Reduced Cortical Folding in Schizophrenia: An MRI Morphometric Study
Abstract
OBJECTIVE: Although well documented, regional brain structural abnormalities in schizophrenia are nonspecific, and morphometric parameters show significant overlapping between patients and healthy comparison subjects. An increasing number of studies have focused on supraregional models involving abnormalities of the neuronal circuitry between cortical regions in schizophrenia. The aim of the present study was to investigate cortical folding as an index of the neuronal wiring in different subtypes of schizophrenia. METHOD: Magnetic resonance imaging measures of gyrification index in intervals of 3.6 mm along the total cerebral cortex were compared in 40 patients with DSM-IV schizophrenia and 20 healthy subjects. Psychopathology was assessed with the Positive and Negative Syndrome Scale, Brief Psychiatric Rating Scale, and the Negative Symptom Rating Scale. RESULTS: The schizophrenia patients showed significantly reduced bilateral cortical folding relative to healthy comparison subjects. Such reductions were more pronounced in those with the disorganized subtype and showed an inverse correlation with negative symptoms and a positive correlation with positive symptoms. The paranoid subtype showed reduced cortical folding that was restricted to the left hemisphere. CONCLUSIONS: These results from a larger patient group confirm a previous report of reduced cortical folding in schizophrenia patients. They also suggest a distinct pattern of abnormality between schizophrenia subtypes regarding the process of cerebral lateralization and are in agreement with the neurodevelopmental hypothesis of schizophrenia.
In the last decades, accumulating evidence from neuroimaging studies has shown that schizophrenia is associated with brain structural abnormalities (1, 2). These abnormalities are already present in first-episode, never treated patients (3, 4), and the absence of gliosis and other neurodegenerative abnormalities (1) favors a neurodevelopmental hypothesis of schizophrenia. Nevertheless, morphometric parameters based on a region-of-interest approach are nonspecific, showing significant overlapping between schizophrenia and other psychiatric conditions and, even for the most robust findings, there have been a significant number of studies reporting negative results. Such inconsistencies raise the question of whether “schizophrenia” is a single disease with different levels of severity along a pathogenetic continuum (5, 6) or whether the psychoses of the schizophrenia spectrum constitute distinct clinical and etiopathological groups (7, 8). An increasing number of studies have focused on a supraregional model involving abnormalities of the neuronal circuitry between multiple cortical regions in schizophrenia (9–12).
In a postmortem study of 61 normal brains, Zilles et al. (13) developed the so-called gyrification index from direct measurements on cortical surfaces as seen in coronal slices (a ratio between total—including sulcal—and superficially exposed cortical surfaces). The gyrification index provides a parameter of interest for schizophrenia, since alterations in cortical folding may reflect an underlying disorder in neural connectivity during the maturation of the brain (14, 15). Kulynych et al. (16), in a magnetic resonance imaging (MRI) study, found gyrification index reductions in the left hemispheres of nine schizophrenic patients compared with nine healthy volunteers (the gyrification index was measured in coronal sections at intervals of 4 mm). Conversely, Vogeley et al. (17) reported gyrification index increases in 12 schizophrenic and schizoaffective patients relative to 12 unaffected siblings when investigating five MRI slices of the right prefrontal cortex.
The purpose of this study was to conduct a detailed investigation of the gyrification index in a series of 50 anterior-to-posterior MRI slices (at intervals of 3.6 mm) from a larger schizophrenic patient group (N=40) relative to healthy comparison subjects (N=20). We also aimed to study the relationship between the gyrification index and schizophrenia symptom scores as well as clinical schizophrenia subtypes.
Method
Subjects
This study was performed at the Institute of Psychiatry of the University of São Paulo. Forty outpatients were diagnosed and classified by schizophrenia subtype according to DSM-IV criteria on the basis of interviews with the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) (18). Exclusion criteria were 1) age lower than 18 or greater than 60 years; 2) history of substance dependence; and 3) history of head trauma, degenerative neurological disorders, or previous treatment with steroid medication. Schizophrenic patients were compared with a healthy group (N=20) that had no history of physical or psychiatric disorders, as determined with the SCID-P. After an explanation of the study, written informed consent was obtained from all subjects before participation. This study was approved by the Ethics Committee of the Faculty of Medicine of the University of São Paulo.
Demographic and clinical data from the two groups are provided in Table 1. Patients and comparison subjects did not differ significantly in terms of gender, age, handedness, and years of education.
Clinical Assessments
Current symptom severity was measured by using the Positive and Negative Syndrome Scale (19), the anchored version of the Brief Psychiatric Rating Scale (BPRS) (20, 21), and the Negative Symptom Rating Scale (22). All patients were receiving antipsychotic medication at the time of the study.
MRI Acquisition and Measurements
Structural MRI scans of the entire brain were obtained by using a 1.5-T Philips Gyroscan S15-ACS scanner (Philips Medical Systems, Andover, Mass.), with T1-weighted fast-field echo continuous coronal slices (thickness=1.2 mm, field of view=240, matrix=256×256). The orientation of the brain was standardized during the acquisition process, with coronal images oriented perpendicularly to the anterior-to-posterior commissural axis. Images were transferred to a SUN workstation and measured manually with the software Gyroview–HR 2.1, with investigators blind to diagnosis.
Gyrification index
The gyrification index was determined according to the method of Zilles et al. (13) as the ratio between the total (including sulcal, inner contour) and the superficially exposed (outer contour) cortical surface, as determined from linear measurements of the coronal lengths for each section/slice at equally spaced intervals along the cerebral cortex. Considering that Zilles et al. calculated the mean gyrification index margin error to be lower than 4% at 4-mm intervals, for practical purposes we have measured gyrification index at 3.6-mm instead of 1.2-mm intervals. The contours were traced externally along the anatomic boundaries of the cortex (outer contour along the superficially exposed cortex; inner contour including sulcal surfaces). In the middle segment of the brain, a line was traced from the curvature of the parahippocampal gyrus to the inferior border of the third ventricle that then followed a midsagittal line until the corpus callosum. Therefore, subcortical and midsagittal structures were not included in gyrification index measurements (Figure 1). Right and left gyrification indexes were calculated as the averages of approximately 50 slices on the right and left hemispheres, respectively. In order to investigate regional differences, an arithmetic mean of the gyrification index was separately calculated for the frontal and for the posterior (temporal-parietal-occipital) regions. Both for this separation and for the comparative superposition of slices between subjects, an index section (cutoff) was used that corresponded to the point at which the temporal lobe becomes attached to the remainder telencephalon, as seen in the coronal plane (the anterior temporal lobe was not included). The following algorithm was used to calculate the asymmetry coefficient of the gyrification index:
(Right GI – Left GI)/0.5 × (Right GI + Left GI)
Brain volume
Brain volume was obtained by the sum of volumes of the hemispheres, whose boundaries matched the outer contour of the gyrification index; consequently, the temporal pole, part of the midbrain, the pons, and the cerebellum were excluded from measurements (Figure 1).
Reliability
All the aforementioned measurements were performed blind to diagnosis by the same rater (P.C.S.). One other rater (E.S.) carried out independent measurements in five randomly selected brains (approximately 50 slices on each hemisphere). The interrater agreement (average measure ICC) was 0.84 for the right gyrification index and 0.87 for the left gyrification index.
Statistical Analysis
Between-group differences were assessed using parametric tests (t tests, one-way analyses of variance) or nonparametric tests (Mann-Whitney, Kruskal-Wallis) depending on the normality distribution. Second, we used a general linear model that controlled for brain volume and sociodemographic variables (gender, age, handedness, and years of education), with Bonferroni correction for multiple comparisons. Correlations between gyrification index and rating scale scores were also calculated, first by bivariate correlation (Spearman correlation), then with partial correlations after we controlled for brain volume and sociodemographic differences.
Results
Schizophrenia Patients Versus Healthy Subjects
The results are summarized in Table 2. Patients showed bilateral gyrification index reductions relative to the healthy subjects, the values of whom matched very closely the results presented by Zilles et al. (right gyrification index: mean=2.55, SD=0.02; left gyrification index: mean=2.57, SD=0.02) (13). After we controlled for demographic variables and brain volume, a significant reduction of the left gyrification index was maintained (F=9.05, df=1, 53, p=0.004), but the right gyrification index reduction in the group of schizophrenic patients was no longer significant (F=3.06, df=1, 53, p<0.09). Although the between-group differences were more pronounced in the posterior (temporal-parietal-occipital) regions, we found an overall bilateral reduction of cortical folding in the schizophrenic group. However, as seen in Figure 2, the gyrification index values show that patients had higher cortical folding in the first (anterior) five slices along the right hemisphere. Indeed, calculating right gyrification index in slices 3, 4, and 5, such as Vogeley and colleagues did in their postmortem study (23), we confirmed their findings: the right gyrification index of patients (mean=2.03, SD=0.07) in this circumscribed area was significantly higher than the right gyrification index of the comparison subjects (mean=1.81, SD=0.1) (t=–3.28, df=4, p=0.03).
Within the schizophrenic group, significant reductions of cortical folding were seen in the left hemisphere in comparison to the right hemisphere (t=–2.86, df=39, p=0.007). No such laterality differences were found in healthy comparison subjects. Mean brain volumes of patients and comparison subjects were not significantly different from each other (t=1.28, df=58, p<0.21).
Comparison by Schizophrenia Subtype
The patients with the disorganized subtype of schizophrenia (N=8) showed significant right gyrification index reductions compared with both healthy comparison subjects (F=5.43, df=3, 56, p=0.001) and patients with the paranoid subtype (F=5.43, df=3, 56, p=0.003). The right gyrification index of patients with the residual subtype of schizophrenia tended to be lower than that of the healthy comparison subjects, but the difference was nonsignificant (F=5.43, df=3, 56, p=0.07). After Bonferroni correction and after gender, age, handedness, education, and brain volume were controlled, only the difference between the healthy subjects and patients with the disorganized subtype of schizophrenia remained statistically significant (F=2.91, df=3, 51, p<0.04).
For the left hemisphere, significant gyrification index reductions relative to healthy comparison subjects were seen in the patients with the disorganized (F=6.53, df=3, 56, p=0.001) and paranoid (F=6.53, df=3, 56, p<0.02) schizophrenia subtypes. Only the difference between patients with the disorganized subtype and healthy comparison subjects retained statistical significance after demographic variables were controlled (F=4.52, df=3, 51, p=0.006).
For the asymmetry coefficient of the gyrification index, patients with the paranoid subtype showed significantly greater right than left gyrification index values relative to the healthy subjects (F=2.17, df=3, 56, p<0.04). No other significant differences in laterality indices were detected.
Gyrification Index Correlations With Demographic Variables and Symptoms
Both right and left gyrification index values showed significant negative correlations with age in the total study group (r=–0.29, df=58, p<0.03 and r=–0.32, df=58, p<0.02, respectively). This effect was more pronounced for the left gyrification index of the comparison subjects (r=–0.51, df=18, p<0.03) and the right gyrification index of patients (r=–0.24, df=38, p<0.16). On the other hand, brain volume was directly correlated with the right gyrification index (r=0.31, df=58, p<0.02), with the largest effect in the group of patients (r=0.28, df=38, p=0.09). Gender, handedness, years of education, duration of illness, and family history of psychosis showed no significant correlations with gyrification index.
The gyrification index showed positive correlations with the severity of symptoms (total scores of Positive and Negative Syndrome Scale and BPRS), especially with paranoid symptoms (delusions, hallucinations, suspiciousness, grandiosity, and somatic concern). There were also significant negative correlations with the deficit symptoms of disorientation and poor attention (Table 3, Figure 3). After age, gender, and brain volume were controlled, the positive correlations of the gyrification index with the BPRS symptoms of suspiciousness, somatic concern, and grandiosity retained statistical significance, but the same did not occur with the correlations with hallucinatory behavior and delusions, which lost statistical significance. Negative symptoms (attention, orientation, and motivation) retained only trends to association with lower gyrification index, even though the negative correlations between gyrification index and total score on the Negative Symptom Rating Scale became more consistent (Table 3).
Discussion
Between-Group Differences
Relative to healthy subjects, schizophrenic patients showed significant left gyrification index reductions (–4.5%) and, to a lesser extent, right gyrification index reductions (–3.3%). Such findings are in agreement with the reduction of cortical folding in left hemispheres of nine male schizophrenic patients as reported by Kulynych et al. (16). On the other hand, in a postmortem study, Vogeley et al. (23) found a prefrontal “hypergyria” in the right hemispheres of 11 male schizophrenic patients. This finding was replicated by the same group (17) in an in vivo MRI study with 12 patients. In the first study (23), the calculation of the mean gyrification index of the prefrontal cortex was restricted to three sections (20 μm in thickness, intervals of 2 mm). In the second study (17), they included three slices of similar topography as well as two other slices 20 and 10 mm anterior to the genu of the corpus callosum. Differences in method make it difficult to compare those results with the findings of our study, since we measured slices at intervals of 3.6 mm along the whole cortex (about 50 slices in each hemisphere). In order to allow a more direct comparison of our data with the postmortem study of Vogeley et al. (23), we repeated our between-group comparison restricting the gyrification index measurement to slices 3, 4, and 5. Like these authors, we found “hypergyria” circumscribed to this right prefrontal area. There is some evidence that schizophrenic patients have the normal counterclockwise torque of the cerebral hemispheres diminished or absent in comparison with healthy individuals (with the right frontal and left occipital lobes more protruding than their counterparts in the opposite hemisphere) (24). According to this view, the apparent right prefrontal hypergyria reported by Vogeley et al. and ourselves could be seen as an artifact of differences in the topographic positioning of the slices of patients and comparison subjects.
In our study, the left hemisphere gyrification index of the schizophrenia patients was significantly lower than the right hemisphere gyrification index. Moreover, except for the disorganized subtype, all statistically significant differences between schizophrenic subgroups and healthy comparison subjects were restricted to the left hemisphere, suggesting that the gyrification index abnormalities have a main effect of left lateralization. Supposing that the reduction of the cortical folding reflects an underlying abnormality of neuronal connectivity, as suggested by some authors (13–15), our results are in agreement with a vast number of studies that have shown a predominance of abnormalities in the left hemisphere of schizophrenic patients (24, 25).
In both groups, we found significant bilateral inverse correlations of the gyrification index with age. In addition, the right gyrification index showed a positive correlation with brain volume. Such results are in disagreement with results reported by previous studies, where no significant correlations were observed between the cortical folding measures and demographic variables (13, 16).
The overall “hypogyria” reported in the present study can be discussed in view of the theories on cortical morphogenesis. The biomechanical hypothesis (26, 27) suggests that cortical folding results from different tensions of growth between the supragranular (layers I, II, and III) and infragranular (layers IV, V, and VI) strata. In a complementary way, the tension-based theory of cortical morphogenesis (28) argues that the mechanical tension along with axons, dendrites, and glial processes exerts a main force in the cortical folding. During corticogenesis, the cortical laminae, initially tethered only by radial glial process, become subsequently anchored by corticocortical and corticosubcortical connections. Considering the viscoelastic properties of axons, areas with more compact corticocortical wiring (larger approximative tension) tend to get together, originating an outward fold (gyrus); on the other hand, regions with poor circuitry (lesser cohesive force) tend to stretch the neuronal fibers, giving rise to inward folds (sulcus). The subplate, a transient fetal structure, contains neuronal populations previous to the migrant neurons to which they establish transient synapses involving neurotransmitters and neuromodulators; these connections are still poorly known but are certainly important for the understanding of corticogenesis (14). Armstrong et al. (15) drew attention to the chronological similarity between subplate and cortical folding. The subplate changes from a bilaminar to a single structure around the 21st ontogenetic week (when the cortex begins its rapid convolutional development) and diminishes in size after birth (when the cortical folding reaches its adult values). Hence, the subplate (whose cells project primarily to middle and superficial layers of cortical plate) could provide the mechanical forces responsible for the cortical folding, formed mainly as a consequence of the corticocortical circuitry.
Previous neuropathologic findings in schizophrenia add evidence in agreement with the above model. With regard to cytoarchitectural features in schizophrenia (see Harrison [1] for a critical review), the pyramidal neurons of the prefrontal cortex and limbic system have been shown to be smaller and more densely packed. The cortex is thinner, especially in laminae II and III, and the reduced volume in superficial layers suggests less extensive or aberrant synaptic connections formed by incoming corticocortical fibers, which are modulated by interaction with subplate cells during corticogenesis.
Gyrification Index: Relationship With Clinical Subtypes and Symptoms
The patients with the DSM-IV disorganized subtype of schizophrenia showed the smallest indexes of cortical folding. However, left gyrification index reduction was not exclusive to this subtype, since the paranoid and residual subtypes also showed left gyrification index reductions that reached statistical significance.
Schizophrenia patients with the paranoid subtype showed similar right gyrification index values to healthy comparison subjects. Indeed, symptom scores that tend to cluster around the paranoid dimension (suspiciousness, delusions, hallucinations, and grandiosity) were associated with an increased gyrification index in our study. On the other hand, the severity of the negative symptoms such as deficits of attention, orientation, and motivation were inversely related to the values of cortical folding. This may be related to the fact that, within the schizophrenic group, the disorganized subtype showed a lower gyrification index, mainly due to reductions in the left hemisphere. However, this interpretation must be made with caution, since the correlation coefficients were relatively modest (rs around –0.35 and p<0.05) and the elevated number of correlations increased the risk of false-positive results.
Several previous neuroimaging studies have found reduced white matter in schizophrenic patients (12, 29–31). Wible et al. (29) and Sanfilipo et al. (31) reported an association between a decreased volume of frontal white matter and the severity of negative symptoms, and Sigmundsson et al. (12) found reduced white matter in the left hemisphere of patients with prominent negative symptoms. Our study showed that the patients with a high prevalence of deficit symptoms (disorganized subtype) had a lower gyrification index. Supposing that a volumetric reduction in the white matter corresponds to a reduced number of axons, lower viscoelastic tension, and therefore a lower cortical folding, our results are in consonance with the findings reported by those authors.
In summary, the hypothesis that abnormalities in cortical folding represent underlying disturbed neuronal connectivity is based on morphogenetic and neuropathologic studies involving the subplate in the genesis of corticocortical and corticothalamic connections as determinant factors of viscoelastic tension responsible for cortical folding. Therefore, the reduced gyrification index observed in schizophrenic patients may reflect abnormalities in corticogenesis and may be related to negative symptoms (e. g., deficits in attention, orientation, and motivation).
However, gyrification index is only an indirect measure of a neuropathological process that is still poorly understood. Additional empirical investigations are needed in order to clarify how the gyrification index could be affected by neurodevelopmental disturbances in humans and to ascertain what kind of cytoarchitectural abnormalities could be related with disturbances in cortical folding.
Our study had limitations that need to be acknowledged. Not all the subtypes of schizophrenia were represented in the study (e.g., the catatonic subtype), and the relatively small number of subjects made the statistical analysis of subgroups problematic. On the other hand, the symptom-based subtypes of DSM have a very modest stability along the course of illness. Patients initially diagnosed as one subtype may display a combination of symptoms that changes over time to another subtype (5), thereby making it difficult to establish the homogeneity of subgroups of schizophrenia.
In conclusion, we found a pattern of reduced cortical folding in patients with schizophrenia that may reflect cytoarchitectonic abnormalities in the neurodevelopmental process. This phenomenon was mainly pronounced in patients with the disorganized subtype, with predominance of negative symptoms. On the other hand, the paranoid subtype showed a relatively preserved gyrification index that correlated positively with the severity of positive symptoms.
In addition, schizophrenic patients showed significantly reduced cortical folding in the left hemisphere compared with the right hemisphere. Such a result may indicate a main effect of left lateralization in schizophrenic patients, a finding that is also in line with abnormal neurodevelopment.
 |
 |
 |
Received Nov. 28, 2001; revised July 31, 2002; accepted March 3, 2003. From the Department and Institute of Psychiatry, the Laboratory of Neuroscience (LIM-27), and the Department of Radiology of the Faculty of Medicine, University of São Paulo. Address reprint requests to Dr. Sallet, Faculty of Medicine, University of São Paulo, R. Dr. Ovidio Pires de Campos s/n, 05403-010, São Paulo SP, Brazil; [email protected] (e-mail). Supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (97/11083-0 and 98/10487-2) to Dr. Sallet and by funding from the Associação Beneficente Alzira Denise Hertzog da Silva.
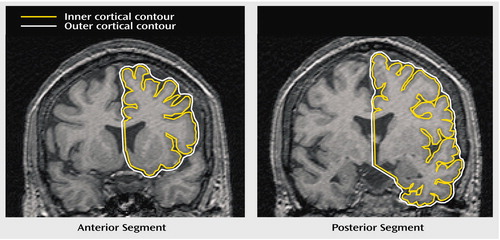
Figure 1. Gyrification Index Measurement in a Schizophrenic Patienta
aThe anterior segment shows the gyrification index for the left frontal region. The posterior segment shows the gyrification index for the left temporal-parietal-occipital region. The gyrification index is the length of the inner cortical contour (yellow line) divided by the length of the outer cortical contour (white line).
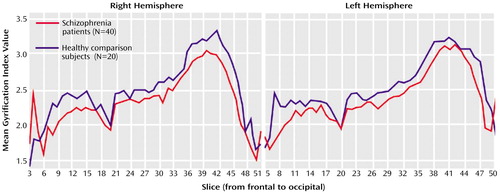
Figure 2. Anterior-to-Posterior Gyrification Index Values in Schizophrenic Patients and Healthy Comparison Subjects Who Underwent MRI Scans for Measurement of Cortical Folding
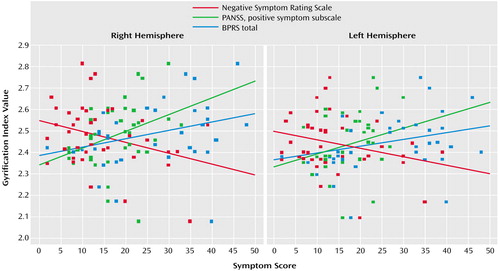
Figure 3. Correlations of Right and Left Gyrification Index Values to Clinical Symptom Scores in Schizophrenic Patients (N=40) and Healthy Comparison Subjects (N=20) Who Underwent MRI Scans for Measurement of Cortical Folding
1. Harrison PJ: The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain 1999; 122:593–624Crossref, Medline, Google Scholar
2. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Crossref, Medline, Google Scholar
3. Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC: A follow-up magnetic resonance imaging study of schizophrenia. Arch Gen Psychiatry 1998; 55:145–152Crossref, Medline, Google Scholar
4. Zipursky RB, Lambe EK, Kapur S, Mikulis DJ: Cerebral gray matter volume deficits in first episode psychosis. Arch Gen Psychiatry 1998; 55:540–546Crossref, Medline, Google Scholar
5. Goldberg TE, Weinberger DR: A case against subtyping in schizophrenia. Schizophr Res 1995; 17:147–152Crossref, Medline, Google Scholar
6. Andreasen NC: A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry 1999; 56:781–787Crossref, Medline, Google Scholar
7. Tsuang MT, Faraone SV: The case for heterogeneity in the etiology of schizophrenia. Schizophr Res 1995; 17:161–175Crossref, Medline, Google Scholar
8. Franzek E, Beckmann H: Different genetic background of schizophrenia spectrum psychoses: a twin study. Am J Psychiatry 1998; 155:76–83Link, Google Scholar
9. Bullmore ET, Woodruff PWR, Wright IC, Rabe-Hesketh S, Howard RJ, Shuriquie N, Murray RM: Does dysplasia cause anatomical dysconnectivity in schizophrenia? Schizophr Res 1998; 30:127–135Crossref, Medline, Google Scholar
10. Andreasen NC, Paradiso S, O’Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203–218Crossref, Medline, Google Scholar
11. McGlashan TH, Hoffman RE: Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry 2000; 57:637–648Crossref, Medline, Google Scholar
12. Sigmundsson T, Suckling J, Maier M, Williams SCR, Bullmore ET, Greenwood KE, Fukuda R, Ron MA, Toone BK: Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 2001; 158:234–243Link, Google Scholar
13. Zilles K, Armstrong E, Schleicher A, Kretschmann H-J: The human pattern of gyrification in the cerebral cortex. Anat Embryol 1988; 179:173–179Crossref, Medline, Google Scholar
14. Rakic P: Specification of cerebral cortical areas. Science 1988; 241:170–176Crossref, Medline, Google Scholar
15. Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K: The ontogeny of human gyrification. Cereb Cortex 1995; 1:56–63Crossref, Google Scholar
16. Kulynych JJ, Luevano LF, Jones DW, Weinberger DR: Cortical abnormality in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatry 1997; 41:995–999Crossref, Medline, Google Scholar
17. Vogeley K, Tepest R, Pfeiffer U, Schneider-Axmann T, Maier W, Honer WG, Falkai P: Right frontal hypergyria differentiation in affected and unaffected siblings from families multiply affected with schizophrenia: a morphometric MRI study. Am J Psychiatry 2001; 158:494–496Link, Google Scholar
18. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
19. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
20. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
21. Woerner M, Manuzza S, Kane J: Anchoring the BPRS: an aid to improve reliability. Psychopharmacol Bull 1988; 24:112–117Medline, Google Scholar
22. Iager A-C, Kirch DG, Wyatt RJ: A negative symptom rating scale. Psychiatr Res 1985; 16:27–36Crossref, Medline, Google Scholar
23. Vogeley K, Schneider-Axmann T, Pfeiffer U, Tepest R, Bayer TA, Bogerts B, Honer WG, Falkai P: Disturbed gyrification of the prefrontal region in male schizophrenic patients: a morphometric postmortem study. Am J Psychiatry 2000; 157:34–39Link, Google Scholar
24. Petty RG: Structural asymmetries of the human brain and their disturbance in schizophrenia. Schizophr Bull 1999; 25:121–139Crossref, Medline, Google Scholar
25. Gruzelier JH: Functional neuropsychophysiological asymmetry in schizophrenia: a review and reorientation. Schizophr Bull 1999; 25:91–120Crossref, Medline, Google Scholar
26. Richman DP, Stewart RM, Hutchison JW, Caviness SV: Mechanical model of brain convolutional development. Science 1975; 189:18–21Crossref, Medline, Google Scholar
27. Armstrong E, Curtis M, Buxhoeveden DP, Fregoe C, Zilles K, Casanova MF, McCarthy WF: Cortical gyrification in the rhesus monkey: a test of the mechanical folding hypothesis. Cereb Cortex 1991; 1:426–432Crossref, Medline, Google Scholar
28. Van Essen DC: A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997; 385:313–318Crossref, Medline, Google Scholar
29. Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW: Prefrontal cortex and schizophrenia: a quantitative magnetic resonance imaging study. Arch Gen Psychiatry 1995; 52:279–288Crossref, Medline, Google Scholar
30. Cannon TI, Van Erp TGM, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Gut RC, Yan M: Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 1998; 55:1084–1091Crossref, Medline, Google Scholar
31. Sanfilipo M, Lafarge T, Rusinek H, Arena L, Loneragan C, Lautin A, Feiner D, Rotrosen J, Wolkin A: Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry 2000; 57:471–480Crossref, Medline, Google Scholar



