Disturbed Gyrification of the Prefrontal Region in Male Schizophrenic Patients: A Morphometric Postmortem Study
Abstract
OBJECTIVE: The goal was to test the hypothesis that abnormalities of gyrification are present in the prefrontal region of postmortem brains from schizophrenic patients. METHOD: The authors compared the prefrontal regions in brains from 24 schizophrenic patients and 24 normal comparison subjects. The gyrification index, the ratio of inner and outer surface contours, was measured bilaterally in three different slices from each brain. Area measurements of gray and white matter were studied separately by planimetric analysis in the same sections. In addition, a gray-to-white-matter ratio and an asymmetry coefficient were computed. RESULTS: The mean gyrification index on the right side was significantly higher in the male schizophrenic patients than in the comparison men. The gyrification index of the female patients was not significantly different from that of the female comparison subjects. Analysis of area measurements revealed no significant differences. CONCLUSIONS: As gyrification is an ontogenetic stable feature unaffected by atrophic processes during aging, the gyrification abnormalities of the prefrontal region provide further evidence of the importance of a neurodevelopmental mechanism in the etiology of schizophrenia, at least in males.
According to the neurodevelopmental hypothesis of schizophrenia, structural brain abnormalities may be one manifestation of a dysconnectivity syndrome in frontotemporolimbic circuits (1–3). The prefrontal region is proposed to be of considerable importance for the pathogenesis of schizophrenia, on the basis of empirical evidence from both neuroimaging (4–8) and postmortem (9–11) studies. The results of magnetic resonance imaging (MRI) studies of frontal volume in schizophrenia are not entirely consistent: there have been reports of both significantly lower volume (5, 7, 12) and no differences (13–15) relative to comparison subjects. In addition, volumetric measures do not generally provide information regarding mechanism. Assessing gyrification as an alternative strategy may, however, provide evidence in postmortem samples for disturbances of development. The central sulcus is observed at about the 20th week of gestation, and the primary fissures are observed near the 26th week (16). Gyrification occurs after the completion of neuronal migration. One model proposes a mechanism related to differential laminar growth in the cortex (17). The gyrification index was proposed by Zilles et al. (18) as a quantitative assessment of gyrification. This measure is calculated as the ratio of the length of the inner perimeter of the cortex (including the depths of the sulci) to the length of the outer perimeter. Gyrification becomes progressively more complex during gestation. The gyrification index reaches a stable plateau value soon after birth and appears to be stable thereafter, even in the presence of atrophic processes affecting gray and white matter at a later age (19). One MRI study (20) applied the gyrification index technique to investigate the left hemisphere in a small group of patients with schizophrenia. A significantly lower than normal mean gyrification index was observed. The gyrification index technique has been most extensively studied and validated in postmortem studies of normal brains, and to our knowledge there have been no postmortem studies of the gyrification index in schizophrenia. The present study was designed to test the hypothesis that disturbances in gyrification could be detected in the prefrontal region of postmortem brains from individuals with schizophrenia.
METHOD
Brain Collection
The prefrontal region was studied in postmortem tissues from 24 patients with schizophrenia and 24 age- and sex-matched comparison subjects, all collected since 1985. Before autopsy, informed consent was obtained from the nearest relative or from the responsible authority in cases of patients under legal care. These procedures were approved by the local ethics committee of the Heinrich-Heine Universität Düsseldorf, where the brains were obtained. Patients with severe systemic illness (metastatic cancer, arteriosclerosis with myocardial infarction or stroke) were excluded from the study. The diagnosis of schizophrenia was established according to DSM-III-R and ICD-9 criteria, on the basis of chart review. All diagnoses of schizophrenia satisfied both the DSM-III-R and ICD-9 criteria.
The anterior boundary of the prefrontal region was defined as the frontal pole, and the posterior boundary was defined as the genu of the corpus callosum. After formalin fixation, the prefrontal region was removed from the hemispheres by using a cut orthogonal to a reference line joining the temporal and occipital poles. The tissue was then embedded in paraffin. Serial sections 20 µm in thickness were then cut, beginning with the level of the genu of the corpus callosum. Modified Heidenhain-Woelcke staining was performed on every 50th slice. We have reported further details previously (21).
Gyrification Index Measurement
The gyrification index, or ratio of the inner to the outer contour of the cortex (18), was measured by tracing an inner contour defined by the complete pial surface of the brain and by tracing an outer contour according to tangential lines connecting the crests of all gyral curvatures (figure 1). The lengths of these contours were determined as the numbers of pixels in the traced lines by means of an image analysis system (Optimas 6.0, Windows 95). The ratio of the length of the inner contour to the length of the outer contour was calculated in each slice for all subjects. Data for the gyrification index were obtained bilaterally from five different slices of the prefrontal region in 48 brains, without knowledge of diagnosis. From the frontal pole, every 100th slice (interslice distance, 2 mm) was examined. Measurements from the most anterior two slices were subsequently excluded, since tangential cuts of gyri appeared to disproportionately alter the gyrification index. The mean gyrification index of slices 3, 4, and 5, covering the prefrontal region, was calculated. Test-retest and interrater reliabilities were above 95% as assessed by t tests for paired samples.
Area Measurements
Area measurements of gray and white matter were determined, without knowledge of diagnosis, by means of planimetric analysis (21) of the same slices used for measurement of the gyrification index. The ratio of gray to white matter was calculated by dividing the gray matter area by the white matter area. Asymmetry coefficients for the total area measurements were obtained according to the formula 2 × (right–left) / (right + left) of Galaburda et al. (22). A standard postmortem shrinkage factor of 2.25 was applied to all measurements to compensate for the effects of dehydration before paraffin embedding (21). Test-retest and interrater reliabilities for these measurements were above 95% as assessed by t tests for paired samples.
Statistical Analysis
Differences between the demographic characteristics of the schizophrenic and comparison groups were assessed by using two-sided t tests. The dependent variables were the gyrification index (left and right), measurements of gray matter area (left and right) and white matter area (left and right), the ratio of gray to white matter (left and right), and the asymmetry coefficient. First, correlative calculations (Pearson, two-sided) were used to investigate possible effects of age, postmortem time, fixation time, and brain weight on the dependent variables. Second, multivariate analysis of covariance (MANCOVA) was used as the initial statistical procedure with the factors diagnosis and sex. Because the dependent variables could depend on the area measurements, total area (total right area plus total left area) was used as a covariate in all MANCOVA calculations. Potentially confounding variables detected in the correlations were included as additional covariates. Third, significant findings from the multivariate analyses were followed by univariate analyses and subsequent t tests for unpaired samples for the appropriate dependent variables.
RESULTS
Demographic Variables
Descriptive statistics and confidence intervals for variables that might influence the measurements are shown in table 1. At first hospitalization the female schizophrenic patients were significantly older than the male patients (t=–2.80, df=21, p=0.01). The brains of the male patients were significantly heavier than the brains of the female patients (t=4.09, df=18, p=0.001). The mean duration of illness for the male patients was longer than that for the female patients; however, this difference was not statistically significant. No significant differences between the diagnostic groups in mean age at death, postmortem delay, or fixation time were observed.
Correlations between the demographic data and the dependent variables revealed significant associations of the left-sided gray and white matter areas to brain weight (gray matter: r=0.34, df=38, p=0.03; white matter: r=0.32, df=38, p=0.04) and of the right-sided gray and white matter areas to age (gray matter: r=–0.33, df=46, p=0.02; white matter: r=–0.29, df=46, p=0.05). No other correlations between the dependent variables and the potentially confounding variables were found.
Gyrification Data
Descriptive statistics on gyrification, including 95% confidence intervals, are listed in table 2. MANCOVA statistics are shown in table 3. For gyrification, only on the right side was a significant diagnosis-by-sex interaction observed, and there was no significant main effect of diagnosis or sex. Analysis of variance also indicated a significant diagnosis-by-sex interaction only on the right side. Subsequent t tests for unpaired samples were performed for the male and female subgroups separately. The male schizophrenic patients differed significantly from the male comparison subjects (t=–2.98, df=20, p=0.007), whereas the female diagnostic groups did not differ significantly. The mean difference was 7%, indicating a relative hypergyria of the right prefrontal region in the male schizophrenic patients (figure 2).
Area Measurements
Descriptive statistics, including 95% confidence intervals, on gray and white matter areas, the ratio of gray to white matter, and asymmetry coefficients are listed in table 2. MANCOVA statistics are shown in table 3. The significant finding of an influence of sex on left gray and white matter area was not corroborated in the univariate analysis. No significant main effect of diagnosis or sex was observed for any of the other measures.
DISCUSSION
The main finding of the present study of the prefrontal region in schizophrenia is the significantly greater than normal gyrification on the right side of brains from male schizophrenic patients. However, especially in the interpretation of the presented negative results, the small number of subjects has to be taken into consideration.
The gyrification index appears to be a reliable measure of gyrification (18, 19), which is stable throughout adult life. The gyrification index did not show any significant relationship with either age or brain weight in the present study group. This confirms that the gyrification index is an ontogenetic stable feature, at least in two different groups of subjects, i.e., the present study group and the group worked up for the anatomical study (19). The difference in the gyrification indexes for the right prefrontal region in the schizophrenic and comparison subjects thus suggests the presence of a developmental abnormality affecting this region in male schizophrenic patients. In the only other available study of the gyrification index in schizophrenia (20), which used MRI, nine right-handed male schizophrenic patients had a significantly lower mean gyrification index for the entire left hemisphere than did comparison subjects (F=5.56, p<0.03). There was no interaction between diagnostic group and cortical region. However, the results of that study are difficult to compare with the present findings for several reasons. First, only the left hemisphere was assessed in the MRI study, and different findings could have been observed on the right side. Second, another issue concerns the differences in resolution between magnetic resonance images acquired as 1.5-mm slices and then reformatted for analysis to 2.0 mm, in the MRI study, and 20-µm-thick postmortem sections, in our study. Third, the existing validation of the significance of the gyrification index is limited to postmortem studies (18, 23). Other investigations of the prefrontal region in schizophrenia demonstrated wide cerebral fissures in the frontal area (4, 24) and an abnormal frontal gyral pattern in a single schizophrenic patient (25). A three-dimensional MRI study could not demonstrate any relevant abnormalities in the frontal lobe (26).
Although abnormalities of the gyrification index are likely to reflect disturbances in processes of brain development, the mechanism of cortical folding is still unclear. A difference in growth “pressure” between cytoarchitectonic layers in the neocortex has been proposed (17). Reports of disorganization of the architecture and distribution of neurons in prefrontal regions in schizophrenia could be consistent with this possibility (27–29). Another proposed mechanism of gyrification is given by a tension-based theory of morphogenesis (30). According to this model, differences in mechanical tension along axons, dendrites, or glial processes connecting different brain regions result in cortical folding. If this model is correct, patients with schizophrenia and abnormalities of gyrification might be expected to have abnormalities of functional neural connectivity that could be demonstrated with imaging techniques such as positron emission tomography or functional MRI.
No abnormalities in gray or white matter area in the prefrontal region were observed in the postmortem brain slices we examined for gyrification in schizophrenia. Previous MRI studies of total prefrontal volume in schizophrenia have shown lower than normal volume (5, 7, 31–35) or no difference from comparison subjects (13–15, 25). The results of MRI studies used to differentiate between gray and white matter volumes have been similarly inconsistent (7, 15, 31, 35, 36). Using the plane-cut method of sectioning the frontal lobe at the frontal border of the corpus callosum, Breier et al. (35) found a significantly lower than normal volume in schizophrenia, which was pronounced in the white matter. In contrast, Zipursky et al. (31) described widespread low volumes in the gray matter. Similarly, Schlaepfer et al. (7) found significantly lower than normal volumes in the gray matter of all heteromodal association areas. Suddath et al. (15) did not find any volume differences when using the plane-cut method. Low volume in the prefrontal region has been correlated with deficits in neuropsychological performance (6, 8, 26, 27).
The present findings of disturbed gyrification in schizophrenia support the concept of a neurodevelopmental component in the mechanism of illness. These abnormalities of cerebral structure could be markers of developmentally impaired connectivity between cortical regions in schizophrenia. Whether these gyrification disturbances also affect the clinical presentation of schizophrenic patients remains to be established through in vivo experiments.
Presented at the 9th Biennial Winter Workshop for Schizophrenia Research, Davos, Switzerland, Feb. 7–14, 1998. Received July 17, 1998; revision received July 13, 1999; accepted July 21, 1999. From the Department of Psychiatry, Friedrich Wilhelms University of Bonn. Address reprint requests to Dr. Vogeley, Department of Psychiatry, Friedrich Wilhelms University of Bonn, Sigmund-Freud-Strasse 25, 53105 Bonn, Germany; [email protected] (e-mail).
 |
 |
 |
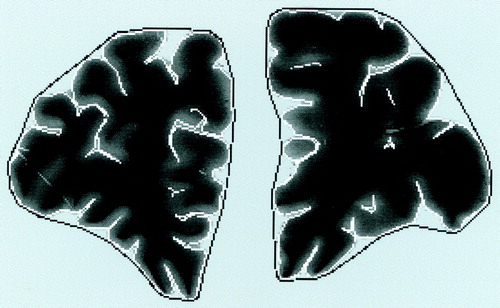
FIGURE 1. Brain Slice of Prefrontal Region in Normal Man Showing Tracings of Cortex Contours Used to Determine Gyrification Indexa
aThe gyrification index is the ratio of the inner contour (white line) to the outer contour (black line). The index was measured with automatized image analysis after manual tracing of the contours and was calculated according to the method of Zilles et al. (18).
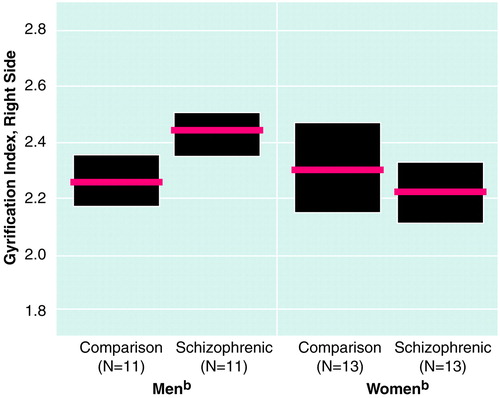
FIGURE 2. Gyrification Indexa in the Prefrontal Region of Postmortem Brains From Male and Female Schizophrenic Patients and Normal Comparison Subjects
aRatio of inner and outer surface contours of the cortex.
bThe red horizontal lines represent mean values, and the shaded boxes are 95% confidence intervals.
1. Ross CA, Pearlson GD: Schizophrenia, the heteromodal association neocortex and development: potential for a neurogenetic approach. Trends Neurosci 1996; 19:171–176Crossref, Medline, Google Scholar
2. Weinberger DR: On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology 1996; 14:1–11Crossref, Medline, Google Scholar
3. Wyatt RJ: Neurodevelopmental abnormalities and schizophrenia. Arch Gen Psychiatry 1996; 53:11–15Crossref, Medline, Google Scholar
4. Shelton RC, Karson CN, Doran AR, Pickar D, Bigelow LB, Weinberger DR: Cerebral structural pathology in schizophrenia: evidence for a selective prefrontal cortical deficit. Am J Psychiatry 1988; 145:154–163Link, Google Scholar
5. Andreasen NC, Nasrallah HA, Dunn V, Olson SC, Grove WM, Ehrhardt JC, Coffman JA, Crossett JH: Structural abnormalities in the frontal system in schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry 1986; 43:136–144Crossref, Medline, Google Scholar
6. Raine A, Lencz T, Reynolds LM, Cooper JE: An evaluation of structural and functional prefrontal deficits in schizophrenia: MRI and neuropsychological measures. Psychiatry Res Neuroimaging 1992; 45:123–137Crossref, Medline, Google Scholar
7. Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD: Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry 1994; 151:842–848Link, Google Scholar
8. Maher BA, Manschreck TC, Woods BT, Yurgelun-Todd DA, Tsuang MT: Frontal brain volume and context effects in short-term recall in schizophrenia. Biol Psychiatry 1995; 37:144–150Crossref, Medline, Google Scholar
9. Benes FM, Bird ED: An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Arch Gen Psychiatry 1987; 44:608–616Crossref, Medline, Google Scholar
10. Benes FM, Davidson J, Bird ED: Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry 1986; 43:31–35Crossref, Medline, Google Scholar
11. Goldman-Rakic PS, Selemon LD: Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997; 23:453–458Crossref, Google Scholar
12. Woods BT, Yurgelun-Todd D, Goldstein JM, Seidman LJ, Tsuang MT: MRI brain abnormalities in chronic schizophrenia: one process or more? Biol Psychiatry 1996; 40:585–596Google Scholar
13. Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW: Prefrontal cortex and schizophrenia: a quantitative magnetic resonance imaging study. Arch Gen Psychiatry 1995; 52:279–288Crossref, Medline, Google Scholar
14. Kelsoe JR, Cadet JL, Pickard D, Weinberger DR: Quantitative neuroanatomy in schizophrenia. Arch Gen Psychiatry 1988; 45:533–541Crossref, Medline, Google Scholar
15. Suddath RL, Casanova MF, Goldberg TE, Daniel DG, Kelsoe JR Jr, Weinberger DR: Temporal lobe pathology in schizophrenia: a quantitative magnetic resonance imaging study. Am J Psychiatry 1989; 146:464–472Link, Google Scholar
16. Sidman RL, Rakic P: Development of the human central nervous system, in Histology and Histopathology of the Nervous System, vol 1. By Haymaker W, Adams RD. Springfield, Ill, Charles C Thomas, 1982, pp 3–145Google Scholar
17. Richman DP, Stewart RM, Hutchinson JW, Caviness VS: Mechanical model of brain convolutional development. Science 1975; 189:18–21Crossref, Medline, Google Scholar
18. Zilles K, Armstrong E, Schleicher A, Kretschmann HJ: The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl) 1988; 179:174–179Crossref, Google Scholar
19. Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K: The ontogeny of human gyrification. Cereb Cortex 1995; 1:56–63Crossref, Google Scholar
20. Kulynych JJ, Luevano LF, Jones DW, Weinberger DR: Cortical abnormalities in schizophrenia: an in vivo application of the gyrification index. Biol Psychiatry 1997; 41:995–999Crossref, Medline, Google Scholar
21. Falkai P, Bogerts B, Schneider T, Greve B, Pfeiffer U, Pilz K, Gonsiorzcyk C, Majtenyi C, Ovary I: Disturbed planum temporale asymmetry in schizophrenia: a quantitative post-mortem study. Schizophr Res 1995; 14:161–176Crossref, Medline, Google Scholar
22. Galaburda AM, Corsiglia J, Rosen GD, Sherman GF: Planum temporale asymmetry, reappraisal since Geschwind and Levitsky. Neuropsychologia 1987; 25:853–868Crossref, Google Scholar
23. Johnstone EC, Bruton CJ, Crow TJ, Frith CD, Owens DG: Clinical correlates of postmortem brain changes in schizophrenia: decreased brain weight and length correlate with indices of early impairment. J Neurol Neurosurg Psychiatry 1994; 57:474–479Crossref, Medline, Google Scholar
24. Weinberger DR, Torrey EF, Neophytides AN, Wyatt RJ: Structural abnormalities of the cerebral cortex in chronic schizophrenia. Arch Gen Psychiatry 1979; 36:935–939Crossref, Medline, Google Scholar
25. Andreasen NC, Ehrhardt JC, Swayze VW II, Alliger RJ, Yuh WT, Cohen G, Ziebell S: Magnetic resonance imaging of the brain in schizophrenia: the pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry 1990; 47:35–44Crossref, Medline, Google Scholar
26. Kikinis R, Shenton ME, Gerig G, Hokama H, Haimson J, O’Donnell BF, Wible CG, McCarley RW, Jolesz FA: Temporal lobe sulco-gyral pattern anomalies in schizophrenia: an in vivo MRI three-dimensional surface rendering study. Neurosci Lett 1994; 182:7–12Crossref, Medline, Google Scholar
27. Falkai P, Bogerts B: The neuropathology of schizophrenia, in Schizophrenia. Edited by Hirsch SR, Weinberger DR. Oxford, UK, Blackwell Scientific, 1995, pp 276–293Google Scholar
28. Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE Jr, Jones EG: Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci 1996; 16:19–30Crossref, Medline, Google Scholar
29. Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE Jr, Jones EG: Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry 1996; 53:425–436Crossref, Medline, Google Scholar
30. Van Essen DC: A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997; 385:313–318Crossref, Medline, Google Scholar
31. Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A: Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry 1992; 49:195–205Crossref, Medline, Google Scholar
32. Breier A, Davis OR, Buchanan RW, Moricle LA, Munson RC: Effects of metabolic perturbation on plasma homovanillic acid in schizophrenia. Arch Gen Psychiatry 1993; 50:541–550Crossref, Medline, Google Scholar
33. Buchanan RW, Breier A, Kirkpatrick B, Elkashef A, Munson RC, Gellad F, Carpenter WT: Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry 1993; 150:59–65Link, Google Scholar
34. Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V II, O’Leary DS, Ehrhardt JC, Yuh WTC: Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA 1994; 272:1763–1769Google Scholar
35. Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F: Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry 1992; 49:921–926Crossref, Medline, Google Scholar
36. Sheline YI, Black KJ, Lin DY, Christensen GE, Gado MH, Brunsden BS, Vannier MW: Stereological MRI volumetry of the frontal lobe. Psychiatry Res Neuroimaging 1996; 67:203–214Crossref, Medline, Google Scholar