley MM, Lang PJ: Measuring emotion: behavior, feeling, and physiology in Cognitive Neuroscience of Emotion. Edited by Lane RD, Nadel L. Oxford, UK, Oxford University Press, 2000, pp 242–276Google Scholar
28. Parker DA, Taylor GJ, Bagby MR: Alexithymia and the recognition of facial expressions of emotion. Psychother Psychosom 1993; 59:197–202Crossref, Medline, Google Scholar
29. Attias J, Bleich A, Furman V, Zinger Y: Event-related potentials in post-traumatic stress disorder of combat origin. Biol Psychiatry 1996; 40:373–381Crossref, Medline, Google Scholar
30. Lang PJ, Öhman A, Vaitl D: The International Affective Picture System: Photographic Slides. Gainesville, University of Florida, Center for Research in Psychophysiology, 1988Google Scholar
31. Berthoz S, Artiges E, Van de Moortele P-F, Poline J-B, Rouquette S, Consoli SM, Martinot J-L: Effect of impaired recognition and expression of emotions on frontocingulate cortices: an fMRI study of men with alexithymia. Am J Psychiatry 2002; 159:961–967Link, Google Scholar
32. Jasper HH: The ten-twenty electrode system of the International Federation. Electroencephalography Clin Neurophysiol 1958; 10:371–375Google Scholar
33. Gratton G, Coles MGH, Donchin E: A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 1983; 55:468–484Crossref, Medline, Google Scholar
34. Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ: Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology 2000; 37:127–152Crossref, Medline, Google Scholar
35. Rockstroh B, Elbert T, Canavan A, Lutzenberger W, Birbaumer N: Slow Cortical Potentials and Behavior. Baltimore, Urban & Schwarzenberg, 1989Google Scholar
36. Donchin E, Ritter W, McCallum WC: Cognitive psychophysiology: the endogenous components of the ERP, in Event-Related Brain Potentials in Man. Edited by Callaway E, Tuetin P, Koslow SH. New York, Academic Press, 1978, pp 349–411Google Scholar
37. Donchin E: Surprise!…Surprise? Psychophysiology 1981; 18:493–513Crossref, Medline, Google Scholar
38. Schapkin SA, Gusev AN, Kuhl J: Categorization of unilaterally presented emotional words: an ERP analysis. Acta Neurobiol Exp 2000; 60:17–28Medline, Google Scholar
39. Sutton S, Braren M, Zubin J, John ER: Evoked potential correlates of stimulus uncertainty. Science 1965; 150:1187–1188Crossref, Medline, Google Scholar
40. Sutton S, Teuting P, Zubin J, John ER: Evoked potential correlates of stimulus uncertainty. Science 1967; 155:1436–1469Crossref, Medline, Google Scholar
41. Scheidt CE, Waller E, Schnock C, Becker-Stoll F, Zimmermann P, Lucking CH, Wirsching M: Alexithymia and attachment representation in idiopathic spasmodic torticollis. J Nerv Ment Dis 1999; 187:47–52Crossref, Medline, Google Scholar
42. Berenbaum H: Childhood abuse, alexithymia and personality disorder. J Psychosom Res 1996; 41:585–595Crossref, Medline, Google Scholar
43. Zlotnick C, Mattia JI, Zimmerman M: The relationship between posttraumatic stress disorder, childhood trauma and alexithymia in an outpatient sample. J Traumatic Stress 2001; 14:177–188Crossref, Google Scholar



