Maternal Lifestyle Factors in Pregnancy Risk of Attention Deficit Hyperactivity Disorder and Associated Behaviors: Review of the Current Evidence
Abstract
OBJECTIVE: The purpose of this review was to examine the literature assessing the relationship between prenatal exposure to nicotine, alcohol, caffeine, and psychosocial stress during pregnancy to the risk of developing behavioral problems related to attention deficit hyperactivity disorder (ADHD) in childhood. METHOD: PubMed, MEDLINE, EMBASE, and PsycINFO were searched systematically. Studies using DSM diagnostic criteria and other validated diagnostic or screening instruments for ADHD and those examining ADHD symptoms were included. A narrative approach was used because the studies differed too much in methods and data sources to permit a quantitative meta-analysis. RESULTS: Twenty-four studies on nicotine (tobacco smoking), nine on alcohol, one on caffeine, and five on psychosocial stress were identified. All were published between 1973 and 2002. In spite of inconsistencies, the studies on nicotine indicated a greater risk of ADHD-related disorders among children whose mothers smoked during pregnancy. Contradictory findings were reported in the alcohol studies, and no conclusion could be reached on the basis of the caffeine study. Results from studies on psychological stress during pregnancy were inconsistent but indicated a possible modest contribution to ADHD symptoms in the offspring. Many studies suffered from methodological shortcomings, such as recall bias, crude or inaccurate exposure assessments, low statistical power, and lack of or insufficient control of confounders. A general lack of information on familial psychopathology also limited the interpretations. CONCLUSIONS: Exposure to tobacco smoke in utero is suspected to be associated with ADHD and ADHD symptoms in children. Other maternal lifestyle factors during pregnancy may also be associated with these disorders. Further studies are needed to reach conclusions.
Attention deficit hyperactivity disorder (ADHD) is one of the most common behavioral disorders in child and adolescent psychiatry. Prevalence varies between 3% and 5% (1), and up to 10% has been reported in recent studies (2). Children with ADHD are characterized by early onset of symptoms of hyperactivity, impulsivity, and poor sustained attention. They show considerable variation in severity of their symptoms, degree of impairment, and presence of comorbid disorders (3). Moreover, the clinical presentation of ADHD varies by gender; boys tend to show more disruptive behavior and a higher frequency of comorbid disorders than girls, which may be one reason boys are overrepresented in clinical settings (4).
Brain imaging suggests that children with ADHD have a dopaminergic midbrain dysfunction at the level of the dopaminergic nuclei (5), decreased regional cerebral blood flow in parts of the prefrontal cortex (6), and alterations in prefrontal cortical asymmetry, right frontal-striatal circuitry, and the cerebellum (7). The mechanisms behind observed differences in the brain and the etiology of ADHD remain unknown (1); however, both genetic and environmental factors have been associated with the severity and maintenance of ADHD (8). Adoption, segregation, and genetic studies suggest an interaction between genetic and environmental factors, such as toxins in utero and pregnancy and delivery complications (1, 9).
According to Barkley (10), as early as 1902 Still proposed that the predisposition to behavioral problems was inherited for some children and a result of prenatal and postnatal injury for others. Pasamanick and co-workers (11) hypothesized that pre- and perinatal injury to the brain could be sufficient to cause childhood behavior disorders. More recently, the programming hypothesis suggests that fetal adaptation to an unfavorable intrauterine environment permanently increases susceptibility to chronic diseases or disorders later in life (12). Apart from the effect of high levels of exposure to alcohol during pregnancy (13), the effects of other agents on brain function remain unknown.
Neurobehavioral changes similar to ADHD symptoms in human children have been found in animals exposed in utero to nicotine, caffeine, ethanol, and stress (14–17). Nicotine, caffeine, and ethanol and its metabolites, as well as stress hormones, cross the placental barrier and reach the human fetal brain. From a public health perspective, it is important to learn to what extent and which of these common lifestyle factors contribute to the development of ADHD and ADHD-related disorders. Preventive actions may limit the poor psychiatric and criminality outcome of ADHD children in adulthood (18, 19).
The purpose of this review is to examine the state of the evidence linking common lifestyle factors during pregnancy such as smoking tobacco, alcohol and caffeine use, and maternal psychological stress to the development of ADHD and ADHD-like symptoms in children.
Method
Literature Search
We identified studies for review from the U.S. National Library of Medicine (PubMed) from 1966 until April 2002, EMBASE from 1990 until January 2002, and PsycINFO from 1966 to February 2002. The following keywords were used to search these databases: pregnancy, prenatal, intrauterine, ADHD, attention, hyperactivity, impulsivity, externalizing behavior, smoking, cigarettes, nicotine, alcohol, caffeine, coffee, stress, psychosocial stress, and psychological distress. The bibliographies of articles were examined to identify further citations.
All human studies concerning prenatal exposure to nicotine, alcohol, caffeine, or psychological stress and an outcome measure that fulfilled one of the following three criteria were included in the review: 1) diagnosis of ADHD in accordance with DSM diagnostic criteria made with either a structured diagnostic interview or a clinical interview, 2) symptoms of inattention, hyperactivity, or impulsivity measured with a validated screening instrument, or 3) neuropsychological tests reflecting inattention, impulsivity, or externalizing behavior. The last two criteria designate subgroups of ADHD. Children’s age at the time of the diagnosis was limited to 4 years and older because diagnosis of ADHD is difficult to determine for younger children (20). Case series were excluded.
Diagnostic Criteria and Assessments
The diagnostic criteria for ADHD have changed and been further developed since the late 1960s. In 1968, DSM-II defined three areas of core symptoms: inattention, hyperactivity, and impulsivity. These were later expanded into an operational list of 16 specific symptoms in 1980 with DSM-III, revised in 1987 (DSM-III-R), and, finally, divided into three subtypes in DSM-IV in 1994. These subtypes are predominantly inattentive, predominantly hyperactive and impulsive, and combined type. These criteria are used today in clinical and research settings.
This review includes studies using a validated diagnostic instrument or a clinical interview assessing DSM-III or DSM-III-R criteria. None of the available studies used DSM-IV criteria. The Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) (21) is a commonly used validated semistructured diagnostic interview for clinicians and is currently considered one of the best diagnostic assessments. Alternatively, specific rating scales can assess DSM criteria for ADHD; Conners’ Parent Rating Scale (22) is one of the best validated.
Several validated questionnaires—the Child Behavior Checklist (23), the Strengths and Difficulties Questionnaire (24), and Rutter’s questionnaires for parents and teachers (25)—are included in this review, as well as their subscales or modified versions for inattention, hyperactivity, and impulsivity. The responses can be calculated into a total problem score or into factor-analytically derived subscales such as externalizing behavior (aggressive, oppositional, and hyperactive symptoms) and inattentive and hyperactive symptoms. Although scores based on inattentive and hyperactive subscales focus mostly on ADHD-like symptoms, these psychometric instruments are designed as screening tools for general psychopathology rather than as diagnostic instruments.
The Child Behavior Checklist (23) is a questionnaire designed to measure multidimensional child behavior rather than ADHD per se. Subscales of hyperactivity and inattention of the Child Behavior Checklist are rather crude measures that have a relatively low correlation with a diagnostic interview like K-SADS. A higher correlation is achieved with the Strengths and Difficulties Questionnaire (26).
Neuropsychological tests are used to provide a cognitive profile and to evaluate executive functions. The Continuous Performance Test (27) is used to test the core symptoms of ADHD (i.e., impulsivity and inattention). The Continuous Performance Test has high sensitivity (83%–90%), but specificity is poorer (59%–61%) when measured against clinical diagnosis (27). As with other rating scales, the test provides one source of information that needs to be integrated with other sources in reaching a final diagnostic decision (27).
The available studies differed vastly in methods, measure of exposure, and outcomes, as well as in the subjects studied, which ranged from unselected samples to highly selected hospital samples. The use of different comparison groups also caused lack of comparability among studies. A variety of diagnostic instruments were used, including DSM-III; DSM-III-R; clinical interviews like K-SADS; questionnaires with different calculations of scores on inattentive, hyperactive, and externalizing behavior symptoms; vigilance scores on the Continuous Performance Test; and nonvalidated outcome measures reflecting child behavior. Assigning a weight to each study, therefore, has no scientific value. Because the requirement for a meta-analysis leading to a pooled risk estimate (i.e., that studies have comparable methods and similar measurements of exposures and endpoints) was not met, we conducted an annotated review.
Results
Evidence of Maternal Smoking During Pregnancy and ADHD
Results from animal studies indicate that hyperactivity in the offspring may result from prenatal nicotine exposure (14) and that the effects are long-lasting (28). Possible mechanisms may be modulation of the dopaminergic system and a greater number of nicotine receptors (29, 30). Human data (31–33) show an association between maternal smoking during pregnancy and low birth weight, preterm delivery, and stillbirth. However, one review (34) reported inconsistent associations between prenatal smoking and long-term intellectual and developmental abilities.
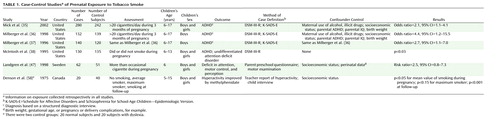
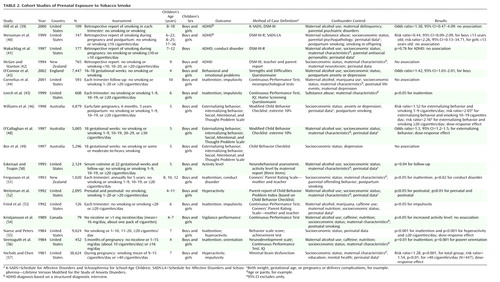
We identified 24 studies that evaluated the association between smoking during pregnancy and ADHD or ADHD symptoms. Eight studies (35–42) used the diagnostic criteria of ADHD as the outcome, and 16 (43–58) studied ADHD subgroups. Deficit in attention, motor control, and perception (47) and minimal brain dysfunction (57) were examined in two of the studies and were included in the review because these diagnoses encompass the core symptoms of ADHD. Table 1 lists the main characteristics of the case-control studies, and Table 2 lists the main characteristics of the cohort studies.
The case-control studies presented in Table 1 provide support for the hypothesis that prenatal exposure to nicotine may result in ADHD symptoms. Milberger et al. (36) attempted to differentiate between genetic vulnerability and prenatal smoking by controlling for parental ADHD and by including siblings of children with ADHD in the model. The results showed that prenatal maternal smoking was associated with a fourfold higher risk of ADHD in the offspring independently of maternal disorder, similar to the risk accounted for by maternal ADHD. Results of the study of Mick et al. (35), based on the 1996 data of Milberger and co-workers (36, 37) and supplemented with data for girls, showed a lower odds ratio of 2.1.
Table 2 presents 18 follow-up studies of maternal smoking during pregnancy (39–46, 48–57). Four of these studies (39–42) classified ADHD according to DSM criteria and collected information on maternal smoking habits during pregnancy retrospectively. Two of the studies (40, 42) assessed smoking as a dichotomized measure. The children were selected from families with alcoholism (39), major depression (40), and other clinical referral symptoms (41). In contrast, McGee and Stanton (42) examined a sample of 765 children whose mothers were randomly selected and recruited at the time of the child’s birth. No association between prenatal smoking and ADHD was found in any of these four studies (39–42).
Eleven of the follow-up studies assessing only prenatal maternal smoking or both prenatal and postnatal smoking indicated an association between smoking and ADHD or ADHD-related symptoms.
Six cohort studies (40, 44, 46, 50–52) and one case-control study (58) aimed at differentiating the effect of in utero and postnatal exposure to smoke in relation to ADHD (40) and ADHD subgroups (44, 46, 50–52, 58). Several studies measured prenatal maternal smoking on an ordinal scale, but one dichotomized groups into no smoking and smoking 10 or more cigarettes per day or less frequently during pregnancy (40). Only one study used maternal serum cotinine to verify smoking status (50).
The results from the studies examining differences between prenatal and postnatal smoking varied. In a study by Weissman et al. (40), postpartum smoking was not associated with ADHD in the offspring. Information on exposure for this study was collected retrospectively (three times over a 10-year period) for a small group ranging widely in age from 6 to 23 years. Similarly, prenatal and postnatal maternal smoking were not found to have any impact on attention deficits or impulsivity in a larger sample of 10-year-olds (44).
Two other studies on relatively large samples of about 2,000 children (50, 52) revealed statistically significant associations between postnatal but not prenatal smoking and hyperactivity (52) and activity level (50). There was a very low frequency of exclusively prenatal or postnatal smokers in the study by Weitzman and co-workers (52), which makes it difficult to disentangle the independent contribution made by each variable. Eskenazi and Trupin (50) primarily designed their study to test cognitive deficits and included only three items on activity level (the child has more energy than most, hates to sit still, and likes to play quietly) rated by the mother. It is unlikely that the selected items were sensitive enough to capture the full concept of hyperactivity.
Statistically significant associations between prenatal smoking and the outcome were reported in two studies concerning externalizing behavior (46) and inattentive symptoms (51). The studies collected data prospectively, had large samples, and controlled for maternal postpartum smoking and socioeconomic status (46, 51). The study by Fergusson and co-workers (51) used teachers as informants in addition to maternal reports.
Eight of the follow-up investigations are particularly important because of large samples, prospective exposure assessments (43, 46, 48, 49, 55–57), and appropriate designs with respect to control for potential confounders such as alcohol (43, 48, 53, 56) and socioeconomic status (43, 46, 48, 49, 53, 55–57). Moreover, a variety of techniques were employed in these studies to assess symptoms, including observational measures of child behavior. These studies reported small but independent effects of smoking on a variety of symptoms related to ADHD in younger children (4–7-year-olds). Four (46, 48, 55, 57) of the eight studies found a dose-response-like association.
Alcohol Consumption During Pregnancy and ADHD in Childhood
Disturbed attention and neuromotor development has been found in monkeys prenatally exposed to moderate levels of ethanol (15). Ethanol enhances migration of nerve cells, which is hypothesized to be involved in behavioral difficulties in childhood. It also interferes with the production of neuroendocrine hormones, which may perturb brain growth (59).
In humans, high levels of alcohol consumption during pregnancy are associated with a greater risk of congenital malformations (60) and possibly stillbirth (61). Alcohol is widely recognized as a teratogenic agent causing CNS dysfunction and impaired mental functioning, including fetal alcohol effect (62) and fetal alcohol syndrome, which incorporates the core symptoms of ADHD (63).
Table 3 presents nine studies investigating the association between prenatal alcohol exposure and ADHD (35, 39) or ADHD subgroups (43, 45, 53, 64–67). Alcohol consumption was significantly associated with ADHD (35) and subgroups of ADHD (64–66) in four of these nine studies, two of which involved high levels of alcohol exposure during pregnancy (35, 65).
A recent case-control study of prenatal exposure to alcohol by Mick and co-workers (35), which also studied smoking, found that twice as many children with ADHD had mothers who either drank alcohol daily or binged heavily during pregnancy (N=10) than children without ADHD (N=5). However, these results were not supported by Hill and colleagues (39), who used dichotomized exposure data collected retrospectively. In this cohort study, the univariate association between prenatal alcohol exposure and ADHD disappeared after the authors adjusted for familial risk of alcoholism, intrauterine exposure to smoking, maternal current alcohol intake, or information on alcohol and parental psychopathology. Studies using the Continuous Performance Test (45, 53, 67) and Conners’ Parent Rating Scale (53) reported no effect of prenatal exposure to alcohol on inattention or impulsivity.
The Pregnancy and Health Study of Streissguth et al. (13) is a birth cohort of 582 children exposed to alcohol in utero. Mothers were categorized as abstainers (light/infrequent drinkers or abstainers) or heavy drinkers (one drink or more per day) during pregnancy. The 4–7-year-old children of heavy drinkers were more likely to exhibit attention deficits and impulsivity on the Continuous Performance Test than were children of abstainers. The association remained statistically significant after adjustment for prenatal maternal smoking, prenatal caffeine intake, and socioeconomic status. In the study by Brown et al. (66), prenatal alcohol exposure was found to be related to externalizing behavior problems, and in the study of Delaney-Black et al. (64) it was related to attention deficits. The largest cohort study, that of O’Connor et al. (43), which involved nearly 7,500 children, did not find an association between alcohol exposure and general behavioral problems.
Caffeine Intake and Psychological Distress
Caffeine may cause a persistent effect on the neurochemical system (16). In some epidemiological studies, fetal exposure to caffeine has been associated with first-trimester spontaneous abortions (68) and low birth weight (69).
We identified no study investigating the association between maternal caffeine exposure during pregnancy and ADHD in childhood. A follow-up study by Barr and Streissguth (70) evaluated the effect of caffeine on Continuous Performance Test performance in 7-year-old children. Ingestion of caffeine (coffee, tea, chocolate) was assessed in early pregnancy and during the fifth month of gestation. Caffeine intake was categorized into three levels equivalent to 0–3, 4–5, and 6 or more cups of coffee per day. Few women were unexposed (0.5%). After adjustment for a large number of potential confounders, including maternal smoking, alcohol intake, and other maternal characteristics, no association was found between exposure to caffeine in utero and the children’s performance on the Continuous Performance Test.
Prenatal stress has been linked to the serotonergic system in late gestation by means of interference with neuron development (71). Cortisol has been linked to interference with neuron development in the serotonergic system during late gestation, and it may also influence the fetal hypothalamic-pituitary-adrenal axis (71).
The maternal psychological state may influence the intrauterine environment by altering the uterine blood flow (72), thereby possibly impeding nutrient supply to the fetus. Maternal stress during pregnancy has been associated with greater prevalence of some congenital malformations (73) and with changes in fetal levels of cortisol, the main hormone associated with stress (74), infant attention regulation (75), schizophrenia (76), social behavior (77), and depression (78).
We identified one study on maternal stress during pregnancy and ADHD (38) and four on ADHD subgroups (43, 79–81), as shown in Table 4. Results from the case-control study (38) showed that mothers of children with ADHD and undifferentiated attention deficit disorder reported psychological stress more frequently during pregnancy. No adjustment for confounding was performed. Thirty years ago, a host of possibly stressful circumstances during pregnancy (measured 1 month postpartum) were found to predict behavioral disturbances in childhood (81). A more recent study (79) used the attention scale on the Child Behavior Checklist and found that children of mothers exposed to stressful family circumstances during pregnancy had a higher likelihood of attention problems. The definition of stressful family circumstances was a combination of genetic and psychosocial factors. Meijer (80) conducted a natural experiment by comparing children of mothers exposed to wartime stress with children born 2 years later. The mothers’ and teachers’ ratings of child behavior problems were higher in the group exposed to war during the early postpartum months rather than during pregnancy. None of these studies assessed the amount of stress experienced during pregnancy with a validated instrument, nor did they control for confounding.
Recently, a large community-based study assessing psychosocial stress during pregnancy was conducted in England (43). After controlling for maternal smoking, alcohol intake (dichotomized variables), and postpartum depression, the authors found that maternal anxiety at week 32, but not at week 18, was associated with hyperactivity and inattention in boys. The results were statistically significant, not only when the upper extreme of the behavioral problem distribution was examined but also when behavioral problems were examined as a continuous variable. Women with high scores on anxiety at gestational week 18 were more likely to drop out of the study. There was no control for parental psychopathology. Furthermore, a high correlation between anxiety scores measured during and after pregnancy makes it difficult to exclude the possibility that the association was due to genetics rather than anxiety in the mother.
Discussion
The reviewed studies indicate that maternal lifestyle during pregnancy may contribute to behavior problems in the offspring. However, because of methodological limitations, these studies are only suggestive; no causal conclusions can be drawn. The majority of studies indicated an association between exposure to tobacco smoke in utero and inattention and hyperactivity, but not all studies found a statistically significant association.
The results for alcohol exposure were inconsistent. Only half of the papers included in the review showed a statistically significant association with inattention and impulsivity. Results from four studies (35, 64–66) support the hypothesis that alcohol contributes to CNS impairment that need not be as severe as fetal alcohol syndrome/fetal alcohol effect. Two of these studies were based on exposure in utero to very high levels of alcohol (35, 65). Studies on high levels of maternal alcohol intake, however, are vulnerable to confounding by social, dietary, and genetic factors. None of the studies adjusted for a family risk of behavioral problems.
Only one study explored the possibility that caffeine exposure in utero could affect child behavior, and the small number of unexposed children hampers the interpretation of the results.
Studies regarding stress showed a small but statistically significant association with disturbances in attention and activity. The study on stress done by O’Connor et. al. (43) in ADHD subgroups is the best of these because of its large sample size of children of the same age, prospective exposure assessment twice during pregnancy, use of a validated scale for psychological stress, and adjustment for potential confounders. The other studies (38, 79–81) did not adjust for potential confounders such as maternal smoking. Smoking may be a potential alternative explanation for the results in these studies in view of the fact that smoking is more common among stressed pregnant women (82).
Methodological Issues
There are several methodological issues common to studies on the lifestyle factors reviewed here. These issues are discussed below with illustrations from the studies under review.
Study design and assessment of exposure
Prospective collection of data minimizes the risk of recall bias and differential misclassification; in contrast, these problems are maximized in studies with retrospectively collected data. Nonetheless, all studies concerning smoking and ADHD (35–42) or alcohol (35, 39) collected exposure information retrospectively.
Selective attrition is another methodological problem that limits the interpretation of results. For example, only 44% of the eligible families participated in one study (39), and women experiencing greater amounts of psychological stress were more likely to drop out in another study (41).
Results must always be judged on the accuracy with which the exposure variables are measured. Dichotomizing smoking as either smoking more than 20 cigarettes or 20 cigarettes or fewer per day, as in some studies (35–37), or dichotomization of alcohol consumption into abstainers versus any alcohol intake during pregnancy (65) may be too crude. Dichotomization (39, 43) may hide a true association, as well as hinder the detection of a possible dose-response effect or a threshold value.
Statistical Issues
The statistical power was limited in many of the studies, particularly the studies on smoking. To detect a 50% increase in ADHD with 80% power, a sample size of 6,000 with 3,000 exposed and 3,000 unexposed children is needed. None of the published studies had that size. Three studies on ADHD subgroups with large sample sizes found a dose-response-like relation with smoking (46, 48, 55). A large cohort sample, as in some studies (43, 55, 57), is especially needed when outcome is rare or the effect is small (83), as we would expect for the reviewed lifestyle factors.
The maternal lifestyle variables studied in this review have all previously been shown to be correlated with each other (84). When studying high-risk populations, multicollinearity may render invalid results (85).
Control of Confounders
Some studies carried out little control (45, 47, 49, 55, 58, 64, 79) or no control (38, 80, 81) for confounding variables. Apart from parental socioeconomic status, the main potential confounders considered in the studies of the single lifestyle factors are maternal alcohol intake and smoking habits. Stratification, rarely used or reported in the reviewed papers, is a useful and simple tool to assess the effect of interactions on outcome. This procedure requires understanding of the possible mechanisms behind and the quality of underlying variables. Highly selected at-risk populations in some studies (36, 37, 39–41, 65) may complicate the issue of confounding further.
Family studies show that 70%–90% of the variance in the ADHD phenotype may be attributed to hereditary factors (9, 86), which, therefore, may be strong independent risk factors for ADHD in offspring. However, few studies (35–37, 39, 41, 43, 45, 46, 49, 51) were able to take familial psychopathology into consideration. Furthermore, little is known about the etiology of ADHD, making it difficult to fully adjust for confounding.
Long-term follow-up studies of children with ADHD have shown a greater risk of adulthood antisocial personality and anxiety disorders, which may be important to assess in addition to ADHD (86). Thus, maternal depression and paternal antisocial behavior (86) could be evaluated in future studies, depending on design issues and sampling.
Smoking is prominent among ADHD patients (87). Cigarette smoke, alcohol, and caffeine are known stimulants of the CNS, and the use of these stimulants reflects certain personality traits (88). Nicotine is a psychomotor stimulant, which reduces the core symptoms of ADHD as well as anxiety and tension (89). Cigarette smoking and drinking alcohol or large amounts of coffee could be pharmacological ways of coping with the psychopathology linked to ADHD. It is possible that maternal smoking during pregnancy may be a proxy variable for the genetic risk of the child to develop ADHD. Adjustment for maternal or parental psychopathology may not solve the problem, but exploring the interaction between ADHD and maternal or parental psychopathology may better enlighten the possible causal paths.
The age of the children at the time of assessment may be important because symptoms become less conspicuous over time and only 30%–40% of children with ADHD retain their diagnosis in adulthood (90). Studies have shown that children with ADHD have a tendency to remit from hyperactive symptoms but to have persistent inattention (91, 92). Therefore, Barkley and Biederman (93) suggested that the age criterion of 7 years for the diagnosis of ADHD be broadened or abandoned. They recommended that the appearance of ADHD symptoms anytime during childhood is a more appropriate criterion for ADHD. In the evaluation of adults with ADHD, the official DSM-IV items are still recommended (91). However, in research, advantages of the age criterion of symptoms ensure a relatively homogeneous sample, reliability of selection criteria across studies, and a high probability of subjects having a valid disorder (93).
Many studies did not distinguish between prenatal and postnatal exposure to tobacco smoke. We believe this to be a minor problem because environmental exposure to tobacco smoke is of much lower magnitude and hardly capable of influencing brain development (94). Prenatal and postnatal smoking are highly correlated and difficult to separate. Because postnatal smoking is most likely on the causal pathway (95), adjustment for postnatal exposure may lead to overadjustment and obscure a potential true association with prenatal exposure. We believe that intrauterine exposure of the child to cigarette smoke may be of importance in ADHD and ADHD-related disorders. This is supported by a recent experimental report (96), which found that exposure to nicotine during pregnancy was associated with behavioral problems in the offspring.
Maternal smoking during pregnancy has been associated with adult criminal outcome, conduct disorders, and substance abuse (40, 97, 98) and with problems in cognitive development (99). Offspring exposed to alcohol in utero exhibit symptoms characterized by deficiencies in regulation of behavior, cognitive flexibility, response inhibition, and planning, as well as lower IQ (100)—symptoms that encompass many different clinical diagnoses. ADHD includes a number of these behavioral aspects, which may be present together because of common etiology or because ADHD is a specific pathogenetic entity. In any case, we would expect that ADHD would be associated with some of the prenatal lifestyle factors because of the familial predisposition of the disorder.
Exposure to several other factors during pregnancy may have effects on long-term development of the child; these include pre-, peri-, and postnatal complications, diseases, trauma, medication, and neurologically compromising events that may occur during development of the nervous system before and after birth (8).
The use of recreational drugs during pregnancy may have serious long-term consequences (101), but the effect of this exposure is difficult to interpret because it is often associated with other putative risk factors like smoking and alcohol abuse (102). Six (35–37, 44, 45, 53) of the 24 studies on smoking and five (35, 45, 53, 64, 66) of the nine studies on alcohol adjusted for illicit drug use during pregnancy but with no effect on the results. A recent review of cocaine abuse during pregnancy concluded that negative effects may be secondary to the effect of smoking or alcohol intake (103).
Conclusions
Studies on maternal lifestyle factors and ADHD or ADHD-like symptoms in the offspring are few and of poor quality. The research is characterized by retrospective assessment of prenatal exposure and lack of diagnostic assessment of ADHD. Few studies adjusted for familial psychopathology. Because of these limitations, no sound conclusion can be drawn regarding the association between maternal lifestyle factors and ADHD and ADHD symptoms in the offspring. However, there may be an association with exposure to tobacco smoke in utero.
This review illustrates the need for more rigorous studies. New studies should avoid recall bias and selection bias, and their designs should result in sufficient statistical power. Follow-up studies based on large community-based samples with prospectively collected exposure measures during pregnancy are preferable. The optimal measures of exposure should include a quantitative measure during each trimester, preferably a biochemical measure (if needed or available in each trimester), to improve the validity of the self-reported measures. Since we do not know if ADHD represents an etiologic entity, studies should focus either on specific behavioral aspects or on a well-defined ADHD phenotype for a broader age category of children.
Information on parental psychopathology is essential to fully exploring the association between ADHD and exposure to maternal lifestyle factors.
 |
 |
 |
 |
Received June 24, 2002; revision received Dec. 16, 2002; accepted Jan. 21, 2003. From the Perinatal Epidemiological Research Unit, Department of Obstetrics and Gynecology, the Psychiatric Hospital for Children and Adolescents, the Department of Child Psychiatry, and the Department of Paediatrics, Aarhus University Hospital, Skejby, Denmark; the Danish Epidemiology Science Center, University of Aarhus, Denmark; the Department of Psychology, University of Uppsala, Sweden; the Department of Public Health Science and General Practice and the Department of Child Psychiatry, University of Oulu, Finland; and the Department of Epidemiology and Public Health, Imperial College Faculty of Medicine, London. Address reprint requests to Dr. Linnet, Perinatal Epidemiological Research Unit, Department of Obstetrics and Gynecology, Aarhus University Hospital, Skejby, 8200 Aarhus N, Denmark; [email protected] (e-mail). Supported by the Nordic Council of Ministers research program Longitudinal Epidemiology (number 020056).
1. Faraone SV, Doyle AE: The nature and heritability of attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin North Am 2001; 10:299-316Crossref, Medline, Google Scholar
2. Rowland AS, Umbach DM, Stallone L, Naftel AJ, Bohlig EM, Sandler DP: Prevalence of medication treatment for attention deficit-hyperactivity disorder among elementary school children in Johnston County, North Carolina. Am J Public Health 2002; 92:231-234Crossref, Medline, Google Scholar
3. Biederman J, Newcorn J, Sprich S: Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry 1991; 148:564-577Link, Google Scholar
4. Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, Wilens TE, Frazier E, Johnson MA: Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry 2002; 159:36-42Link, Google Scholar
5. Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, Cohen RM: High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry 1999; 156:1209-1215Abstract, Google Scholar
6. Spalletta G, Pasini A, Pau F, Guido G, Menghini L, Caltagirone C: Prefrontal blood flow dysregulation in drug naive ADHD children without structural abnormalities. J Neural Transm 2001; 108:1203-1216Crossref, Medline, Google Scholar
7. Giedd JN, Blumenthal J, Molloy E, Castellanos FX: Brain imaging of attention deficit/hyperactivity disorder. Ann NY Acad Sci 2001; 931:33-49Crossref, Medline, Google Scholar
8. Barkley RA: Etiologies, in Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, Guilford, 1998, pp 164-185Google Scholar
9. Faraone SV, Biederman J: Neurobiology of attention-deficit hyperactivity disorder. Biol Psychiatry 1998; 44:951-958Crossref, Medline, Google Scholar
10. Barkley RA: History, in Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, Guilford, 1998, pp 3-55Google Scholar
11. Pasamanick B, Rodgers ME, Lilienfeld AM: Pregnancy experience and the development of behavior disorders in children. Am J Psychiatry 1956; 112:613-618Link, Google Scholar
12. Barker DJ: In utero programming of chronic disease. Clin Sci (Lond) 1998; 95:115-128Crossref, Medline, Google Scholar
13. Streissguth AP, Sampson PD, Barr HM: Neurobehavioral dose-response effects of prenatal alcohol exposure in humans from infancy to adulthood. Ann NY Acad Sci 1989; 562:145-158Crossref, Medline, Google Scholar
14. Eriksson P, Ankarberg E, Fredriksson A: Exposure to nicotine during a defined period in neonatal life induces permanent changes in brain nicotinic receptors and in behaviour of adult mice. Brain Res 2000; 853:41-48Crossref, Medline, Google Scholar
15. Clarke AS, Schneider ML: Effects of prenatal stress on behavior in adolescent rhesus monkeys. Ann NY Acad Sci 1997; 807:490-491Crossref, Medline, Google Scholar
16. Sobotka TJ: Neurobehavioral effects of prenatal caffeine. Ann NY Acad Sci 1989; 562:327-339Crossref, Medline, Google Scholar
17. DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TR: Fetal neurobehavioral development. Child Dev 1996; 67:2553-2567Crossref, Medline, Google Scholar
18. Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH: Conduct problems, gender and adult psychiatric outcome of children with attention-deficit hyperactivity disorder. Br J Psychiatry 2002; 181:416-421Crossref, Medline, Google Scholar
19. Satterfield JH, Schell A: A prospective study of hyperactive boys with conduct problems and normal boys: adolescent and adult criminality. J Am Acad Child Adolesc Psychiatry 1997; 36:1726-1735Crossref, Medline, Google Scholar
20. Barkley RA: Developmental course, adult outcome, and clinic-referred ADHD adults, in Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, Guilford, 1998, pp 186-224Google Scholar
21. Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrazi MA, Davies M: The assessment of affective disorders in children and adolescents by semistructured interview: test-retest reliability of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present Episode Version. Arch Gen Psychiatry 1985; 42:696-702Crossref, Medline, Google Scholar
22. Conners CK: Manual for Conners’ Rating Scales. Toronto, Multi-Health Systems, 1989Google Scholar
23. Achenbach TM, Edelbrock C: Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington, University of Vermont, Department of Psychiatry, 1983Google Scholar
24. Goodman R: The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry 1997; 38:581-586Crossref, Medline, Google Scholar
25. Rutter M, Tizard J, Whitmore K: Education, Health and Behaviour. London, Longmans, 1970Google Scholar
26. Goodman R, Scott S: Comparing the Strengths and Difficulties Questionnaire and the Child Behavior Checklist: is small beautiful? J Abnorm Child Psychol 1999; 27:17-24Crossref, Medline, Google Scholar
27. Barkley RA: Tests and observational measures, in Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, Guilford, 1998, pp 294-311Google Scholar
28. Ajarem JS, Ahmad M: Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav 1998; 59:313-318Crossref, Medline, Google Scholar
29. Marks MJ, Grady SR, Collins AC: Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther 1993; 266:1268-1276Medline, Google Scholar
30. Slotkin TA, Lappi SE, Seidler FJ: Impact of fetal nicotine exposure on development of rat brain regions: critical sensitive periods or effects of withdrawal? Brain Res Bull 1993; 31:319-328Crossref, Medline, Google Scholar
31. Shah NR, Bracken MB: A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol 2000; 182:465-472Crossref, Medline, Google Scholar
32. Wisborg K, Henriksen TB, Hedegaard M, Secher NJ: Smoking during pregnancy and preterm birth. Br J Obstet Gynaecol 1996; 103:800-805Crossref, Medline, Google Scholar
33. Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ: Exposure to tobacco smoke in utero and the risk of stillbirth and death in the first year of life. Am J Epidemiol 2001; 154:322-327Crossref, Medline, Google Scholar
34. Olds D: Tobacco exposure and impaired development: a review of the evidence. Ment Retard Dev Disabil Res Rev 1997; 3:257-269Crossref, Google Scholar
35. Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S: Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry 2002; 41:378-385Crossref, Medline, Google Scholar
36. Milberger S, Biederman J, Faraone SV, Jones J: Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol 1998; 27:352-358Crossref, Medline, Google Scholar
37. Milberger S, Biederman J, Faraone SV, Chen L, Jones J: Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry 1996; 153:1138-1142Link, Google Scholar
38. McIntosh DE, Mulkins RS, Dean RS: Utilization of maternal perinatal risk indicators in the differential diagnosis of ADHD and UADD children. Int J Neurosci 1995; 81:35-46Crossref, Medline, Google Scholar
39. Hill SY, Lowers L, Locke-Wellman J, Shen SA: Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. J Stud Alcohol 2000; 61:661-668Crossref, Medline, Google Scholar
40. Weissman MM, Warner V, Wickramaratne PJ, Kandel DB: Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry 1999; 38:892-899Crossref, Medline, Google Scholar
41. Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL: Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry 1997; 54:670-676Crossref, Medline, Google Scholar
42. McGee R, Stanton WR: Smoking in pregnancy and child development to age 9 years. J Paediatr Child Health 1994; 30:263-268Crossref, Medline, Google Scholar
43. O’Connor TG, Heron J, Golding J, Beveridge M, Glover V: Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years: report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry 2002; 180:502-508Crossref, Medline, Google Scholar
44. Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA: Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J Dev Behav Pediatr 2001; 22:217-225Crossref, Medline, Google Scholar
45. Leech SL, Richardson GA, Goldschmidt L, Day NL: Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol 1999; 21:109-118Crossref, Medline, Google Scholar
46. Williams GM, O’Callaghan M, Najman JM, Bor W, Andersen MJ, Richards D, Chunley U: Maternal cigarette smoking and child psychiatric morbidity: a longitudinal study. Pediatrics 1998; 102:e11Google Scholar
47. Landgren M, Kjellman B, Gillberg C: Attention deficit disorder with developmental coordination disorders. Arch Dis Child 1998; 79:207-212Crossref, Medline, Google Scholar
48. O’Callaghan MJ, Williams GM, Andersen MJ, Bor W, Najman JM: Obstetric and perinatal factors as predictors of child behaviour at 5 years. J Paediatr Child Health 1997; 33:497-503Crossref, Medline, Google Scholar
49. Bor W, Najman JM, Andersen MJ, O’Callaghan M, Williams GM, Behrens BC: The relationship between low family income and psychological disturbance in young children: an Australian longitudinal study. Aust NZ J Psychiatry 1997; 31:664-675Crossref, Medline, Google Scholar
50. Eskenazi B, Trupin LS: Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure, II: effects on neurodevelopment at age 5 years. Am J Epidemiol 1995; 142(9 suppl):S19-S29Google Scholar
51. Fergusson DM, Horwood LJ, Lynskey MT: Maternal smoking before and after pregnancy: effects on behavioral outcomes in middle childhood. Pediatrics 1993; 92:815-822Medline, Google Scholar
52. Weitzman M, Gortmaker S, Sobol A: Maternal smoking and behavior problems of children. Pediatrics 1992; 90:342-349Medline, Google Scholar
53. Fried PA, Watkinson B, Gray R: A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol 1992; 14:299-311Crossref, Medline, Google Scholar
54. Kristjansson EA, Fried PA, Watkinson B: Maternal smoking during pregnancy affects children’s vigilance performance. Drug Alcohol Depend 1989; 24:11-19Crossref, Medline, Google Scholar
55. Naeye RL, Peters EC: Mental development of children whose mothers smoked during pregnancy. Obstet Gynecol 1984; 64:601-607Medline, Google Scholar
56. Streissguth AP, Martin DC, Barr HM, Sandman BM, Kirchner GL, Darby BL: Intrauterine alcohol and nicotine exposure: attention and reaction time in 4-year-old children. Dev Psychol 1984; 20:533-541Crossref, Google Scholar
57. Nichols PL, Chen TC: Minimal Brain Dysfunction: A Prospective Study. Hillsdale, NJ, Lawrence Erlbaum Associates, 1981Google Scholar
58. Denson R, Nanson JL, McWatters MA: Hyperkinesis and maternal smoking. Can Psychiatr Assoc J 1975; 20:183-187Crossref, Medline, Google Scholar
59. Pratt OE: Introduction: what do we know of the mechanisms of alcohol damage in utero? Ciba Found Symp 1984; 105:1-7Medline, Google Scholar
60. Kesmodel U: [Alcohol and pregnancy]. Ugeskr Laeger 1999; 161:4989-4994 (Danish)Medline, Google Scholar
61. Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ: Moderate alcohol intake during pregnancy and the risk of stillbirth and death in the first year of life. Am J Epidemiol 2002; 155:305-312Crossref, Medline, Google Scholar
62. Aronson M, Hagberg B, Gillberg C: Attention deficits and autistic spectrum problems in children exposed to alcohol during gestation: a follow-up study. Dev Med Child Neurol 1997; 39:583-587Crossref, Medline, Google Scholar
63. Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE: A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res 1997; 21:150-161Crossref, Medline, Google Scholar
64. Delaney-Black V, Covington C, Templin T, Ager J, Nordstrom-Klee B, Martier S, Leddick L, Czerwinski RH, Sokol RJ: Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics 2000; 106:782-791Crossref, Medline, Google Scholar
65. Streissguth AP, Barr HM, Sampson PD, Bookstein FL: Prenatal alcohol and offspring development: the first fourteen years. Drug Alcohol Depend 1994; 36:89-99Crossref, Medline, Google Scholar
66. Brown RT, Coles CD, Smith IE, Platzman KA, Silverstein J, Erickson S, Falek A: Effects of prenatal alcohol exposure at school age, II: attention and behavior. Neurotoxicol Teratol 1991; 13:369-376Crossref, Medline, Google Scholar
67. Boyd TA, Ernhart CB, Greene TH, Sokol RJ, Martier S: Prenatal alcohol exposure and sustained attention in the preschool years. Neurotoxicol Teratol 1991; 13:49-55Crossref, Medline, Google Scholar
68. Cnattingius S, Signorello LB, Anneren G, Clausson B, Ekbom A, Ljunger E, Blot WJ, McLaughlin JK, Petersson G, Rane A, Granath F: Caffeine intake and the risk of first-trimester spontaneous abortion. N Engl J Med 2000; 343:1839-1845Crossref, Medline, Google Scholar
69. Fortier I, Marcoux S, Beaulac-Baillargeon L: Relation of caffeine intake during pregnancy to intrauterine growth retardation and preterm birth. Am J Epidemiol 1993; 137:931-940Crossref, Medline, Google Scholar
70. Barr HM, Streissguth AP: Caffeine use during pregnancy and child outcome: a 7-year prospective study. Neurotoxicol Teratol 1991; 13:441-448Crossref, Medline, Google Scholar
71. Peters DA: Effects of maternal stress during different gestational periods on the serotonergic system in adult rat offspring. Pharmacol Biochem Behav 1988; 31:839-843Crossref, Medline, Google Scholar
72. Teixeira JM, Fisk NM, Glover V: Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. Br Med J 1999; 318:153-157Crossref, Medline, Google Scholar
73. Hansen D, Moller H, Olsen J: Severe periconceptional life events and the sex ratio in offspring: follow up study based on five national registers. Br Med J 1999; 319:548-549Crossref, Medline, Google Scholar
74. Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V: Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab 2001; 86:104-109Medline, Google Scholar
75. Huizink AC, de Medina PG, Mulder EJ, Visser GH, Buitelaar JK: Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry 2002; 41:1078-1085Crossref, Medline, Google Scholar
76. van Os J, Selten JP: Prenatal exposure to maternal stress and subsequent schizophrenia: the May 1940 invasion of the Netherlands. Br J Psychiatry 1998; 172:324-326Crossref, Medline, Google Scholar
77. Watson JB, Mednick SA, Huttunen M, Wang X: Prenatal teratogens and the development of adult mental illness. Dev Psychopathol 1999; 11:457-466Crossref, Medline, Google Scholar
78. Neugebauer R, Hoek HW, Susser E: Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA 1999; 282:455-462Crossref, Medline, Google Scholar
79. Laucht M, Esser G, Baving L, Gerhold M, Hoesch I, Ihle W, Steigleider P, Stock B, Stoehr RM, Weindrich D, Schmidt MH: Behavioral sequelae of perinatal insults and early family adversity at 8 years of age. J Am Acad Child Adolesc Psychiatry 2000; 39:1229-1237Crossref, Medline, Google Scholar
80. Meijer A: Child psychiatric sequelae of maternal war stress. Acta Psychiatr Scand 1985; 72:505-511Crossref, Medline, Google Scholar
81. Stott DH: Follow-up study from birth of the effects of prenatal stresses. Dev Med Child Neurol 1973; 15:770-787Crossref, Medline, Google Scholar
82. Stewart DE, Streiner DL: Cigarette smoking during pregnancy. Can J Psychiatry 1995; 40:603-607Crossref, Medline, Google Scholar
83. Tabachnick BG, Fidell LS: Using Multivariate Statistics, 2nd ed. New York, Harper & Row, 1989, p 746Google Scholar
84. Pirie PL, Lando H, Curry SJ, McBride CM, Grothaus LC: Tobacco, alcohol, and caffeine use and cessation in early pregnancy. Am J Prev Med 2000; 18:54-61Crossref, Medline, Google Scholar
85. Cohen J, Cohen P: Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1983, p 545Google Scholar
86. Faraone SV, Doyle AE: Genetic influences on attention deficit hyperactivity disorder. Curr Psychiatry Rep 2000; 2:143-146Crossref, Medline, Google Scholar
87. Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS: Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse 1995; 7:373-378Crossref, Medline, Google Scholar
88. Haines AP, Imeson JD, Meade TW: Psychoneurotic profiles of smokers and non-smokers. Br Med J 1980; 280:1422Crossref, Medline, Google Scholar
89. Pomerleau OF, Pomerleau CS: Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation. Neurosci Biobehav Rev 1984; 8:503-513Crossref, Medline, Google Scholar
90. Mannuzza S, Klein RG, Bonagura N, Malloy P, Giampino TL, Addalli KA: Hyperactive boys almost grown up, V: replication of psychiatric status. Arch Gen Psychiatry 1991; 48:77-83Crossref, Medline, Google Scholar
91. Barkley RA: Assessment of adults with ADHD, in Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York, Guilford, 1998, pp 345-372Google Scholar
92. Biederman J, Faraone S, Milberger S, Curtis S, Chen L, Marrs A, Ouellette C, Moore P, Spencer T: Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry 1996; 35:343-351Crossref, Medline, Google Scholar
93. Barkley RA, Biederman J: Toward a broader definition of the age-of-onset criterion for attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:1204-1210Crossref, Medline, Google Scholar
94. Watson R: Passive smoking is major threat. Br Med J 1998; 316:9Medline, Google Scholar
95. Wisborg K, Henriksen TB, Obel C, Skajaa E, Ostergaard JR: Smoking during pregnancy and hospitalization of the child. Pediatrics 1999; 104:e46Google Scholar
96. Roy TS, Seidler FJ, Slotkin TA: Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J Pharmacol Exp Ther 2002; 300:124-133Crossref, Medline, Google Scholar
97. Räsänen P, Hakko H, Isohanni M, Hodgins S, Järvelin M-R, Tiihonen J: Maternal smoking during pregnancy and risk of criminal behavior among adult male offspring in the Northern Finland 1966 Birth Cohort. Am J Psychiatry 1999; 156:857-863Link, Google Scholar
98. Brennan PA, Grekin ER, Mednick SA: Maternal smoking during pregnancy and adult male criminal outcomes. Arch Gen Psychiatry 1999; 56:215-219Crossref, Medline, Google Scholar
99. Ernst M, Moolchan ET, Robinson ML: Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry 2001; 40:630-641Crossref, Medline, Google Scholar
100. Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP: Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol 2000; 18:331-354Crossref, Medline, Google Scholar
101. Lester BM, LaGasse LL, Seifer R: Cocaine exposure and children: the meaning of subtle effects. Science 1998; 282:633-634Crossref, Medline, Google Scholar
102. Streissguth AP, Grant TM, Barr HM, Brown ZA, Martin JC, Mayock DE, Ramey SL, Moore L: Cocaine and the use of alcohol and other drugs during pregnancy. Am J Obstet Gynecol 1991; 164:1239-1243Crossref, Medline, Google Scholar
103. Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B: Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA 2001; 285:1613-1625Crossref, Medline, Google Scholar



