Neural Correlates of Episodic Encoding and Recognition of Words in Unmedicated Patients During an Acute Episode of Schizophrenia: A Functional MRI Study
Abstract
OBJECTIVE: Memory impairment has been well documented in schizophrenia. In a previous study, the authors investigated patterns of brain activity during episodic encoding and recognition of words in remitted, stable schizophrenia outpatients being treated with novel antipsychotics. The same procedure was used in this study to investigate unmedicated patients during an acute episode of schizophrenia. METHOD: Functional magnetic resonance imaging was used to study regional brain activation in 10 unmedicated patients experiencing an acute episode of schizophrenia and 10 healthy comparison subjects during performance of a modified version of the words subtest of Warrington’s Recognition Memory Test. RESULTS: Despite intact recognition performance, patients with schizophrenia showed reduced activation of anterior prefrontal, posterior cingulate, and retrosplenial areas relative to comparison subjects during word encoding. During word recognition, reduced activation was found in the patients’ dorsolateral prefrontal and limbic/paralimbic regions. On the other hand, higher metabolism in bilateral anterior prefrontal cortices was observed. CONCLUSIONS: The results suggest that different neural pathways are engaged during episodic encoding and recognition of words in patients experiencing an acute episode of schizophrenia relative to healthy comparison subjects. Furthermore, acute psychosis may prevent practice effects, reflected in a failure to engage brain regions associated with successful episodic memory retrieval in healthy subjects.
Previous research indicates that verbal learning and memory deficits are among the most severe cognitive deficits observed in schizophrenia patients (1, 2). In a previous functional magnetic resonance imaging (fMRI) study of outpatients with remitted schizophrenia, we found attenuated frontotemporal activation during episodic encoding and recognition of words relative to healthy subjects (3). However, it needs to be considered that all patients investigated in this study were treated with second-generation antipsychotics, which are believed to have beneficial effects on cognitive functioning (4). Furthermore, only patients who had been clinically stable for a period of at least 6 months were included in the study. To address these limitations, we investigated activation patterns in unmedicated patients during an acute episode of schizophrenia.
Despite neuropsychological evidence that schizophrenia patients are impaired in the encoding of verbal information (5), we hypothesized that recognition memory for words would be preserved (6). Given previous neuroimaging research (7, 8), we further hypothesized that unmedicated patients with schizophrenia would fail to engage dorsolateral prefrontal and limbic/paralimbic regions during the performance of a verbal memory task and that they would instead recruit nonspecialized brain systems.
Method
Participants
Originally, 20 German-speaking male patients with schizophrenia were recruited from the inpatient units of the Department of Psychiatry of Innsbruck University Clinics while they were experiencing psychotic symptoms (i.e., hallucinations, delusions, thought disorder). Unfortunately, there was a large number of unexpected dropouts (50%): five patients did not tolerate the MRI procedure, and an additional five patients had to be excluded from the statistical analysis because of excessive movement (exceeding 0.4 voxels). This led to a final sample of 10 patients. All of them fulfilled ICD-10 criteria for schizophrenia. Six patients were experiencing their first episode of the illness and were neuroleptic naïve; four had suffered from at least two episodes of schizophrenia (range=2–4 episodes) and were experiencing an acute exacerbation of positive symptoms due to noncompliance with antipsychotic medication.
Except anxiolytics of benzodiazepine derivatives, which were paused on the day of the study, none of the patients received any psychotropic medication at the time of the study. Patients were investigated within 1–2 days after admission to the inpatient unit. They were followed longitudinally, and the diagnosis of schizophrenia was confirmed 6 months after participation in this study through diagnostic conference and thorough medical record review. In addition, the Positive and Negative Syndrome Scale (9) was given on the day of the study to evaluate symptom severity. Ratings were completed by psychiatrists belonging to a trained research team. All patients were physically healthy at the time of the study, and none had a history of head trauma, serious medical illness, or substance abuse. The comparison subjects were 10 German-speaking, age- and sex-matched healthy male volunteers. They had no history of substance abuse or other medical, psychiatric, or neurological disorder that might affect central nervous system function. All participants were right handed, as measured with the Edinburgh Handedness Scale (10), and signed informed consent forms in accordance with the Ethics Committee of the Medical Faculty of Innsbruck University. In order to guarantee task understanding and to make our data comparable to those of our previous study (3), all subjects performed the words subtest of Warrington’s Recognition Memory Test (11) 15 minutes before scanning.
Cognitive Task
During fMRI scanning, encoding and recognition conditions were tested by using a modified version of the words subtest of Warrington’s Recognition Memory Test. Originally, this task consists of one-syllable, high-frequency words presented at a rate of 3 seconds each. For the encoding task, the subjects are presented with a list of 50 common words and are instructed to indicate whether they like or dislike the item represented by each word. Recognition is subsequently assessed by asking the subject to choose the target word when presented in a forced-choice procedure and paired with a similar distractor item.
fMRI Examination
A blocked periodic design was used in which a 40-second rest condition was followed by an activation condition of equal length. During both the encoding and the recognition tasks, the cycle of alternation between conditions was repeated three times in the course of 4 minutes each. The resting baseline reference task was a standard condition during which subjects were instructed to lie still and remain quiet with their eyes open (12). During this condition, a blank white screen appeared. The activation condition consisted of a modified version of the words subtest of the Recognition Memory Test. The stimulus material was projected on a computer screen placed in front of the MRI scanner. Stimuli were presented at a rate of 3 seconds each and were visible to the participant by means of an angled mirror placed above the head coil. Words were centered on the screen and presented as black block letters against a white background. During the encoding task, subjects were instructed to press one of two keys with their right index or middle finger to indicate whether the item was more “pleasant” or “unpleasant.” This task used all 50 items out of the original test. The recognition task consisted of 25 of the 50 items that were studied during the encoding task and 25 distractor items, which were presented in random order. Subjects pressed one of two keys to indicate whether or not they had seen the word during the encoding task. Responses were recorded by hand and were classified as correct or incorrect.
Scanning Procedures and Analysis
fMRI was performed on a 1.5-T scanner (Magnetom VISION, Siemens, Erlangen, Germany) (gradient rise time=300 μsec, 25 mT/msec) by using a circularly polarized head coil (field of view=250 mm). For functional imaging, a T2-weighted, single-shot, echo-planar sequence was used (echo time=64 msec, echo spacing=1.64 msec, field of view=250 mm, matrix=64×128, slice thickness=3 mm, voxel dimension=2.94×1.95×4.5 mm3, flip angle=90°). A total of 120 blood-oxygen-level-dependent contrast-sensitive images with an interslice gap of 0.5 mm were acquired at each of 15 planes parallel to the intercommissural line (13, 14). Rest conditions were alternated with activation conditions, each consisting of 10 images at 4-second intervals (repetition time). Before each time series, five dummy images were collected to reach scanner equilibrium. These images were excluded from the following analyses.
Image analysis was performed offline on a SPARC Ultra 1 workstation (Sun Microsystems, Santa Clara, Calif.) by using MATLAB and SPM 99 statistical parametric mapping software (15–17). The 60 volume images of each task were automatically realigned to the first image of the time series to correct for head movement between scans. Low-frequency artifacts assumed to arise from cardiorespiratory and other cyclical components were removed with high-pass filtering (0.5 cycle/min) of the time series. The functional data sets from each subject were slightly smoothed with a Gaussian filter with a root-mean-square radius of 4×4×9 mm (for the x, y, and z axes, respectively) to suppress high-frequency noise in the images and to improve signal-to-noise ratio. The alternating periods of rest and activation were modeled by using a simple, smoothed, delayed half-sine-wave reference vector to take into account the delayed cerebral blood flow (CBF) changes after stimulus presentation. Significantly activated voxels were searched for using the “general linear model” approach for time-series data. For this, we defined a design matrix comprising contrast testing for significant activation during the activation versus rest condition. The within-group t statistics were expressed as standardized z scores in final projection maps and thresholded at p<0.05, corrected for multiple comparisons (18, 19), with at least eight contiguous voxels activated. Anatomical location of the activated foci was assigned with the aid of the atlas of Talairach and Tournoux (20). In a final processing step, we performed group analyses and subtracted “activation minus rest in patients” from “activation minus rest in comparison subjects” and vice-versa, thresholded at p<0.05, corrected for multiple comparisons.
Group comparisons with respect to behavioral data (age, years of education, task performance) were performed by using the Mann-Whitney U test.
Results
The demographic characteristics of the study group are summarized in Table 1. There was no significant difference between groups in terms of age, years of education, or task performance. Recognition levels were high for both groups (comparison subjects: mean=91.2% correct [SD=5.5]; patients: mean=77.2% correct [SD=22.6]).
Word Encoding
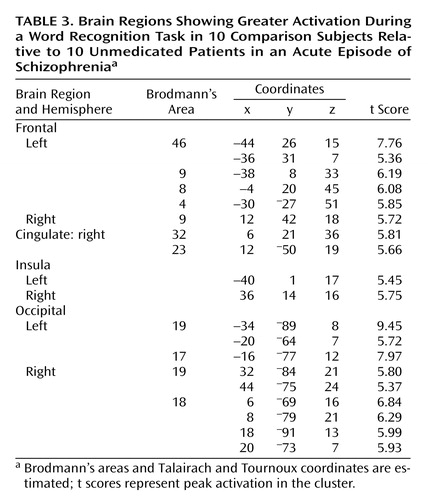
Regarding frontal lobe activation, comparison subjects showed effects in bilateral ventrolateral (bilateral Brodmann’s area 44, right Brodmann’s area 47) and anterior (bilateral Brodmann’s area 10) regions and in the left anterior cingulate (Brodmann’s area 32). Effects were also seen in the sensorimotor cortex (bilateral Brodmann’s area 6, left Brodmann’s area 4), in the left angular gyrus (Brodmann’s area 39), and in the left lenticular nucleus. In patients, activation was detected in the left precentral gyrus (Brodmann’s area 6) and in the left precuneus (Brodmann’s area 31).
Differences in task-related activation were examined by subtracting the CBF values of patients from those of healthy subjects (Table 2, Figure 1). The comparison group had relatively greater activation in the right anterior prefrontal cortex (Brodmann’s area 10), bilateral posterior cingulate (left Brodmann’s area 31, right Brodmann’s area 23), left retrosplenial area (Brodmann’s area 30), and left cuneus (Brodmann’s area 18). The subtraction of activation values of healthy subjects from those of patients yielded no significant differences.
Word Recognition
Word recognition activated a widespread area in both hemispheres. The comparison subjects showed bilateral ventrolateral (bilateral Brodmann’s area 47, right Brodmann’s areas 44 and 45) and left-hemisphere dorsolateral (Brodmann’s area 46) and dorsal (Brodmann’s area 8) prefrontal activations. Further effects were found in left sensorimotor (Brodmann’s areas 4, 6, 2, and 40) regions, bilateral anterior cingulate (Brodmann’s area 32), bilateral lateral temporal cortices (left Brodmann’s area 21, bilateral Brodmann’s area 22), left precuneus (Brodmann’s areas 7 and 19), and bilateral occipital cortices (bilateral Brodmann’s area 18, right Brodmann’s areas 19 and 17). Additional effects were detected in the left thalamus and subthalamic nucleus and in bilateral lenticular nuclei. In patients, activation was seen in bilateral anterior (Brodmann’s area 10) and dorsolateral prefrontal (Brodmann’s area 9) regions, in bilateral precentral gyri (Brodmann’s area 6), and in the left ventrolateral prefrontal cortex (Brodmann’s area 44). In addition, activation was found in the right anterior cingulate (Brodmann’s area 32), the right primary auditory cortex (Brodmann’s area 41), left precuneus (Brodmann’s area 7) and inferior parietal lobule (Brodmann’s area 40), bilateral occipital cortices (bilateral Brodmann’s area 18, left Brodmann’s area 17, right Brodmann’s area 19), in the left thalamus, bilateral subthalamic nuclei, and in the left lenticular nucleus.
When subtracting the activation values of patients from those of healthy subjects, the comparison group had relatively greater activation in bilateral dorsolateral prefrontal cortices (bilateral Brodmann’s area 9, left Brodmann’s area 46), left dorsal prefrontal (Brodmann’s area 8) and motor (Brodmann’s area 4) regions, right cingulate gyrus (Brodmann’s areas 32 and 23), bilateral insula, and in bilateral occipital cortices (bilateral Brodmann’s area 19, left Brodmann’s area 17, right Brodmann’s area 18) (Table 3, Figure 2). On the other hand, patients with schizophrenia showed a greater activation in bilateral anterior prefrontal cortices (Brodmann’s area 10) and in the left Brodmann’s area 6 relative to comparison subjects (Table 4, Figure 2).
Direct comparisons of the same task between patients and healthy subjects revealed the same activation patterns as the task minus rest comparisons.
Discussion
This study examined the neural correlates of episodic encoding and recognition of words in unmedicated, acutely ill individuals with schizophrenia. During encoding, patients with schizophrenia showed a failure to engage frontal, posterior cingulate, and retrosplenial regions. Recognition on the other hand was associated with reduced activation in dorsolateral prefrontal and limbic/paralimbic (cingulate, insula) regions, despite intact recognition performance relative to healthy subjects. In contrast, a higher metabolism in bilateral anterior prefrontal cortices was observed in schizophrenia subjects.
The areas activated by these two tasks in healthy volunteers have been described previously (21–33). During recognition, relatively lower activity was seen in the patients’ dorsolateral prefrontal cortex. This region has been associated with verbal working memory in healthy individuals (34), and correspondingly, impairments of working memory and executive functions in schizophrenia have been associated with decreased blood flow in dorsolateral prefrontal cortex (35). Our finding of reduced activation in this region in patients might, therefore, be related to impaired working memory in schizophrenia. On the other hand, recognition levels were comparable in both groups. This finding is in agreement with our previous study in clinically stable schizophrenia patients treated with second-generation antipsychotics (3). We suggest that, despite acute psychosis, our patients’ working memory abilities permitted successful encoding and that reduced dorsolateral prefrontal activation among our patient group may represent executive difficulties.
Our finding of reduced activity in the patients’ anterior prefrontal, posterior cingulate, and retrosplenial cortices during encoding seems surprising at first sight, since these areas have mainly been associated with episodic memory retrieval (36). However, when interpreting our data, one has to consider that in the current study all material was familiar, since all subjects had already performed the words subtest of Warrington’s Recognition Memory Test before scanning. As the primary scope of this study was to look for differences of functional networks employed to process the tasks in question and not to look for cognitive differences between patients with schizophrenia and healthy comparison subjects, this procedure was chosen in order to guarantee task understanding. Obviously, we have to assume retrieval processes, which might have occurred while the participants performed the encoding task in the scanner. Consequently, our finding of relatively greater right anterior prefrontal activation during the encoding task in the comparison group may represent retrieval processes. Accordingly, our finding of enhanced activity in bilateral anterior prefrontal cortices among the schizophrenia group during the recognition task may refer to retrieval success (37). We speculate that the relatively small time period between the encoding and recognition tasks during scanning enabled our patients to maintain the encoded information in working memory and to engage retrieval processes. Since we did not observe such findings in remitted patients with schizophrenia (3), acute psychosis may have prevented practice effects in the present patient group. This hypothesis agrees with our finding of greater posterior midline activation in the comparison group during the encoding task, which has also consistently been associated with successful episodic memory retrieval (36).
Reduced activation was also found in the anterior cingulate of patients during performance of the recognition task. Disturbances in this brain region have been reported in patients with schizophrenia during several cognitive tasks (8, 38–41). Next to its participation in an “attentional system,” this structure has been proposed to contribute to the recognition of newly learned material (42). Furthermore, the anterior cingulate has been said to play a prominent role in the executive control of cognition (43). The lack of anterior cingulate activation in our patient group may therefore reflect an attentional deficit on one hand and executive difficulties on the other.
Patients also showed reduced bilateral insula activation during the recognition task. This structure has been associated with semantic encoding, short-term storage of auditory-verbal material, and internal phonological processing of written words in healthy individuals (44–46). Deficits in insular activity of schizophrenia patients have also been previously reported by other groups (8, 47). However, our finding should be viewed with caution given the fact that comparison subjects did not show insular activation during the within-group analysis. Still, our results underscore the importance of this structure for the pathophysiology of schizophrenia.
The current study has several limitations. First, we have studied a small group of subjects, and all subjects were male. Gender differences in the functional organization of the brain for working memory have been shown in healthy individuals (48), while in schizophrenia, further studies are needed to elucidate the impact of gender on memory performance and brain activation. Second, the current study included patients with schizophrenia who were at different stages of the illness (first-episode and multiple-episode patients), and only the first-episode patients were neuroleptic naive. We cannot exclude an influence of previous antipsychotic exposure on the cerebral activation pattern in the multiple-episode patient group, even though they were free of antipsychotics at the time of study. Third, all subjects had already carried out the task before scanning. Activation patterns observed in our group may therefore be different from those that would have emerged without this test run. On the other hand, use of a prescan test may eliminate artificial findings resulting from mere differences in being able to follow test procedures between study participants.
Last, we chose to contrast encoding and recognition with a resting baseline reference condition rather than performing serial subtractions of increasingly complex reference tasks (49) and were therefore not able to control for nonmemory functions. This procedure might be responsible for the lack of visual cortex activation for the task versus rest comparisons for both patients and comparison subjects during the encoding task, since subjects may have used visual imagery to rehearse the presented words, which would have canceled out visual activation during the presentation of the words. However, the use of active reference conditions has the potential limitation of confounding the interpretation of activation effects if cognitive components interact between one or more reference tasks (50). Nevertheless, including a motor baseline to our resting baseline task in order to control for motor activation during the acquisition and recognition conditions would have strengthened the interpretation of our findings.
Our findings are of considerable clinical relevance insofar as verbal memory has been found to be related to community outcome, social problem solving, and the acquisition of social skills in patients with schizophrenia (51). One challenge for future imaging studies will be to find out whether and to what extent cognitive dysfunction in schizophrenia is caused, improved, or worsened by medication. This calls for longitudinal studies that investigate patients before and after the implementation of treatment.
 |
 |
 |
 |
Received Nov. 6, 2002; revision received April 16, 2003; accepted April 24, 2003. From the Department of Biological Psychiatry and the Department of Radiology, Innsbruck University Clinics. Address reprint requests to Dr. Hofer, Department of Biological Psychiatry, Innsbruck University Clinics, Anichstrasse 35, A-6020 Innsbruck, Austria; [email protected] (e-mail). Supported by grant 7359 from the Österreichische Nationalbank (National Bank of Austria).
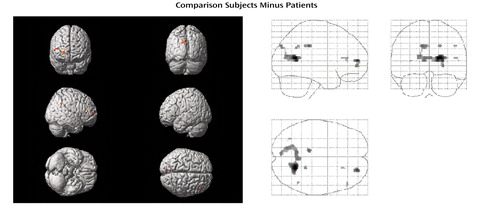
Figure 1. Differences in Brain Activation During a Word Encoding Task Between 10 Healthy Subjects and 10 Unmedicated Patients in an Acute Episode of Schizophreniaa
aColored areas exceed a corrected p value of 0.05 with eight or more contiguous voxels activated. For localization of activation, see Table 2.
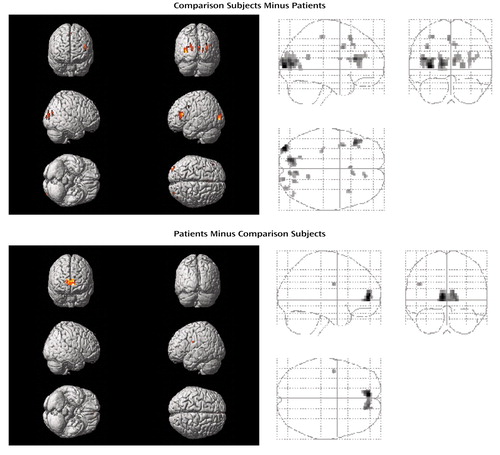
Figure 2. Differences in Brain Activation During a Word Recognition Task Between 10 Healthy Subjects and 10 Unmedicated Patients in an Acute Episode of Schizophreniaa
aColored areas exceed a corrected p value of 0.05 with eight or more contiguous voxels activated. For localization of activation, see Tables 3 and 4.
1. Saykin AJ, Gur RC, Gur RE, Mozley D, Mozley LH, Resnick SM, Kester B, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
2. Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC: Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry 1994; 51:124–131Crossref, Medline, Google Scholar
3. Hofer A, Weiss EM, Golaszewski SM, Siedentopf CM, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW: An fMRI study of episodic encoding and recognition of words in patients with schizophrenia in remission. Am J Psychiatry 2003; 160:911–918Link, Google Scholar
4. Weiss EM, Bilder RM, Fleischhacker WW: The effects of second-generation antipsychotics on cognitive functioning and psychosocial outcome in schizophrenia. Psychopharmacology (Berl) 2002; 162:11–17Crossref, Medline, Google Scholar
5. Harvey PD, Earle-Boyer EA, Wielgus MS, Levinson JC: Encoding memory and thought disorder in schizophrenia and mania. Schizophr Bull 1986; 12:252–261Crossref, Medline, Google Scholar
6. Tracy JI, Mattson R, King C, Bundick T, Celenza MA, Glosser G: A comparison of memory for verbal and non-verbal material in schizophrenia. Schizophr Res 2001; 50:199–211Crossref, Medline, Google Scholar
7. Hazlett EA, Buchsbaum MS, Jeu LA, Nenadic I, Fleischman MB, Shihabuddin L, Haznedar MM, Harvey PD: Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophr Res 2000; 43:33–46Crossref, Medline, Google Scholar
8. Crespo-Facorro B, Wiser AK, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Neural basis of novel and well-learned recognition memory in schizophrenia: a positron emission tomography study. Hum Brain Mapp 2001; 12:219–231Crossref, Medline, Google Scholar
9. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
10. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
11. Warrington EK: Recognition Memory Test. Windsor, UK, Nelson, 1984Google Scholar
12. Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M: Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science 1982; 217:659–661Crossref, Medline, Google Scholar
13. Kwong KK: Functional magnetic resonance imaging with echo planar imaging. Magn Reson Q 1995; 11:1–20Medline, Google Scholar
14. Ogawa S, Lee TM, Kay AR, Tank DW: Brain magnetic resonance imaging with contrast dependent blood oxygenation. Proc Natl Acad Sci USA 1990; 87:9868–9872Crossref, Medline, Google Scholar
15. Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RSJ: Spatial registration and normalization of images. Hum Brain Mapp 1995; 2:165–189Crossref, Google Scholar
16. Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ: Statistical parametric mapping in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
17. Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SCR, Frackowiak RSJ, Turner R: Analysis of fMRI time series revisited. Neuroimage 1995; 2:45–53Crossref, Medline, Google Scholar
18. Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC: Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1994; 1:214–220Google Scholar
19. Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD: Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 1995; 1:223–235Google Scholar
20. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
21. Kapur S, Tulving E, Cabeza R, McIntosh AR, Houle S, Craik FIM: The neural correlates of intentional learning of verbal materials: a PET study in humans. Brain Res Cogn Brain Res 1996; 4:243–249Crossref, Medline, Google Scholar
22. McDermott KB, Ojemann JG, Petersen SE, Ollinger JM, Snyder AZ, Akbudak E, Conturo TE, Raichle ME: Direct comparison of episodic encoding and retrieval of words: an event-related fMRI study. Memory 1999; 7:661–678Crossref, Medline, Google Scholar
23. Kirchhoff BA, Wagner AD, Maril A, Stern CE: Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci 2000; 20:6173–6180Crossref, Medline, Google Scholar
24. Halsband U, Krause BJ, Schmidt D, Herzog H, Tellmann L, Müller-Gärtner HW: Encoding and retrieval in declarative learning: a positron emission tomography study. Behav Brain Res 1998; 97:69–78Crossref, Medline, Google Scholar
25. Mottaghy FM, Shah NJ, Krause BJ, Schmidt D, Halsband U, Jäncke L, Müller-Gärtner HW: Neuronal correlates of encoding and retrieval in episodic memory during a paired-word association learning task: a functional magnetic resonance imaging study. Exp Brain Res 1999; 128:332–342Crossref, Medline, Google Scholar
26. Lee ACH, Robbins TW, Pickard JD, Owen AM: Asymmetric frontal activation during episodic memory encoding: the effects of stimulus type on encoding and retrieval. Neuropsychologia 2000; 38:677–692Crossref, Medline, Google Scholar
27. Ragland JD, Gur RC, Lazarev MG, Smith RJ, Schroeder L, Raz J, Turetsky BI, Alavi A, Gur RE: Hemispheric activation of anterior and inferior prefrontal cortex during verbal encoding and recognition: a PET study of healthy volunteers. Neuroimage 2000; 11:624–633Crossref, Medline, Google Scholar
28. Otten LJ, Henson RNA, Rugg MD: Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain 2001; 124:399–412Crossref, Medline, Google Scholar
29. Wagner AD, Desmond JE, Glover GH, Gabrieli JDE: Prefrontal cortex and recognition memory: functional MRI evidence of context-dependent retrieval processes. Brain 1998; 121:1985–2002Crossref, Medline, Google Scholar
30. Saykin AJ, Johnson SC, Flashman LA, McAllister TW, Sparling M, Darcey TM, Moritz CH, Guerin SJ, Weaver J, Mamourian A: Functional differentiation of medial temporal and frontal regions involved in processing novel and familiar words: an fMRI study. Brain 1999; 122:1963–1971Crossref, Medline, Google Scholar
31. Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD: Short-term and long-term verbal memory: a positron emission tomography study. Proc Natl Acad Sci USA 1995; 92:5111–5115Crossref, Medline, Google Scholar
32. Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ: Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci 1999; 19:3962–3972Crossref, Medline, Google Scholar
33. Kim JJ, Andreasen NC, O’Leary DS, Wiser AK, Boles Ponto LL, Watkins GL, Hichwa RD: Direct comparison of the neural substrates of recognition memory for words and faces. Brain 1999; 122:1069–1083Crossref, Medline, Google Scholar
34. Petrides M, Alivisatos B, Meyer E, Evans AC: Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA 1993; 90:878–882Crossref, Medline, Google Scholar
35. Berman KF, Zec RF, Weinberger DR: Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, II: role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry 1986; 43:126–135Crossref, Medline, Google Scholar
36. Cabeza R, Nyberg L: Imaging cognition, II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 2000; 12:1–47Crossref, Medline, Google Scholar
37. Tulving E, Markowitch HJ, Craik FE, Habib R, Houle S: Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex 1996; 6:71–79Crossref, Medline, Google Scholar
38. Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Recalling word lists reveals “cognitive dysmetria” in schizophrenia: a positron emission tomography study. Am J Psychiatry 1999; 156:386–392Abstract, Google Scholar
39. Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel BV Jr, Lohr J, Wu J, Haier RJ, Bunney WE Jr: Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry 1997; 154:682–684Link, Google Scholar
40. Dolan RJ, Fletcher P, Frith CD, Friston KJ, Frackowiak RSJ, Grasby PM: Dopaminergic modulation of impaired cognitive activation in the anterior cingulate cortex in schizophrenia. Nature 1995; 378:180–182Crossref, Medline, Google Scholar
41. Carter CS, Mintun M, Nichols T, Cohen JD: Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry 1997; 154:1670–1675Link, Google Scholar
42. Meunier M, Bachevalier J, Mishkin M: Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia 1997; 35:999–1015Crossref, Medline, Google Scholar
43. D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M: The neural basis of the central executive system of working memory. Nature 1995; 378:279–281Crossref, Medline, Google Scholar
44. Abdullaev YG, Posner MI: Event-related brain potential imaging of semantic encoding during processing single words. Neuroimage 1998; 7:1–13Crossref, Medline, Google Scholar
45. Augustine JR: Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev 1996; 22:229–244Crossref, Medline, Google Scholar
46. Paulesu E, Frith CD, Frackowiak RSJ: The neural correlates of the verbal component of working memory. Nature 1993; 362:342–344Crossref, Medline, Google Scholar
47. Curtis VA, Bullmore ET, Brammer MJ, Wright IC, Williams SCR, Morris RG, Sharma TS, Murray RM, McGuire PK: Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 1998; 155:1056–1063Link, Google Scholar
48. Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L: Gender differences in the functional organization of the brain for working memory. Neuroreport 2000; 11:2581–2585Crossref, Medline, Google Scholar
49. Posner MI, Petersen SE, Fox PT, Raichle ME: Localization of cognitive operations in the human brain. Science 1988; 240:1627–1631Crossref, Medline, Google Scholar
50. Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RSJ, Dolan RJ: The trouble with cognitive subtraction. Neuroimage 1996; 4:97–104Crossref, Medline, Google Scholar
51. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Link, Google Scholar



