Emotions in Unmedicated Patients With Schizophrenia During Evaluation With Positron Emission Tomography
Abstract
OBJECTIVE: Schizophrenia is currently conceptualized as a disease of functional neural connectivity, leading to symptoms that affect aspects of mental activity, including perception, attention, memory, and emotion. The neural substrates of its emotional components have not been extensively studied with functional neuroimaging. Previous neuroimaging studies have examined medicated patients with schizophrenia. The authors measured regional cerebral blood flow (rCBF) during performance of a task that required unmedicated patients to recognize the emotional valence of visual images and to determine whether they were pleasant or unpleasant. METHOD: The authors examined rCBF in 17 healthy volunteers and 18 schizophrenia patients who had not received antipsychotic medications for at least 3 weeks during responses to pleasant and unpleasant visual stimuli. Areas of relative increases or decreases in rCBF were measured by using the [15O]H2O method. RESULTS: When patients consciously evaluated the unpleasant images, they did not activate the phylogenetically older fear-danger recognition circuit (e.g., the amygdala) used by the healthy volunteers, although they correctly rated them as unpleasant. Likewise, the patients showed no activation in areas of the prefrontal cortex normally used to recognize the images as pleasant and were unable to recognize them as such. Areas of decreased CBF were widely distributed and comprised subcortical regions such as the thalamus and cerebellum. CONCLUSIONS: This failure of the neural systems used to support emotional attribution is consistent with pervasive problems in experiencing emotions by schizophrenia patients. The widely distributed nature of the abnormalities suggests the importance of subcortical nodes in overall dysfunctional connectivity.
Schizophrenia causes pervasive mental changes that include the ability to think clearly, perceive correctly, and experience and express emotions intensely. The neural basis of its cognitive disturbances (e.g., memory deficits, attentional impairments, hallucinations) has been intensively explored, using the tools of functional neuroimaging (1–6). Emotional abnormalities are among the most striking features of this illness. Patients sometimes appear to have lost the ability to feel. For example, they may respond impassively when told of the death of a loved one. They may also have difficulty interpreting the emotional significance of events or situations, which may in turn lead to delusional interpretations of their meaning. The neural mechanisms of emotional disturbance in schizophrenia have been less well investigated than other aspects of the illness (7, 8). However, several functional neuroimaging studies have begun to examine this issue (9–13). In general, but not always (11), patients with schizophrenia fail to activate limbic and paralimbic regions when either correctly or incorrectly performing affective tasks.
The interpretation of these previous studies is, however, limited because all studies have included patients who were taking different or multiple neuroleptic medications during the functional imaging experiment (9–14). The effects of neuroleptic (also known as antipsychotic) medications on affective functioning in schizophrenia are multifaceted. Neuroleptics may either reduce or exacerbate the emotional difficulties of patients, depending on the specific receptor profile and its interaction with affect-regulating neurotransmitter systems. They also have effects on psychopathology, produce neurological side effects, and influence cerebral blood flow (CBF). Therefore, because of the importance of emotion in schizophrenia and to circumvent the confounding of treatment with neuroleptic medications, we conducted a functional imaging study of patients with schizophrenia after a period of withdrawal of neuroleptics of at least 3 weeks. The present study of medication-free patients examined one facet of emotion: the ability to attribute the correct emotional meaning to images (pictures of objects, people, and situations) that are commonly seen in everyday life.
Many studies have now shown that “normal” emotion is not a unitary construct (15). Processing emotion-laden stimuli has evaluative, experiential, and attributive components. Emotions also have direction and valence, such as positive or negative, pleasant or unpleasant, happy or sad. Phylogenetically, emotions may also have levels, ranging from more primitive emotions that promote survival (fear of dangerous stimuli, sexual desire) to emotions that are “higher” and less obviously adaptive (feeling altruistic, generous, or loving). Animal, human lesion, and functional imaging studies have begun to dissect the various neural substrates of these components of emotion (16). For example, the subcortical limbic system (including the amygdala) is devoted to the evaluation of negative stimuli, particularly for potential danger (17). The prefrontal cortex is a pivotal node for the evaluation of pleasant and rewarding stimuli (18).
We have previously used positron emission tomography (PET) to study the neural correlates of attributing the emotional valence of positive and negative visual stimuli in normal healthy volunteers (19). We now have extended this work to the study of schizophrenia. Appraisal of the emotional significance of a stimulus is a critical stage in emotional processing, and this ability is often impaired in patients with schizophrenia. In this study, we compared patients’ response to visual images that reflect situations or objects common in everyday life that represent extremes of emotional valence. Regional CBF (rCBF) was measured while 18 patients with DSM-IV schizophrenia and 17 healthy volunteers formulated a judgment (attribution) of the pleasantness or unpleasantness of these stimuli.
Method
Subjects
Eighteen patients suffering from DSM-IV schizophrenia were studied. Diagnoses were based on a structured interview, the Comprehensive Assessment of Symptoms and History (20). The patients with schizophrenia were all medication free for at least 3 weeks, a period after which oral neuroleptics are no longer measurably present in the brain (21). None had received depot neuroleptics in the past 6 months. Six patients were drug naive, and 12 had discontinued medications themselves, had them discontinued by a referring physician, or had them withdrawn while on the inpatient unit of the Iowa Mental Health Clinical Research Center after giving written informed consent and being fully informed about the risk of relapse. Three had comorbid substance abuse (one with amphetamine dependence, one with cannabis abuse, and one with amphetamine abuse and cannabis dependence); there were no other comorbid diagnoses. Sixteen patients were men, and two were women. Their average age was 30.0 years (SD=8.9), and their mean education level was 12.9 years (SD=3.3). Thirteen were right-handed, two were left-handed, and three used both hands. Symptom ratings obtained on the day of scanning indicated that symptom levels were in the mild to moderate range, a level that did not interfere with their ability to perform the tasks. Symptom dimensions (22, 23) were negative (mean=2.53, SD=1.19), positive (psychotic) (mean=2.53, SD=1.19), and disorganized (mean=2.55, SD=1.43). Two subjects experienced auditory hallucinations during all three imaging sessions, as determined by debriefing after each scan acquisition.
Seventeen healthy volunteers, recruited from the community by newspaper advertising, were studied. They were screened with an abbreviated version of the Comprehensive Assessment of Symptoms and History to rule out psychiatric, neurological, and general medical illness, including substance abuse. Nine were women, and seven were men. All were right-handed. Their average age was 29.5 years (SD=7.56), and their average educational achievement was 14.3 years (SD=1.7). All subjects gave written informed consent for this protocol, as approved by the University of Iowa Human Subjects Institutional Review Board. Findings regarding these 17 healthy volunteers were reported elsewhere (19).
Affective Tasks
The emotion attribution protocol used in this study was fully described elsewhere (19). In brief, two sets of stimuli (comprising 18 pleasant and 18 unpleasant pictures) were selected from the International Affective Picture System (24) to achieve a wide range of emotional content for each type of valence. Pleasant content depicted such emotions as success, beauty, or appetite, whereas unpleasant content included emotions such as fear, disgust, or disappointment. The pictures were screened before a large number of normal local volunteers who were of an age equivalent to those in this study and rated for valence. The pictures with the smallest standard deviations were selected and presented again to a similar large group of volunteers. Based on their ratings, 18 pictures were selected for each condition, chosen to achieve pleasant or unpleasant valence, and matched for types of content (e.g., people, objects, scenes). A complete list of pictures can be found in a previous study (19). Mean image luminance was 12.37 foot-candles (SD=0.49) for the set of unpleasant pictures and 12.47 (SD=0.49) for the pleasant set. Before presentation of the pictures and the imaging, the subjects were familiarized with the use of the visual analogue scale. Before the imaging, they were instructed as follows:
For this condition, I want you to relax and watch the pictures on the monitor. A new picture will appear every 2–3 seconds. Watch them until they stop, but continue looking at the screen until I tell you we are finished. Then you will be rating the pictures as a group with the scale we discussed earlier.
Picture displays began 10 seconds before the arrival of the bolus in the brain. After the images were acquired, the subjects looked at a –7 (extremely unpleasant)-to-0 (neutral)-to-7 (extremely pleasant) visual analogue scale that was held by a research technician and made their ratings by pointing to a specific number. The order of presentation was “pleasant” followed by “unpleasant” to minimize possible carryover effects of intensity (25). Mean normative arousal scores (range=0–9) were 5.73 (SD=0.74) for the unpleasant set and 4.69 (SD=1.03) for the pleasant set. These two conditions were part of a larger PET study that also included exposure to odors. The results of the olfaction study have been reported elsewhere (26).
Neuroimaging Acquisition and Processing
rCBF was measured by using the bolus [15O]H2O method (27). The subjects were oriented in the PET scanner with laser light guides aligned at the orbital meatal line of the brain. The center of the most rostral slice was indicated by the laser guides. Fifteen slices (6.5 mm, center to center), with an intrinsic in-plane resolution of 6.5 mm full width at half maximum and a 10-cm axial field of view, were acquired. The images were reconstructed by using a Butterworth filter (cutoff frequency=0.35 Nyquist). CBF was determined by using [15O]H2O (a 50 mCi/injection) and methods described previously (28). For each injection, arterial blood was sampled from time=0 (injection) to time=100 seconds. The imaging was initiated at the time of injection and consisted of 20 frames at 5 seconds per frame for a total of 100 seconds. The parametric (i.e., CBF) image was created by using a 40-second summed image (initial=40 seconds immediately after the bolus transit) and an arterial input function. A preliminary (“sham”) injection, to determine the bolus arrival time, was employed to establish stimulus timing (29). An 18-mm Hanning filter was applied to the PET images to eliminate residual anatomical variability. Magnetic resonance imaging (MRI) consisted of contiguous coronal slices (1.5-mm thick) acquired on a 1.5-T GE Signa scanner (General Electric Systems, Milwaukee). The technical parameters of the MRI acquisition were as follows: spoiled gradient recall acquisition sequence, flip angle=40°, TE=5 msec, TR=24 msec, number of excitations=2. MRI was analyzed with locally developed software (BRAINS, University of Iowa, Iowa City). All brains were realigned parallel to the anterior commissure-posterior commissure line and the interhemispheric fissure to ensure comparability of head position across subjects. Alignment also placed the images in standard Talairach space (30). Images from multiple subjects were coregistered and resliced in three orthogonal planes to produce a three-dimensional data set that was used for visualization and analysis (31).
Image Analysis
The data presented here are based on a nonparametric randomization analysis, with use of the statistical methods described previously (32). The analysis shown compares the response of patients and comparison subjects on the two most extreme emotion-attribution tasks: very pleasant versus very unpleasant pictures. This comparison was powerful for comparing patients and comparison subjects because it examined the extremes of emotional response. Randomization analysis relies on across-task and across-group comparisons for unpleasant minus pleasant pictures in patients as opposed to unpleasant minus pleasant pictures in comparison subjects. We used an uncorrected alpha of 0.005.
Results
Although the patients with schizophrenia had “normal” ratings on the unpleasant pictures, they displayed difficulty in attributing the correct valence to the pleasant pictures. Pleasant stimuli were rated by the patients as mean=3.62 (SD=4.04) and by the comparison subjects as mean=6.11 (SD=1.11) (t=2.44, df=31, p=0.02, two-tailed). On the other hand, the unpleasant stimuli were rated by the patients as mean=–4.37 (SD=4.48) and by the comparison subjects as mean=–4.87 (SD=4.09) (t=–0.32, df=31, p>0.70).
In the patients, direct comparison (33) of unpleasant and pleasant tasks yielded a paucity of findings, reflecting their muted emotional responsiveness. There was an area of relatively increased CBF associated with response to unpleasant stimuli in the right ventral lateral prefrontal cortex (Brodmann’s area 47, tmax=4.54, 153 voxels; Talairach coordinates: x=32, y=37, z=–10) and one area of increased CBF in the right ventral medial prefrontal cortex (Brodmann’s area 10, tmax=–4.09, 95 voxels; Talairach coordinates: x=2, y=48, z=–18) in response to the pleasant task. Direct task comparisons among healthy volunteers can be found elsewhere (19).
Our randomization analysis indicated that when they were compared with normal healthy comparison subjects, the patients with schizophrenia exhibited widespread decreases in CBF when they assessed and rated the emotional valence of the unpleasant pictures (Table 1 and Figure 1). Most of these regions had increased CBF in the normal comparison subjects when they rated the unpleasant stimuli. The normal subjects had activation in the left amygdala, the cerebellum, and the secondary visual cortex in the unpleasant/pleasant comparison (19). The decreases in CBF in the patients were the inverse of the normal response and reflected a failure to activate the regions normally used in performance of this emotional task.
One group of regions showing an abnormally decreased response to the unpleasant pictures included the limbic and paralimbic structures implicated in the assessment and appraisal of danger: the amygdala, hippocampus, and medial ventral prefrontal cortex. Other regions showing decreased activity in schizophrenia included the visual association cortices. In addition to these decreases in the limbic, paralimbic, and association cortex, the patients also showed decreases in CBF in the thalamus and cerebellum while appraising the unpleasant pictures, indicating that the abnormalities occurring during emotional appraisal arise from a widely distributed network that includes subcortical regions.
A different gestalt emerged when the CBF patterns of the patients and comparison subjects were compared during appraisal of the pleasant stimuli (Table 1 and Figure 2). Again, the pattern observed in the patients was an inverse of the pattern observed in the healthy volunteers. Whereas the healthy comparison subjects showed activations in the medial, orbital, and dorsal lateral frontal cortex (19), the patients had decreases in multiple regions of the prefrontal cortex, reflecting a lack of activation of the networks used for pleasure recognition and attribution. In addition, they also had decreases in CBF in the insular cortex, an allocortical region that is connected to the limbic system, consists of multimodal sensory integration regions, and is thought to play a role in perceiving relationships between the external world and the internal milieu (34).
We did not find areas of increased CBF in patients relative to comparison subjects during attribution of valence to either unpleasant or pleasant stimuli.
Correlations were calculated for the relationship between symptom severity and ratings of emotional valence in the patients with schizophrenia. There was a significant inverse correlation between the ratings of the unpleasant pictures and the positive symptom dimension, which is comprised of delusions and hallucinations (r=–0.72, N=16, p<0.002). That is, the more severe the positive symptoms, the higher the tendency to rate the unpleasant stimuli as unpleasant. There was also a significant direct correlation between ratings of the unpleasant pictures and the negative symptom dimension (r=0.55, N=16, p<0.03). That is, the more severe the negative symptoms, the lower the ability to recognize the negative emotional valence of the pictures and instead to interpret them more positively.
Discussion
To our knowledge, this is the first study to report on the functional neuroanatomy of affective functioning of nonmedicated patients with schizophrenia. These extensive decreases in CBF during valence attribution to affectively laden visual stimuli suggest impairment in the systems of the brain used for recognition of the emotional significance of people, situations, and objects. This study confirms the results of previous studies (9–14) and extends the findings to nonmedicated patients. This neural response is consistent with the clinical presentation of schizophrenia. The emotional deficits of schizophrenia are well-recognized phenomena (7, 35–41). However, the neural basis of these deficits has not yet been fully mapped. A pivotal aspect of appropriate affective reactions is the ability to attribute the correct emotional value to events and situations. This study advances the neurobiological understanding of this core aspect of the psychopathology of patients with schizophrenia.
CBF and Neural Response to Unpleasant Stimuli
The patients with schizophrenia were unable to engage neural nodes known to be responsible for processing negatively charged affective stimuli, including the amygdala, the hippocampus, and the ventral medial frontal cortex. Functional imaging studies have reported amygdala abnormalities in patients with schizophrenia who responded with the appropriate affect to sadness-inducing human faces (42) and in patients who failed to respond to fearful faces (43). Hence, our study is consistent with previous reports of functional abnormality in the amygdala of patients during probes by negative stimuli. In the present study, the findings concerning the amygdala were left lateralized. This phenomenon may be due to the nature of our stimuli. The human amygdala may respond with right-lateralized activity to affect-laden human faces and with left-lateralized activity to nonhuman scenes (44) or in circumstances requiring a cognitive representation of fear (45). Our study helps clarify that the processing of faces is likely not the only causative factor for abnormal amygdala function (46). Adding to the mapping of amygdala dysfunction is another difference between studies. In the study by Schneider et al. (42), subjects were asked to rate their subjective mood rather than attribute a valence to stimuli, unlike the study by Phillips et al. (43) and ours. In agreement with previous studies (10), in our study, failure to engage the amygdala was consistent with the appropriate attribution of valence. It is worth noting, however, that at least two studies did not show reduced amygdala response to affective stimuli (9, 11). These findings may be explained on the basis of active neuroleptic medication in patients during task performance.
Animal and human studies have demonstrated that the amygdala plays a key role in the affective evaluation of negative or dangerous situations (17, 19, 47–53). It has been called “the hub in the wheel of fear,” since it acts as a router that permits a rapid and adaptive response to dangerous stimuli and a slower, more reflective response after the meaning of the stimulus has been assessed (17). The slow response is mediated by the hippocampus, which responds to unexpected stimuli as part of a novelty-assessment network by retrieving stored contextual and emotional experiences that permit attribution of meaning or valence to the stimuli (54, 55). The ventral frontal cortex is also part of this emotion appraisal network (18, 56, 57). Other regions showing decreased activity in schizophrenia include the visual association cortices, which also participate in determination of meaning through recognizing the specific nature of perceptions, such as in faces or objects (48).
Abnormalities in the thalamus are consistent with a growing number of postmortem, in vivo morphometric, and functional imaging studies that describe primary abnormalities in the thalamus in schizophrenia (58–64). Functional imaging studies have also shown that the thalamus is associated with visually induced negative affect (65, 66).
An abnormality in midline cerebellar activity in patients during an emotional valence recognition task is consistent with the posited role of the cerebellum in emotion (67, 68). Cerebellar midline structures (i.e., the vermis), together with other older cerebellar regions (i.e., the fastigial nuclei and the floccular nodular lobule) have been regarded as the equivalent of the limbic cerebellum (69) and have been found to be abnormal in schizophrenia (70). A large decrease in CBF was also observed, however, in the cerebellar cortex. Failure to recruit the cerebellum, along with other nodes in the cortical-cerebellar-thalamic-cortical circuit, completes an impairment posited to be the central unifying mechanism of symptom production and cognitive disturbance in schizophrenia (71). Therefore, the present study extends to emotion processing the findings of previous imaging studies obtained by using cognitive paradigms that did not include emotion (72).
This study did not find areas of relative increase in CBF in patients with schizophrenia relative to comparison subjects in either task. This paucity of activation (the “silent brain”) is consistent with our within-group analysis showing little differential activation in patients responding to unpleasant versus pleasant stimuli. However, consistent with the data of Taylor et al. (9), small areas of increased CBF were found in the ventral prefrontal cortex in association with correct interpretation of unpleasant stimuli and in response to pleasant stimuli in the within-group analyses of the patients.
CBF and Neural Response to Pleasant Stimuli
Although the normal comparison subjects activated an archaic danger-recognition system when rating the unpleasant pictures, they activated multiple sites in the phylogenetically younger network of pleasure-recognition sites in the prefrontal cortex and in the insular cortex when rating the pleasant pictures (19). In contrast, as shown in Table 1 and Figure 2, the patients with schizophrenia had pervasive decreases in CBF in multiple regions of the prefrontal cortex while attempting to attribute the correct emotional valence to pleasant stimuli. These results are consistent with numerous studies that have previously demonstrated both structural and functional abnormalities in the prefrontal cortex in schizophrenia (73–84).
The patients also had decreased CBF in the insular cortex while attempting to attribute the correct emotional valence to pleasant stimuli. Decreased CBF in the insula in patients with schizophrenia is consistent with previous studies that have shown functional and structural abnormalities in the insular cortex in schizophrenia (85–87). The insular cortex is a multimodal integration region that is involved in the linking of sensory experiences with their appropriate emotional responses (88, 89). Previous functional neuroimaging studies have reported abnormalities in CBF in the insula during diverse emotional states, including the experiencing of aversive stimuli, nociception, anticipatory anxiety, and mood provocation (89–92). Insular gray matter volume and cortical surface size are inversely related to the severity of psychotic symptoms in schizophrenia (93).
These results indicate that patients with schizophrenia have at least two facets of neurofunctional deficits. The first one, which might be called condition independent, has been observed in patients, regardless of the mental task at hand, and entails deficits in frontal-thalamic-cerebellar circuitry. The results of this study are similar to those obtained in others in which patients performed “pure” cognitive tasks (94, 95). On the other hand, condition-specific deficits were also observed during this study of emotion. These were seen in regions governing affect regulation. Condition- or task-specific neurofunctional deficits are often found together with task-independent deficits in functional neuroimaging studies of schizophrenia (26, 96).
Clinical Significance
The results observed at the neural level, by means of measurements of CBF, are consistent with the clinical picture of schizophrenia, especially when examined in the context of the ratings of emotional valence given by the patients. The blunting of subjective experience and emotional expression are common symptoms in schizophrenia and are clinically referred to as part of the group of negative symptoms (anhedonia, asociality, and affective blunting). The patients in this study had difficulty in attributing the correct valence to pleasant pictures. They lacked the “normal” ability to recognize pleasant stimuli. However, they were able to attribute correctly an unpleasant or negative valence to the unpleasant pictures. Maintaining an intact behavioral response to aversive stimuli conveys survival advantages for people with schizophrenia, but their inability to recognize and attribute a positive valence for pleasant pictures is reflective of the suffering that they experience during survival.
Examination of the correlations between symptom patterns and the ability to attribute the correct emotional valence to the stimuli added to the clinical perspectives of this study. The patients who had higher levels of positive symptoms (delusions and hallucinations) were more likely to give the unpleasant pictures a more negative rating, seeing them as extremely unpleasant. This suggests that these patients had an unusual sensitivity to negative stimuli, making them more vulnerable to misinterpretation of their emotional meaning. This unusual sensitivity may form part of the basis for misperceptions and delusional interpretations. On the other hand, the patients who had higher scores on the negative dimension (i.e., anhedonia, affective blunting, asociality) also had difficulty in giving the correct (negative) rating to the unpleasant pictures, instead giving them a relatively more positive rating. This correlation between severity of negative symptoms and an inability to recognize the negative emotional valence of unpleasant pictures may explain other facets of the clinical experience of schizophrenia. Specifically, this rating pattern is consistent with clinical manifestations, such as both the blunting of affect and inappropriate affect.
In summary, we found that when patients evaluated the unpleasant pictures, they did not engage the limbic and paralimbic regions used by the healthy volunteers, even though they correctly rated them as unpleasant. The patients also failed to activate the areas of the prefrontal cortex that are normally used to recognize images as pleasant, but in addition, they were unable to recognize them as pleasant. This network of decreases in CBF during attribution of a positive or negative valence to pleasant or unpleasant visual stimuli in patients with schizophrenia suggests a failure in the functional brain systems used for recognition of the emotional significance of people, situations, and objects.
 |
Received May 29, 2002; revisions received Feb. 11 and June 12, 2003; accepted June 20, 2003. From the Mental Health Clinical Research Center, Department of Psychiatry, and the PET Center, Department of Radiology, University of Iowa College of Medicine, Iowa City; the Psychiatry Department, University Hospital Marques de Valdecilla School of Medicine, University of Cantabria, Santander, Spain; and the MIND Institute and the Departments of Psychiatry and Neurology, University of New Mexico, Albuquerque. Address reprint requests to Dr. Paradiso, Department of Psychiatry, University of Iowa, 2-200 Psychiatry Research—MEB, Iowa City, IA 52242-1057; [email protected] (e-mail). Funded in part by NIMH grants MH-31593, MH-40856, and MH-43271, an NIMH Research Scientist Award (MH-00625), and an Established Investigator Award from the National Alliance for Research on Schizophrenia and Depression; Dr. Paradiso is supported by the Edward Mallinckrodt, Jr., Foundation.
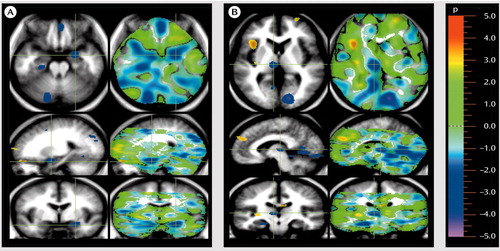
Figure 1. Regions in Which Cerebral Blood Flow (CBF) Was Lower in Unmedicated Patients With Schizophrenia Than in Healthy Volunteers During Rating of Unpleasant Pictures for Emotional Valencea
aThe patients show wide distribution of decreases in regional CBF during performance of this task. The images follow radiological convention and show location as if one were standing at the foot of the patient’s bed (transaxial views at the top) or facing the patient (coronal views at the bottom). Sagittal images (in the middle) are also shown. The images depict a randomization analysis, with areas of significant decreases in CBF (in blue) in patients at an alpha=0.005 threshold, registered on average magnetic resonance imaging for all subjects to permit anatomic localization. Green crosshairs in image A were placed on an area of decreased CBF in the amygdala, which is seen in all three planes. In addition, areas of decreased CBF are seen in the ventral medial cortex (top), hippocampus (top), and cerebellum (top). Other areas of decreased CBF are shown in image B, including the thalamus (under the crosshairs), the occipital, lingual, and fusiform gyri (top and middle), and the hippocampus (bottom).
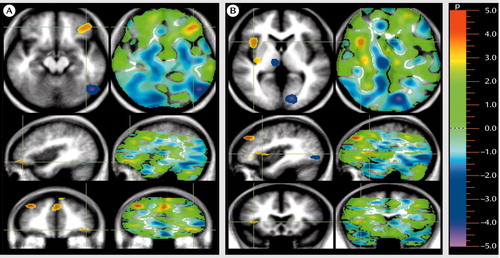
Figure 2. Regions in Which Cerebral Blood Flow (CBF) Was Lower in Unmedicated Patients With Schizophrenia Than in Healthy Volunteers During Rating of Pleasant Pictures for Emotional Valencea
aThe patients show wide distribution of decreases in regional CBF during performance of this task. Areas of significant decreases in CBF are shown in yellow or red (alpha=0.005). In image A, the crosshairs were placed on an area of decreased CBF in the left ventral lateral prefrontal cortex (in all three planes). Other areas of decreased CBF included the medial prefrontal cortex (bottom), and the right dorsal lateral prefrontal cortex (bottom). In image B, the crosshairs were placed directly on areas of decreased CBF in the insula, which is shown in two locations in the uppermost image.
1. Spence SA, Liddle PF, Stefan MD, Hellewell JS, Sharma T, Friston KJ, Hirsch SR, Frith CD, Murray RM, Deakin JF, Grasby PM: Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk: focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry 2000; 176:52–60Crossref, Medline, Google Scholar
2. Manoach DS, Halpern EF, Kramer TS, Chang Y, Goff DC, Rauch SL, Kennedy DN, Gollub RL: Test-retest reliability of a functional MRI working memory paradigm in normal and schizophrenic subjects. Am J Psychiatry 2001; 158:955–958Link, Google Scholar
3. Wible CG, Kubicki M, Yoo S-S, Kacher DF, Salisbury DF, Anderson MC, Shenton ME, Hirayasu Y, Kikinis R, Jolesz FA, McCarley RW: A functional magnetic resonance imaging study of auditory mismatch in schizophrenia. Am J Psychiatry 2001; 158:938–943Link, Google Scholar
4. Nordahl TE, Carter CS, Salo RE, Kraft L, Baldo J, Salamat S, Robertson L, Kusubov N: Anterior cingulate metabolism correlates with Stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology 2001; 25:139–148Crossref, Medline, Google Scholar
5. Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK: Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 2000; 57:1033–1038Crossref, Medline, Google Scholar
6. Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel BV Jr, Lohr J, Wu J, Haier RJ, Bunney WE Jr: Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry 1997; 154:682–684Link, Google Scholar
7. Mandal MK, Pandey R, Prasad AB: Facial expressions of emotions and schizophrenia: a review. Schizophr Bull 1998; 24:399–412Crossref, Medline, Google Scholar
8. Hellewell JS, Whittaker JF: Affect perception and social knowledge in schizophrenia, in Handbook of Social Functioning in Schizophrenia. Edited by Mueser K, Rarrier N. Boston, Allyn & Bacon, 1998, pp 197–212Google Scholar
9. Taylor SF, Liberzon I, Decker LR, Koeppe RA: A functional anatomic study of emotion in schizophrenia. Schizophr Res 2002; 58:159–172Crossref, Medline, Google Scholar
10. Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Kohler C, Alsop D, Maldjian J, Ragland JD, Gur RC: An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry 2002; 159:1992–1999Link, Google Scholar
11. Kosaka H, Omori M, Murata T, Iidaka T, Yamada H, Okada T, Takahashi T, Sadato N, Itoh H, Yonekura Y, Wada Y: Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr Res 2002; 57:87–95Crossref, Medline, Google Scholar
12. Schneider F, Weiss U, Kessler C, Salloum JB, Posse S, Grodd W, Muller-Gartner HW: Differential amygdala activation in schizophrenia during sadness. Schizophr Res 1998; 34:133–142Crossref, Medline, Google Scholar
13. Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, Williams SC, David AS: A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res 1999; 92:11–31Crossref, Medline, Google Scholar
14. Streit M, Ioannides A, Sinnemann T, Wölwer W, Dammers J, Zilles K, Gaebel W: Disturbed facial affect recognition in patients with schizophrenia associated with hypoactivity in distributed brain regions: a magnetoencephalographic study. Am J Psychiatry 2001; 158:1429–1436Link, Google Scholar
15. LeDoux J: Emotion, in Handbook of Physiology, vol 5. Edited by Plum R. Bethesda, Md, American Psychological Society, 1987, pp 419–460Google Scholar
16. Gazzaniga M (ed): The New Cognitive Neurosciences. Cambridge, Mass, MIT Press, 2000Google Scholar
17. LeDoux J: The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York, Simon & Schuster, 1996Google Scholar
18. Davidson RJ, Putnam KM, Larson CL: Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 2000; 289:591–594Crossref, Medline, Google Scholar
19. Paradiso S, Johnson DL, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry 1999; 156:1618–1629Link, Google Scholar
20. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
21. Perry PJ, Alexander B, Liskow BI: Psychotropic Drug Handbook. Washington, DC, American Psychiatric Association Press, 1997Google Scholar
22. Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M: Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch Gen Psychiatry 1995; 52:341–351Crossref, Medline, Google Scholar
23. Flaum M, O’Leary DS, Swayze VW II, Miller DD, Arndt S, Andreasen NC: Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. J Psychiatr Res 1995; 29:261–276Crossref, Medline, Google Scholar
24. Lang PJ, Ohman A, Vaitl D: The International Affective Picture System (photographic slides). Gainesville, University of Florida, 1988Google Scholar
25. Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR: Response and habituation of the human amygdala during visual processing of facial expression. Neuron 1996; 17:875–887Crossref, Medline, Google Scholar
26. Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, Hichwa RD: Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA 2001; 286:427–435Crossref, Medline, Google Scholar
27. Herscovitch P, Markham J, Raichle ME: Brain flow measured with intravenous H215O, I: theory and error analysis. J Nucl Med 1983; 24:782–789Medline, Google Scholar
28. Hichwa RD, Boles Ponto LL, Watkins GL: Clinical blood flow measurement with [15O] water and positron emission tomography (PET), in Chemists’ Views of Imaging Centers. Edited by Emran AM. New York, Plenum, 1995, pp 401–417Google Scholar
29. Hurtig RR, Hichwa RD, O’Leary DS, Boles Ponto LL, Narayana S, Watkins GL, Andreasen NC: Effects of timing and duration of cognitive activation in [15O]water PET studies. J Cereb Blood Flow Metab 1994; 14:423–430Crossref, Medline, Google Scholar
30. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
31. Levin DN, Pelizzari CA, Chen GTY, Chen CT, Cooper MD: Retrospective geometric correlations of MR, CT, and PET images. Radiology 1988; 169:817–823Crossref, Medline, Google Scholar
32. Arndt S, Cizadlo T, Andreasen NC, Heckel D, Gold S, O’Leary DS: Tests for comparing images based on randomization and permutation methods. J Cereb Blood Flow 1996; 16:1271–1279Crossref, Medline, Google Scholar
33. Worsley KJ, Evans AC, Marrett S, Neelin P: A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1992; 12:900–918Crossref, Medline, Google Scholar
34. Mesulam M, Mufson E: The insula of Reil in man and monkey: architectonic connectivity and function, in Cerebral Cortex. Edited by Peters A, Jones EG. New York, Plenum, 1985, pp 179–226Google Scholar
35. Iscoe I, Veldman D: Perception of an emotional continuum by schizophrenics, normal adults and children. J Clin Psychol 1963; 19:272–276Crossref, Medline, Google Scholar
36. Izard C: Paranoid schizophrenic and normal subjects’ perception of photography of human faces. J Consult Psychol 1959; 23:119–124Crossref, Medline, Google Scholar
37. Mandal MK, Palchoudhury S: Identifying the components of facial emotion and schizophrenia. Psychopathology 1989; 22:295–300Crossref, Medline, Google Scholar
38. Pillowski I, Bassett D: Schizophrenia and the response to facial emotions. Compr Psychiatry 1980; 21:236–244Crossref, Medline, Google Scholar
39. Schneider F, Gur RC, Gur RE, Shtasel DL: Emotional processing in schizophrenia: neurobehavioral probes in relation to psychopathology. Schizophr Res 1995; 17:67–75Crossref, Medline, Google Scholar
40. Streit M, Wolwer W, Gaebel W: Facial-affect recognition and visual scanning behaviour in the course of schizophrenia. Schizophr Res 1997; 24:311–317Crossref, Medline, Google Scholar
41. Walker E, Marwit SJ, Emory E: A cross-sectional study of emotion recognition in schizophrenics. J Abnorm Psychol 1980; 89:428–436Crossref, Medline, Google Scholar
42. Schneider F, Weiss U, Kessler C, Salloum JB, Posse S, Grodd W, Muller-Gartner HW: Differential amygdala activation in schizophrenia during sadness. Schizophr Res 1998; 34:133–142Crossref, Medline, Google Scholar
43. Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, Williams SC, David AS: A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res 1999; 92:11–31Crossref, Medline, Google Scholar
44. Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR: The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 2002; 17:317–323Crossref, Medline, Google Scholar
45. Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M: Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 2001; 4:437–441Crossref, Medline, Google Scholar
46. Whittaker JF, Deakin JF, Tomenson B: Face processing in schizophrenia: defining the deficit. Psychol Med 2001; 31:499–507Crossref, Medline, Google Scholar
47. Zald DH, Pardo JV: Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA 1997; 94:4119–4124Crossref, Medline, Google Scholar
48. Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA: Human amygdala activation detected with echo-planar functional magnetic resonance imaging. Neuroreport 1996; 7:1765–1769Crossref, Medline, Google Scholar
49. Morris JS, Ohman A, Dolan RJ: Conscious and unconscious emotional learning in the human amygdala. Nature 1998; 393:467–470Crossref, Medline, Google Scholar
50. Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA: Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 1998; 18:411–418Crossref, Medline, Google Scholar
51. Adolphs R, Tranel D, Damasio H, Damasio A: Fear and the human amygdala. J Neurosci 1995; 15:5879–5891Crossref, Medline, Google Scholar
52. Broks P, Young AW, Maratos EJ, Coffey PJ, Calder AJ, Isaac CL, Mayes AR, Hodges JR, Montaldi D, Cezayirli E, Roberts N, Hadley D: Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia 1998; 36:59–70Crossref, Medline, Google Scholar
53. Kalin NH, Shelton SE, Davidson RJ, Kelley AE: The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci 2001; 21:2067–2074Crossref, Medline, Google Scholar
54. Tulving E, Markowitsch HJ, Craik FIM, Habib R, Houle S: Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex 1996; 6:71–79Crossref, Medline, Google Scholar
55. Knight RT: Contribution of human hippocampal region to novelty detection. Nature 1996; 383:256–259Crossref, Medline, Google Scholar
56. Rolls ET: The orbitofrontal cortex. Phil Trans R Soc Lond B Biol Sci 1996; 351:1433–1443Crossref, Medline, Google Scholar
57. Paradiso S, Chemerinski E, Yazici KM, Tartaro A, Robinson RG: Frontal lobe syndrome reassessed: comparison of patients with lateral or medial frontal brain damage. J Neurol Neurosurg Psychiatry 1999; 67:664–667Crossref, Medline, Google Scholar
58. Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS: Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry 2001; 158:618–624Link, Google Scholar
59. Popken GJ, Bunney WE Jr, Potkin SG, Jones EG: Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA 2000; 97:9276–9280Crossref, Medline, Google Scholar
60. Danos P, Baumann B, Bernstein HG, Stauch R, Krell D, Falkai P, Bogerts B: The ventral lateral posterior nucleus of the thalamus in schizophrenia: a post-mortem study. Psychiatry Res 2002; 114:1–9Crossref, Medline, Google Scholar
61. Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L: Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 2002; 159:59–65Link, Google Scholar
62. Jensen JE, Al-Semaan YM, Williamson PC, Neufeld RW, Menon RS, Schaeffer B, Densmore M, Drost DJ: Region-specific changes in phospholipid metabolism in chronic, medicated schizophrenia: (31)P-MRS study at 4.0 Tesla. Br J Psychiatry 2002; 180:39–44Crossref, Medline, Google Scholar
63. Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL, Evans AC, Kahn RS: Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry 2001; 58:1118–1125Crossref, Medline, Google Scholar
64. Auer DP, Wilke M, Grabner A, Heidenreich JO, Bronisch T, Wetter TC: Reduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentation. Schizophr Res 2001; 52:87–99Crossref, Medline, Google Scholar
65. Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun L-S, Chen K: Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 1997; 154:918–925Link, Google Scholar
66. Paradiso S, Robinson RG, Andreasen NC, Downhill JE, Davidson RJ, Kirchner PT, Watkins GL, Boles Ponto LL, Hichwa RD: Emotional activation of limbic circuitry in elderly normal subjects in a PET study. Am J Psychiatry 1997; 154:384–389Link, Google Scholar
67. Schutter DJ, van Honk J, d’Alfonso AA, Peper JS, Panksepp j: High frequency repetitive transcranial magnetic over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: a pilot study in humans. Neurosci Letts 2003; 336:73–76Crossref, Medline, Google Scholar
68. Schmahmann JD: An emerging concept: the cerebellar contribution to higher function. Arch Neurol 1991; 48:1178–1187Crossref, Medline, Google Scholar
69. Schmahmann JD, Sherman JC: The cerebellar cognitive affective syndrome. Brain 1998; 121:561–579Crossref, Medline, Google Scholar
70. Deicken RF, Feiwell R, Schuff N, Soher B: Evidence for altered cerebellar vermis neuronal integrity in schizophrenia. Psychiatry Res 2001; 107:125–134Crossref, Medline, Google Scholar
71. Andreasen NC: A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry 1999; 56:781–787Crossref, Medline, Google Scholar
72. Meyer-Lindenberg A, Poline J-B, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF: Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 2001; 158:1809–1817Link, Google Scholar
73. Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC: Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry 2000; 57:761–768Crossref, Medline, Google Scholar
74. Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T: An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res 2001; 52:47–55Crossref, Medline, Google Scholar
75. Convit A, Wolf OT, de Leon MJ, Patalinjug M, Kandil E, Caraos C, Scherer A, Saint Louis LA, Cancro R: Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Res 2001; 107:61–73Crossref, Medline, Google Scholar
76. Fallgatter AJ: Electrophysiology of the prefrontal cortex in healthy controls and schizophrenic patients: a review. J Neural Transm 2001; 108:679–694Crossref, Medline, Google Scholar
77. McGlashan TH, Hoffman RE: Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry 2000; 57:637–648Crossref, Medline, Google Scholar
78. Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE: Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 2001; 50:825–844Crossref, Medline, Google Scholar
79. Knable MB, Weinberger DR: Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol 1997; 11:123–131Crossref, Medline, Google Scholar
80. Andreasen NC: Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science 1997; 275:1586–1593Crossref, Medline, Google Scholar
81. Lewis DA, Anderson SA: The functional architecture of the prefrontal cortex and schizophrenia. Psychol Med 1995; 25:887–894Crossref, Medline, Google Scholar
82. Friston KJ, Frith CD: Schizophrenia: a disconnection syndrome? Clin Neuroscience 1995; 3:89–97Medline, Google Scholar
83. Goldman-Rakic PS, Selemon LD: Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997; 23:437–458Crossref, Medline, Google Scholar
84. Dolan RJ, Fletcher PC, McKenna P, Friston KJ, Frith CD: Abnormal neural integration related to cognition in schizophrenia. Acta Psychiatr Scand Suppl 1999; 395:58–67Crossref, Medline, Google Scholar
85. Curtis VA, Bullmore ET, Brammer MJ, Wright IC, Williams SCR, Morris RG, Sharma TS, Murray RM, McGuire PK: Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 1998; 155:1056–1063Link, Google Scholar
86. Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS Jr, Faraone SV, Tsuang MT: Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry 1999; 56:537–547Crossref, Medline, Google Scholar
87. Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK: Mapping of grey matter changes in schizophrenia. Schizophr Res 1999; 35:1–14Crossref, Medline, Google Scholar
88. Mesulam MM, Mufson EJ: Insula of the old world monkey, I: architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol 1982; 212:1–22Crossref, Medline, Google Scholar
89. Kosslyn SM, Shin LM, Thompson WL, McNally RJ, Rauch SL, Pitman RK, Alpert NM: Neural effects of visualizing and perceiving aversive stimuli: a PET investigation. Neuroreport 1996; 7:1569–1576Crossref, Medline, Google Scholar
90. Casey KL: Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci USA 1999; 96:7668–7674Crossref, Medline, Google Scholar
91. Chua P, Krams M, Toni I, Passingham R, Dolan R: A functional anatomy of anticipatory anxiety. Neuroimage 1999; 9(part 1):563–571Google Scholar
92. Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675–682Abstract, Google Scholar
93. Crespo-Facorro B, Kim J, Andreasen NC, O’Leary DS, Bockholt HJ, Magnotta V: Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophr Res 2000; 46:35–43Crossref, Medline, Google Scholar
94. Crespo-Facorro B, Kim J, Andreasen NC, O’Leary DS, Bockholt HJ, Magnotta V: Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophr Res 2000; 46:35–43; correction, 2001; 51:183–184Google Scholar
95. Wiser AK, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Dysfunctional cortico-cerebellar circuits cause “cognitive dysmetria” in schizophrenia. Neuroreport 1998; 9:1895–1899Crossref, Medline, Google Scholar
96. Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Boles Ponto LL, Watkins GL, Hichwa RD: Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 1996; 93:9985–9990Crossref, Medline, Google Scholar



