Different Genetic Background of Schizophrenia Spectrum Psychoses: A Twin Study
Abstract
OBJECTIVE: The authors report on a systematic twin study of index twins suffering from schizophrenia spectrum psychoses. Using different diagnostic systems, they examined twin concordance, family history, and the frequency and severity of the birth complications of 22 monozygotic and 23 dizygotic twin pairs. METHOD: All twins in the region of Lower Franconia, Germany, born after 1930 and hospitalized for psychiatric disease were ascertained. The zygosity diagnoses were based on molecular genetic methodology and a zygosity questionnaire. Two psychiatrists, working independently, formulated diagnoses according to DSM-III-R criteria and Leonhard's nosology. RESULTS: There were substantially different concordance rates with regard to diagnostic subgroups, and monozygotic concordance was significantly higher than dizygotic concordance in only two of the following five subgroups (subgroups 1 and 3): 1) strict schizophrenia according to DSM-III-R: monozygotic, 85.7%, dizygotic, 25.0% 2) schizophreniform, schizoaffective, and delusional (paranoid) disorders and psychotic disorder not otherwise specified according to DSM-III-R: monozygotic, 47.1%, dizygotic, 30.8% 3) unsystematic schizophrenia according to Leonhard: monozygotic, 88.9%, dizygotic, 25.0% 4) systematic schizophrenia according to Leonhard: monozygotic pairs lacking, dizygotic, 0% 5) cycloid psychoses according to Leonhard: monozygotic, 38.5%, dizygotic, 36.4%. In the case of cycloid psychoses and conditions less prominent in DSM-III-R schizophreniform, schizoaffective, and delusional (paranoid) disorders and psychotic disorder not otherwise specified, the affected twins had suffered significantly more severe birth complications than their healthy partners. Not one of the 37 monozygotic twins was diagnosed as having systematic schizophrenia, whereas six of the 25 dizygotic index twins received this diagnosis. CONCLUSIONS: The results of the study suggest that schizophrenia spectrum psychoses may consist of clinically and etiologically heterogeneous subgroups with different genetic backgrounds. (Am J Psychiatry 1998; 155:76–83)
The diagnostic concept of schizophrenia has been open to dispute right from the start, and this situation has not changed markedly since the introduction of modern operational and atheoretical diagnostic systems. As the following quotation succinctly points out, striking progress in schizophrenia research has yet to be made: “We have now at our disposal, powerful genetic, biochemical, and brain-imaging technology. Nevertheless, there is an increasing gap between these developments and the growth in our understanding of the etiology of schizophrenia” (1).
Leonhard's classification of endogenous psychoses is largely unknown, even though a number of studies point to its validity and reliability (2–10). This classification is highly operationalized and based on the sophisticated clinical descriptions and hierarchical symptom patterns occurring during the long-term course of psychiatric diseases (11, 12). A diagnosis based on Leonhard's classification can be made only if all the clinical features fit; i.e., the diagnosis may not be made if characteristic symptoms are lacking. This is the main difference between Leonhard's classification and the operational diagnostic systems that require the presence of some but not all or specific symptoms of a symptom cluster. According to Leonhard, psychoses exhibiting “schizophrenic symptoms” (DSM-III-R criterion A) have to be divided into three distinct clinical and nosological subgroups: cycloid psychoses and unsystematic and systematic schizophrenias.
Cycloid psychoses run a phasic and prognostically favorable long-term course that is similar to manic-depressive disease. “Schizophrenic symptoms” are frequently present during the acute phases. Complete remissions and the absence of residual symptoms are characteristic features of cycloid psychoses. Because of their polymorphous clinical pictures, they are found in many diagnostic categories when DSM-III-R criteria are used, ranging from bipolar mood disorders with psychotic symptoms to strictly defined schizophrenia. Cycloid psychoses exhibit some resemblance to unsystematic schizophrenias, especially in the early stages of the disease. Unsystematic schizophrenias, however, generally lead to residual states of varying severity and can easily be distinguished from cycloid psychoses by experienced and thoroughly trained psychiatrists. Unsystematic schizophrenias are also distributed in a wide range of DSM-III-R diagnostic categories. For the most part, however, they fulfill the “strict” DSM-III-R criteria for schizophrenia. Systematic schizophrenias usually begin insidiously and run a chronic, progressive course without remission. The irreversible, treatment-resistant residual states are clinically well defined and can be reliably distinguished from unsystematic schizophrenia (6, 13). According to DSM-III-R, almost all systematic schizophrenias meet the strict criteria for schizophrenia. Family studies have revealed high familial loading with identical psychoses for unsystematic schizophrenias but low familial loading for systematic schizophrenias and cycloid psychoses (3, 11, 12).
In recent literature, the conflicting results obtained in schizophrenia research are generally thought to be an indication of polygenic and heterogenic inheritance combined with various adverse environmental factors (14, 15). Nevertheless, the question of whether schizophrenia and quasi-schizophrenic psychoses do, in fact, represent a continuum of diseases without any clear dividing lines between them or are, instead, distinct disease entities with various etiologies has yet to be answered.
Using the twin-study approach, we sought to shed new light on this urgent question. Our diagnoses were established by using DSM-III-R criteria and Leonhard's classification of endogenous psychoses (11, 12). We studied twin concordance for monozygotic and dizygotic twins, familial loading with mental disease, and the frequency and severity of birth complications.
METHOD
Twin Ascertainment
Lower Franconia is a region of Bavaria the population of which is characterized by low fluctuation due to new arrivals and emigration. There are three psychiatric hospitals within this region, the Department of Psychiatry of Wuerzburg University, the State Hospital Lohr/Main, and the State Hospital Werneck; almost every patient requiring hospital treatment of mental disorders is admitted to one of them. Given the lack of a psychosis register in Germany, twin ascertainment was representative within limits; i.e., the sample comprised all twin individuals born after 1930 who had been admitted to one of the region's psychiatric hospitals at least once.
The psychiatric diagnoses at the three hospitals have been documented uniformly according to ICD-9. We began by selecting all twins with ICD-9 diagnoses of schizophrenia (categories 295.0–295.9) and paranoid syndromes (297.0–297.9). To enter the study, the index twins then had to meet DSM-III-R criteria for the following disorders: schizophrenia (295.1, 295.2, 295.3, 295.6, 295.9), delusional (paranoid) disorder (297.10), psychotic disorder not otherwise specified (298), schizophreniform disorder (295.4), and schizoaffective disorder (295.7). These categories thus represented, by definition, psychoses of the schizophrenia spectrum. This diagnostic limitation excluded from the study patients with cycloid psychosis who met DSM-III-R criteria for bipolar mood disorder with psychotic symptoms.
Only same-sex twin pairs entered the study. Zygosity diagnoses were established by using molecular genetic methodology with high polymorphous microsatellites (16) and a zygosity questionnaire (17).
Diagnosis
Two experienced psychiatrists carried out the diagnostic work by personally examining the twins. The first author contacted the twins by letter and by phone. After written informed consent was obtained, most of the twins were visited at their homes; the others came to the hospital.
The first author diagnosed one of the twin partners, and not until he had formulated his diagnosis was the twin's partner presented to the second author. The second author received no information on the first author's diagnosis until he had committed himself to a specific diagnosis according to DSM-III-R and Leonhard's classification. He received all the relevant information except for family history, history of the mother's deliveries, and mental state of the twin's partner. Since the second author knew only one partner of a twin pair during his diagnostic work, he was blind to the twins' zygosity. Because the first author knew both twins in each pair, he could draw conclusions regarding zygosity by comparing the twins' appearance. Like the second author, however, he did not know the zygosity diagnosis made according to the molecular genetic method. This methodological approach minimized the likelihood of the diagnosis of one twin influencing the diagnosis of his or her co-twin, as would have been possible if both twins in a given twin pair had been diagnosed by the same investigator.
The Schedule for Affective Disorders and Schizophrenia—Lifetime Version Modified for the Study of Anxiety Disorders (SADS-LA), a semistructured interview, was used for DSM-III-R diagnoses. The Leonhard diagnoses were based on Leonhard's original descriptions of endogenous psychoses. We have achieved high kappa values in previous reliability studies by using DSM-III-R criteria and Leonhard's classification (4, 6).
Determination of Twin Concordance
Twin concordance can be calculated by either a pairwise or probandwise method. The pairwise method is appropriate for statistical evaluation in comparisons of monozygotic and dizygotic concordance rates. Probandwise calculation implies that pairs with two index twins have to be counted twice. This method of calculation presupposes that the index twins have been systematically ascertained and allows a direct comparison of concordance rates with the values for familial loading in family studies (18).
The concordance criteria were gradated according to the definitions of Gottesman and Shields (19, 20) and Fischer et al. (21) into three groups: K1 (strict definition), K2 (middle definition), and K3 (broad definition).
Concordance groups according to DSM-III-R. K1 (strict): The co-twin's diagnosis is identical to that of the index twin. K2 (middle): The index twin has received a diagnosis of schizophrenia, and the co-twin has received a diagnosis from the categories 295.4, 295.7, 297, or 298. K3 (broad): The co-twin suffered or suffers from some other relevant DSM-III-R mental disorder, except for mental retardation and dementia. A twin pair was defined as discordant if the co-twin had never suffered from a mental disorder and was found to be healthy at the time of investigation.
Concordance groups according to Leonhard. K1 (strict): The co-twin's diagnosis is identical to that of the index twin. K2 (middle): The diagnosis of the co-twin is not identical to the index twin's, but the co-twin suffered from another schizophrenia spectrum psychosis. The K3 (broad) definition and discordance criteria were the same as those for DSM-III-R.
Family History
The investigation of the twin pairs' familial loading with mental disorders was based on the family history method (22). All living family members who had been said to suffer from mental disorders were personally examined through use of a semistructured interview (SADS-LA). All hospital records of living and deceased family members who had been hospitalized for mental disorders were made available. All living mothers (78%) and fathers (38%) were personally examined with the SADS-LA. Only data on parents and siblings were used to evaluate familial loading. Ill co-twins were not counted.
Birth Complications
In order to obtain information on delivery, we reviewed hospital records, questioned the twins themselves, and interviewed all living mothers. This approach has been estimated to yield sufficiently valid and reliable results (23–28). Birth complications were rated according to a widely used severity-weight allocation scale for specific complications (29). We then compared the healthy with the ill twins in the discordant pairs and the more severely affected twins with their co-twins in the concordant pairs. The definition of which twin was the more severely affected was based on age at onset of the disease, the frequency and duration of hospitalization, social competence (e.g., professional activity), and the general clinical impression at the time of the follow-up investigation.
RESULTS
Twin Ascertainment
From 1960 to 1989 we systematically screened over 25,000 hospital records and found 371 index twins who had been hospitalized for mental disorders at least once in the region of Lower Franconia. This means that one of every 67 patients was found to be a twin. Because this is approximately in line with the frequency of twins in the general population, we obviously did not lose a substantial proportion of the twins (30).
According to the records, 50 co-twins were deceased; of the remaining 321 pairs, 204 were of the same sex and 117 of the opposite sex. According to the charts, 101 same-sex index twins suffered from endogenous psychoses, whereas the others had received diagnoses such as neurosis, personality disorder, alcohol or drug abuse, dementia, and other organic brain diseases.
Sixty-three index twins of 52 twin pairs had received one of the following ICD-9 diagnoses: schizophrenic psychoses (categories 295.0 to 295.9) or paranoid syndromes (297.0 to 297.9). From 1989 onward, the State Hospital Lohr/Main registered, in a computer program, all twins admitted. By 1994 this had resulted in an additional 12 same-sex twin pairs with 14 index twins meeting the primary inclusion criteria of the study. In four of the 64 same-sex twin pairs then available, one partner was dead. Probands from 10 of the remaining 60 pairs refused to participate in the study. In five pairs, the index twins did not meet DSM-III-R criteria for any one of the schizophrenia spectrum psychoses. What follows, therefore, are the data for 45 same-sex twin pairs (22 female, 23 male).
Demographic Data
The mean age of the 45 twin pairs at the time of the investigation was 40 years (SD=13, range=22–65). The mean age of the 22 monozygotic twin pairs was 41 years (SD=12, range=22–63), and that of the 23 dizygotic twin pairs was 40 years (SD=14, range=20–65). The mean age at the time of the first hospitalization of the ill twins (N=62) was 20 years (SD=8) for men (N=31) and 22 years (SD=10) for women (N=31). The onset of the disease and first hospitalization typically took place at the same time. For 58 (93.5%) of the 62 psychotic probands, there was an interval of more than 5 years between the onset of the disease and the follow-up investigation. For 26 of them (41.9%), there was an interval of more than 19 years.
Zygosity Diagnoses
We succeeded in obtaining blood samples from both twins in 39 pairs (87%). The zygosity diagnoses made on the basis of the molecular genetic and questionnaire methods were identical in 38 cases (97.4%). The twins of five pairs refused to give blood samples. In one twin, the blood obtained was not sufficient for molecular genetic analysis. In six (13%) of the 45 pairs, therefore, zygosity diagnoses were based solely on the zygosity questionnaire. There were 22 monozygotic and 23 dizygotic twin pairs.
Twin Concordance
Among the 17 concordant pairs in which both partners of a twin pair had developed psychoses of the schizophrenia spectrum, there was an interval of less than 1 year in four pairs (23.5%), of less than 5 years in nine pairs (52.9%), and of less than 9 years in three pairs (17.6%). In only one pair (5.9%) was there an interval of 16 years.
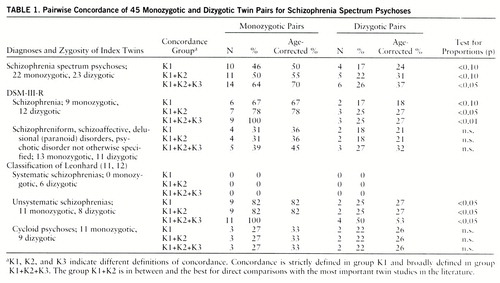
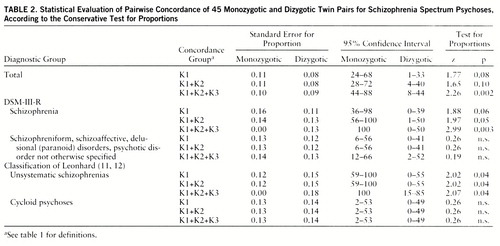
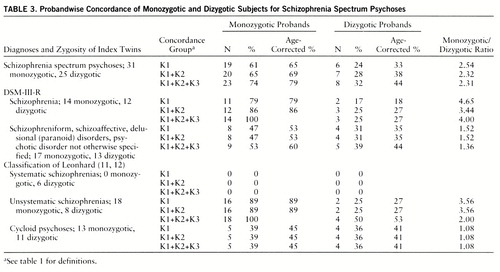
Table 1 shows pairwise concordance, and table 2 presents all components of statistical evaluation of these data (test for proportions). Table 3 presents probandwise concordance. Four twin pairs were concordant according to the K3 criteria. The four co-twins from this group all received a DSM-III-R axis II diagnosis (personality disorder), and one of them had also suffered from chronic insomnia for more than 10 years.
The probandwise and pairwise concordance of the total group for schizophrenia spectrum psychoses (group K1+K2) differed only nonsignificantly from the concordance rate of a meta-analysis of twin studies between 1928 and 1991 (probandwise concordance for monozygotic twins: 65% in our study, as opposed to 58% in the meta-analysis; dizygotic twins: 28% versus 15% pairwise concordance for monozygotic twins: 50% versus 55% dizygotic twins: 22% versus 11%).
Using the conservative test for proportions, we obtained statistically significant differences at the 5% level between monozygotic and dizygotic concordance rates in the group of unsystematic schizophrenias according to Leonhard's classification and in the group of twins who met the strict DSM-III-R criteria for schizophrenia. When we used one-tailed Fisher's exact probability test with exact p values (31), the statistically significant differences were even more evident. For schizophrenia spectrum psychoses, group K1: p=0.02, K1+K2: p=0.02, K1+K2+K3: p=0.006; for strict DSM schizophrenia, K1: p=0.01, K1+K2: p=0.01, K1+K2+K3: p=0.0002; for unsystematic schizophrenia, K1: p=0.008, K1+K2: p=0.008, K1+K2+K3: p=0.006.
A broad definition of concordance (group K1+K2+K3) in the monozygotic pairs led to an increase in concordance rates up to 100% for both unsystematic schizophrenia and DSM-III-R schizophrenia. In the dizygotic pairs it did not change the concordance rate for DSM-III-R schizophrenia but led to an increase in the rate for unsystematic schizophrenia from 25% to 50%.
The concordance rates for monozygotic pairs in the group of DSM-III-R categories 295.4, 295.7, 297, and 298 and in the group of cycloid psychoses were substantially lower and were not different to a statistically significant degree from the dizygotic concordance rates. In the group of cycloid psychoses, the dizygotic rate was just as high as the monozygotic rate (probandwise concordance: monozygotic twins, 39% dizygotic twins, 36%). Broadening the definition of concordance did not change these rates.
The group with strict DSM-III-R schizophrenia differed significantly from the group comprising the DSM-III-R diagnoses 295.4, 295.7, 297, and 298 with respect to concordance rates (contingency test: χ2=9.17, df=3, p<0.05). When we tested more conservatively and compared monozygotic and dizygotic concordance rates separately, the higher monozygotic concordance rate for strict DSM-III-R schizophrenia than for other spectrum psychoses only approached significance (χ2=3.01, df=1, p<0.09). No difference emerged when dizygotic rates were compared.
The concordance rates for the groups with unsystematic schizophrenia and cycloid psychoses also differed significantly (contingency test: χ2=11.08, df=3, p<0.01). Even when tested more conservatively, the monozygotic concordance rate was significantly higher for unsystematic schizophrenia than for cycloid psychosis (χ2=5.3, df=1, p<0.05). No difference emerged when dizygotic rates of both groups were compared.
Not one of the 37 psychotic monozygotic twins had been diagnosed as having systematic schizophrenia, whereas six of the 25 psychotic dizygotic twins had received this diagnosis. This difference was statistically significant (test for proportions, z=2.67, p<0.01). Every dizygotic pair with an index twin with systematic schizophrenia was discordant.
Table 4 shows the distribution of psychotic twins diagnosed according to Leonhard within the various DSM-III-R categories. Each of the twins with systematic schizophrenia also met DSM-III-R criteria for schizophrenia. Only 65% of the twins with unsystematic schizophrenia, however, were diagnosed as schizophrenic according to DSM-III-R. The remaining 35% covered a wide range of DSM-III-R diagnoses. Twins with cycloid psychosis were similarly distributed throughout the whole spectrum of DSM-III-R diagnoses, and 16% of them met DSM-III-R criteria for schizophrenia.
Family History
Table 5 presents the family history data of the 45 twin pairs with regard to diagnoses. We differentiated among familial loading with schizophrenia spectrum psychosis, affective psychosis, suicide without prior psychiatric diagnosis, and no psychosis but other DSM-III-R mental disorder (except for mental retardation and dementia).
There were more schizophrenia spectrum psychoses and fewer affective psychoses among parents and siblings in the group with DSM-III-R schizophrenia than in the group comprising DSM-III-R categories 295.4, 295.7, 297, and 298. This difference was not statistically significant, however. On the other hand, twins with unsystematic schizophrenia had significantly more parents and siblings suffering from schizophrenia spectrum psychosis and from no psychosis but other mental disorder than did twins with cycloid psychosis (contingency test, χ2=9.21, df=3, p<0.05), and there was neither a parent nor a sibling suffering from a schizophrenia spectrum psychosis in the families of twins with cycloid psychosis.
History of Delivery
There were no differences with regard to birth weight either in the group as a whole or in the various diagnostic groups. The rate and severity of birth complications for the ill or more severely affected twins in the cycloid psychosis group and the group comprising DSM-III-R categories 295.4, 295.7, 297, and 298, however, were significantly higher than for their partners (whole group: z=2.00, p=0.05; cycloid psychoses: z=2.52, p=0.01; categories 295.4, 295.7, 297, and 298: z=2.25, p=0.02; Wilcoxon matched-pairs signed rank test). The dizygotic index twins suffering from systematic schizophrenia according to Leonhard (N=6) had complications that were three times more frequent and more severe than those of their healthy co-twins. There was no difference between the ill or more severely affected twins and their partners in the groups with DSM-III-R schizophrenia and unsystematic schizophrenia.
DISCUSSION
Until now, the most important twin studies focusing on schizophrenia have reported significantly higher concordance rates among monozygotic than dizygotic twins. Some studies, however, have reported substantially different concordance rates for monozygotic twin pairs, whereas the concordance rates for dizygotic twins do not vary substantially (30). These studies have obviously been dominated by various patient groups that have either raised or lowered the monozygotic concordance rates. Some authors have reported significantly different monozygotic concordance rates with regard to the severity of illness in the index twins. Index twins suffering from mild or transient schizophrenia have been found to have ill co-twins in only 17%–33% of cases, whereas pairs in which index twins exhibit severe residual psychopathology have had concordance rates of 75%–100% (19, 32–34). We found that significantly more monozygotic concordant than dizygotic concordant twins met the strict DSM-III-R criteria for schizophrenia. Fewer monozygotic concordant twins met criteria for other DSM-III-R diagnoses (295.4, 295.7, 297, and 298) than met strict criteria for schizophrenia; and although more monozygotic concordant than dizygotic concordant twins met criteria for these other diagnoses, the difference was not significant. These findings point to a tendency toward quantitatively higher genetic loading in individuals with severe residual psychopathology than in others (35, 36).
When Leonhard's criteria were used, however, striking differences in the concordance rates for unsystematic schizophrenias (unfavorable long-term course with residual states of varying severity) and cycloid psychoses (favorable long-term course without residual psychopathology) emerged. There were high monozygotic rates and significantly lower dizygotic rates in unsystematic schizophrenias and low monozygotic rates and nearly exactly the same dizygotic rates (monozygotic: 39%, dizygotic: 36%) in cycloid psychoses. We found that the monozygotic concordance rate of unsystematic schizophrenia was significantly higher than that of cycloid psychoses. This points to a qualitatively higher genetic loading in individuals with unsystematic schizophrenia than in individuals suffering from cycloid psychosis.
Broadening the definition of concordance did not change concordance rates in the cycloid psychosis group and the group comprising DSM-III-R diagnoses 295.4, 295.7, 297, and 298. With regard to strict DSM-III-R schizophrenia and unsystematic schizophrenia according to Leonhard's classification, however, monozygotic concordance rose as high as 100%. Farmer et al. (37) also reported that the highest monozygotic/dizygotic concordance rates resulted from a broadening of the definition of concordance.
It is striking that of the 37 psychotic monozygotic probands, no twins have been found with a diagnosis of systematic schizophrenia, whereas six (24%) of 25 psychotic dizygotic index twins suffered from systematic schizophrenia (p<0.01).
Leonhard investigated 69 psychotic monozygotic twins and did not find systematic schizophrenia either, while this was the case in 12 (25.5%) of his 47 psychotic dizygotic twins (12). Because Leonhard did not carry out a systematic twin ascertainment, he may have overlooked monozygotic twins with systematic schizophrenia. Despite our systematic twin ascertainment, however, we did not find any monozygotic twins with systematic schizophrenia either. Even so, an interpretation of this finding remains obscure; it is an argument against the theory that “confusion of ego identity” (38, 39) is a causal factor for systematic schizophrenia.
There was no statistically significant difference in the frequency of schizophrenia, affective psychoses, and other mental disturbances among the parents and siblings of twins with strict DSM-III-R schizophrenia and those of the group comprising the DSM-III-R categories 295.4, 295.7, 297, and 298. Twins with unsystematic schizophrenia, however, had significantly more parents and siblings suffering from schizophrenia spectrum psychoses and no psychosis but other mental disorder than did twins with cycloid psychosis, and it is striking that there were no schizophrenia spectrum psychoses at all among the parents and siblings of twins with cycloid psychosis. The fact that 7% of the 100 parents and siblings of twins with cycloid psychoses suffered from affective psychoses may indicate that there is a genetic loading for affective psychoses in patients with cycloid psychoses. On the other hand, equal concordance rates for monozygotic and dizygotic twins point to a mainly environmental cause of these illnesses, and the assumption that cycloid psychoses are merely a variant of manic-depressive disorder is not tenable, since manic-depressive disorder exhibits high monozygotic and low dizygotic concordance rates (40).
Birth complications, as “environmental” stressors, seem to contribute to the etiology of cycloid psychoses. The rate and severity of such complications were significantly higher among the ill or more severely affected twins than among their partners. Eighty-four percent of the twins with cycloid psychoses had been allocated to the group comprising DSM-III-R categories 295.4, 295.7, 297, and 298. This explains the finding that even in this diagnostically heterogeneous group, the ill or more severely affected twins suffered more severe birth complications than their partners. No such difference was apparent in the groups with DSM-III-R schizophrenia and Leonhard's unsystematic schizophrenia. The six dizygotic index twins with systematic schizophrenia suffered three times as many complications as their healthy partners. This corroborates the findings of Stöber et al. (41–43) that pregnancy-birth complications may play an important role in the etiology of systematic schizophrenia and cycloid psychoses. These findings, however, should not be overstated, since the data on delivery were based mainly on retrospective reports. The latter is true, however, of the group of patients as a whole, and the fact that significant results were obtained only in a circumscribed clinical subgroup indicates that the data are important. According to Galton's rule, the results of our study indicate that DSM-III-R schizophrenia and unsystematic schizophrenia according to Leonhard are primarily genetically determined. In cycloid psychoses, however, heredity seems to be very low, and environmental factors such as birth complications may be of some etiological importance. The group comprising DSM-III-R categories 295.4, 295.7, 297, and 298 falls in between these two categories; i.e., genetic and environmental factors have to be weighted equally.
Special Leonhard diagnoses are no match for special DSM-III-R categories. While it is true that all the twins diagnosed as having systematic schizophrenia also met DSM-III-R criteria for schizophrenia, unsystematic schizophrenias as well as cycloid psychoses were distributed variously over a wide range of DSM-III-R categories. Despite some overlap, therefore, an equation of DSM-III-R schizophrenia with Leonhard's unsystematic and systematic schizophrenia or of cycloid psychoses with DSM-III-R categories 295.4, 295.7, 297, and 298 is simply not permissible.
The findings of this twin study provide evidence that schizophrenia and quasi-schizophrenic psychoses comprise a spectrum of genetically heterogeneous diseases. When DSM-III-R criteria were used, there was a tendency for strict schizophrenia to be more genetically loaded than the heterogeneous diagnostic group of schizophreniform, schizoaffective, delusional, and psychotic disorders not otherwise specified.
When Leonhard's classification was used, there was statistically significant evidence that cycloid psychoses and unsystematic and systematic schizophrenias are genetically different groups. In the case of cycloid psychoses and systematic schizophrenias, genetic loading seems to be low, while environmental factors may play an important role in etiology. Unsystematic schizophrenias, however, seem to be predominantly inherited, and environmental stressors are not prominent.
 |
 |
 |
 |
 |
Received Oct. 28, 1996; revisions received March 10 and July 31, 1997; accepted Aug. 8, 1997. From the Department of Psychiatry, University of Wuerzburg. Address reprint requests to Dr. Franzek, Department of Psychiatry, University of Wuerzburg, Fuechsleinstraße 15, D-97080 Wuerzburg, Germany. Supported by the Deutsche Forschungsgemeinschaft. The authors thank Dr. Armin Schmidtke and Dr. Werner Strik for their statistical advice and evaluation of the data and Dr. Gerd Jungkunz and Dr. Albrecht Schottky for their assistance in twin ascertainment.
1. Parnas J: Schizophrenia: etiological factors in the light of longitudinal high-risk research, in Etiology of Mental Disorders. Edited by Kringlen E, Lavik NJ, Torgersen S. Oslo, University Press of Oslo, 1990, pp 49–61Google Scholar
2. Astrup C: The Chronic Schizophrenias. Oslo, UniversitÄtsforlaget, 1979Google Scholar
3. Beckmann H, Franzek E, Stöber G: Genetic heterogeneity in catatonic schizophrenia: a family study. Am J Med Genet Neuropsychiatr Genet 1996; 67:289–300Crossref, Medline, Google Scholar
4. Franzek E, Beckmann H: Schizophrenia: not a disease entity? a study of 57 long-term hospitalized chronic schizophrenics. Eur J Psychiatry 1992; 6:97–108Google Scholar
5. Lindvall M, Hagnell O, Öhman R: Epidemiology of cycloid psychosis—a prospective longitudinal study of incidence and risk in the 1947 cohort of the Lundby study. Eur Arch Psychiatry Clin Neurosci 1986; 236:109–119Crossref, Google Scholar
6. Pfuhlmann B, Franzek E, Stöber G, Cetkovich-Bakmas M, Beckmann H: On interrater reliability for Leonhard's classification of endogenous psychoses. Psychopathology 1997; 30:100–105Crossref, Medline, Google Scholar
7. Stöber G, Franzek E, Lesch HP, Beckmann H: Periodic catatonia: a schizophrenic subtype with major gene effect and anticipation. Eur Arch Psychiatry Clin Neurosci 1995; 245:135–141Crossref, Medline, Google Scholar
8. Strik WK, Dierks T, Franzek E, Maurer K, Beckmann H: Differences in P300-amplitude and topography between cycloid psychosis and schizophrenia in Leonhard's classification. Acta Psychiatr Scand 1993; 87:179–183Crossref, Medline, Google Scholar
9. Ungvari GS: The Wernicke-Kleist-Leonhard school of psychiatry. Biol Psychiatry 1993; 34:749–752Crossref, Medline, Google Scholar
10. Warkentin S, Nilsson A, Karlson S, Risberg J, Franzen G, Gustafson L: Cycloid psychosis: regional blood flow correlates of a psychotic episode. Acta Psychiatr Scand 1992; 85:23–29Crossref, Medline, Google Scholar
11. Leonhard K: The Classification of Endogenous Psychoses, 5th ed. New York, Irvington, 1979Google Scholar
12. Leonhard K: Aufteilung der endogenen Psychosen und ihre differenzierte Ätiologie, 7th ed. New York, Thieme, 1995Google Scholar
13. Beckmann H, Fritze J, Franzek E: The influence of neuroleptics on specific syndromes and symptoms in schizophrenics with unfavourable long-term course. Neuropsychobiology 1992; 26:50–58Crossref, Medline, Google Scholar
14. Kendler KS, McGuire M, Gruenberg AM, Walsh D: Clinical heterogeneity in schizophrenia and the pattern of psychopathology in relatives: results from an epidemiologically based family study. Acta Psychiatr Scand 1994; 89:294–300Crossref, Medline, Google Scholar
15. Maier W, Lichtermann D, Minges J, Hallmayer J, Heun R, Benkert O, Levinson DF: Continuity and discontinuity of affective disorder and schizophrenia: results of a controlled family study. Arch Gen Psychiatry 1993; 50:871–883Crossref, Medline, Google Scholar
16. Erdmann J, Nöthen M, Stratmann M, Fimmers R, Franzek E, Propping P: The use of microsatellites in zygosity diagnosis of twins. Acta Genet Med Gemellol 1993; 42:45–51Crossref, Medline, Google Scholar
17. Torgersen S: The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol 1979; 28:225–236Crossref, Medline, Google Scholar
18. McGue M: When assessing twin concordance, use the probandwise not the pairwise rate. Schizophr Bull 1992; 18:171–176Crossref, Medline, Google Scholar
19. Gottesman II, Shields J: Schizophrenia in twins: 16 years' consecutive admissions to a psychiatric clinic. Br J Psychiatry 1966; 112:809–818Crossref, Medline, Google Scholar
20. Gottesman II, Shields J: Schizophrenia and Genetics: A Twin Study Vantage Point. New York, Academic Press, 1972Google Scholar
21. Fischer M, Harvald B, Hauge M: A Danish twin study of schizophrenia. Br J Psychiatry 1969; 115:981–990Crossref, Medline, Google Scholar
22. Andreasen NC, Rice J, Endicott J, Reich T, Coryell W: The family history approach to diagnosis: how useful is it? Arch Gen Psychiatry 1986; 43:421–429Google Scholar
23. Franzek E, Stöber G: Maternal infectious diseases during pregnancy and obstetric complications in the etiology of distinct subtypes of schizophrenia: further evidence from maternal hospital records. Eur Psychiatry 1995; 10:326–330Crossref, Medline, Google Scholar
24. Gayle HD, Yip R, Frank MJ, Nieburg P, Binkin NJ: Validation of maternally reported birth weights among 46,637 Tennessee WIC Program participants. Public Health Rep 1988; 103:143–146Medline, Google Scholar
25. Little RE: Birthweight and gestational age: mother's estimates compared with state and hospital records. Am J Public Health 1986; 76:1350–1351Crossref, Medline, Google Scholar
26. Fingerhut LA, Kleinman JC: Comparability of reporting between the birth certificate and the 1980 National Natality Survey. Vit Health Stat 2 1985; 99:1–34Google Scholar
27. O'Callaghan E, Larkin C, Waddington JL: Obstetric complications in schizophrenia and the validity of maternal recall. Psychol Med 1990; 20:89–94Crossref, Medline, Google Scholar
28. Wenar C, Coulter JB: A reliability study of developmental histories. Child Dev 1962; 33:453–462Medline, Google Scholar
29. Parnas J, Schulsinger F, Teasdale TW, Schulsinger H, Feldman PM, Mednick SA: Perinatal complications and clinical outcome within the schizophrenic spectrum. Br J Psychiatry 1982; 140:416–420Crossref, Medline, Google Scholar
30. Propping P: Psychiatrische Genetik: Befunde und Konzepte. Berlin, Springer, 1989Google Scholar
31. Mehta G, Patel N: StatXact-Turbo. Cambridge, Mass, Cytel Software Corp, 1992Google Scholar
32. Gottesman II, Shields J: Schizophrenia: The Epigenetic Puzzle. Cambridge, England, Cambridge University Press, 1982Google Scholar
33. Inouye E: Similarity and dissimilarity of schizophrenia in twins, in Proceedings of the Third World Congress on Psychiatry, Vol 1. Montreal, University of Toronto Press, 1961, pp 542–530Google Scholar
34. Kallmann FJ: The genetic theory of schizophrenia: an analysis of 691 schizophrenic twin index families. Am J Psychiatry 1946; 103:309–322Link, Google Scholar
35. Kringlen E: Genetic aspects with emphasis on twin studies, in Etiology of Mental Disorders. Edited by Kringlen E, Lavik NJ, Torgersen S. Oslo, University Press of Oslo, 1990, pp 63–79Google Scholar
36. Onstad S, Skre I, Edvardsen J, Torgersen S, Kringlen E: Mental disorders in first-degree relatives of schizophrenics. Acta Psychiatr Scand 1991; 83:463–467Crossref, Medline, Google Scholar
37. Farmer AE, McGuffin P, Gottesman II: Twin concordance for DSM-III schizophrenia. Arch Gen Psychiatry 1987; 44:634–641Crossref, Medline, Google Scholar
38. Allen MG, Pollin W: Schizophrenia in twins and the diffuse ego boundary hypothesis. Am J Psychiatry 1970; 127:437–442Link, Google Scholar
39. Jackson DD: A critique of the literature on the genetics of schizophrenia, in The Study of Schizophrenia. Edited by Jackson DD. New York, Basic Books, 1959, pp 37–90Google Scholar
40. Bertelsen A, Harvald B, Hauge M: A Danish twin study of manic-depressive disorders. Br J Psychiatry 1977; 130:330–351Crossref, Medline, Google Scholar
41. Stöber G, Franzek E, Beckmann H: The role of maternal infectious diseases during pregnancy in the etiology of schizophrenia in the offspring. Eur Psychiatry 1992; 7:147–152Google Scholar
42. Stöber G, Franzek E, Beckmann H: Schwangerschafts—und Geburtskomplikationen—ihr Stellenwert in der Entstehung schizophrener Psychosen. Fortschr Neurol Psychiatr 1993; 61:329–337Crossref, Medline, Google Scholar
43. Stöber G, Franzek E, Beckmann H: Schwangerschaftsinfektionen bei Müttern von chronisch Schizophrenen: die Bedeutung einer differenzierten Nosologie. Nervenarzt 1994; 65:175–182Medline, Google Scholar



