Neuropsychological Deficits in Psychotic Versus Nonpsychotic Major Depression and No Mental Illness
Abstract
OBJECTIVE: At least three studies have indicated that patients with psychotic major depression studied under non-drug-free conditions differ from patients with nonpsychotic major depression and healthy comparison subjects on several measures of neuropsychological performance. The current study explored specific impairments in cognitive function in subjects with psychotic major depression, subjects with nonpsychotic major depression, and healthy comparison subjects studied under drug-free conditions.METHOD: A battery of neuropsychological tests was administered to 11 patients with psychotic major depression, 32 patients with nonpsychotic major depression, and 23 normal comparison subjects under drug-free conditions. The three groups did not differ statistically in age, sex, or level of education. To ensure that participants had minimal levels of severity and endogenicity, all patients were required to have a score of at least 20 on the 21-item Hamilton Depression Rating Scale and a score of at least 7 on the Core Endogenomorphic Scale, which uses eight items from the Hamilton depression scale.RESULTS: Patients with psychotic major depression demonstrated significantly greater impairment than patients with nonpsychotic major depression and/or comparison subjects in attention and response inhibition (as measured by the Stroop color-word subscale score) as well as in verbal declarative memory (as measured by the Paragraph Recall Test).CONCLUSIONS: These data indicate that patients with psychotic major depression demonstrate impairment in functions thought to be mediated by the frontal cortex and mediotemporal lobes.
Over the past decade, data have emerged to support psychotic major depression as a discrete disorder separate from nonpsychotic major depression (see reference 1 for review). Several years ago (2), we reported relationships between abnormalities in brain imaging and neuropsychological deficits in patients with psychotic major depression. More recently, two groups have reported that patients with psychotic major depression are similar to those with schizophrenia and that both differ from patients with nonpsychotic major depression on a number of cognitive measures.
Jeste et al. (3) reported that both patients with psychotic major depression and patients with schizophrenia demonstrated significantly poorer performance than did subjects with nonpsychotic major depression on tests in the following cognitive areas: learning (story learning), sequencing and psychomotor speed (Trail Making Test A and B and WAIS-R Digit Symbol), fine motor coordination (grooved pegboard, nondominant hand) and attention (digit vigilance). In contrast, memory retention was similar across the groups. Nelson et al. (4) reported that patients with psychotic major depression and patients with schizophrenia demonstrated significant impairment in attention as measured by the Continuous Performance Test, but patients with nonpsychotic major depression and normal comparison subjects did not. In both studies, differences between patients with psychotic major depression and patients with nonpsychotic major depression could not be attributed to differences in severity of depressive symptoms. Kim et al. (5) recently reported that elderly patients with psychotic major depression demonstrated significant impairment on the Wisconsin Card Sorting Test. These studies all point to substantial cognitive impairment in psychotic major depression. However, since virtually all previously studied patients had been medicated, it is unclear whether some of the cognitive deficits in psychotic patients could be attributable to possible effects of medication.
One major biological finding in psychotic major depression has been excessive hypothalamic-pituitary-adrenal (HPA) axis activity that is reflected in high levels of 24-hour urinary free cortisol (6), high rates of dexamethasone nonsuppression (7–11), and high postdexamethasone cortisol levels (8, 11). In patients with Cushing’s disease and in affectively ill patients, elevated cortisol activity has been reported to be associated with neuropsychological test impairment on a variety of tests by our group and others (2, 12–14). Repeated administration of glucocorticoids to healthy comparison subjects has been reported to result in impairment in verbal/declarative memory (14–16) and, recently, in executive function or working memory (17). These data suggest that hypercortisolemia could contribute to the cognitive deficits seen in some depressed patients.
In this paper, we present neuropsychological data on patients with psychotic major depression, patients with nonpsychotic major depression, and healthy comparison subjects studied under drug-free conditions. A psychometric test battery was administered to assess attention, memory, and psychomotor function. The current data point to difficulties in attention, response inhibition, and verbal declarative memory (both immediate and delayed) in patients with psychotic major depression and are consistent with the hypothesis that glucocorticoid excess may play a role in specific cognitive disturbances in depressed patients.
Method
Participants included 11 patients with psychotic major depression, 32 with nonpsychotic major depression, and 23 healthy comparison subjects. Patients met the following inclusion criteria: diagnosis of major depression, a baseline total score of 20 or higher on the 21-item Hamilton Depression Rating Scale, and a score of at least 7 on the Core Endogenomorphic Scale (18), which uses eight items of the 21-item Hamilton depression scale. The latter two criteria were designed to ensure inclusion of patients with psychotic major depression and patients with nonpsychotic major depression with similar minimum levels of endogenous-type symptoms. In addition, all patients were medically healthy and had been drug-free for at least 2 weeks, had received no fluoxetine or depot neuroleptics for at least 6 weeks, had no drug or alcohol abuse for 1 year, and were able to provide informed consent.
This study was approved by the institutional review boards of Stanford University Medical Center, Brigham and Women’s Hospital, and Washington University Medical Center. Written informed consent was obtained from each subject after all procedures had been fully explained. Diagnoses were ascertained by using the Structured Clinical Interview for DSM-III-R (19) as well as questions on psychosis from the Schedule for Affective Disorders and Schizophrenia (20).
Healthy comparison subjects had no history of axis I or axis II psychiatric disorder, had been taking no medications for at least 2 weeks, and had not used alcohol for at least 7 days. All subjects were English speaking, and none was color-blind.
Before admission to the general clinical research centers for neuroendocrine studies at the respective centers, a neuropsychological battery was administered at 11:00 a.m. The battery included measures of word knowledge (Vocabulary subtest of the WAIS-R [21]); attention response inhibition (Stroop Color and Word Test [22]); attention and motor speed (Trail Making Test A [23]); sequencing (Trail Making Test B [23]); psychomotor speed (Digit Symbol subtest of the WAIS-R and Ms and Ns); visuospatial construction (Block Design subtest of the WAIS-R); verbal memory (Paragraph Recall Test [15]); and visual memory (Visual Reproduction subtest of the Wechsler Memory Scale—Revised [24]). The test battery was administered at 11:00 a.m. because that time was 2–3 hours after eating and preceded admission for the HPA axis protocol.
At 6:00 p.m. the following day, the Paragraph Recall Test was readministered. The Paragraph Recall Test was performed at this time to allow for assessment of the acute effects of hydrocortisone or ovine-corticotropin-releasing hormone administered at 7:00 p.m. These results will be presented at a later time. In this test, an audiotaped paragraph-long story is presented to the subject, who is asked to immediately repeat the content as exactly as possible. A second paragraph is presented under the same conditions immediately thereafter, and the subject is again asked to repeat the content of this paragraph. After a 25-minute delay, subjects are again asked to retell the paragraphs in the same sequence. However, subjects had not been initially informed that they would be tested 25 minutes later. The responses were tape-recorded and scored for exact bits of information reported as well as errors of both contamination (i.e., reporting story content from other previously presented paragraphs) and confabulation (i.e., reporting story content that had never been presented). Paragraphs were selected on the basis of their neutral affect.
Data Analysis
Test results on the Trail Making Test A and B, WAIS-R subtests, Stroop Color and Word Test, and Visual Reproduction subtest from the Wechsler Memory Scale—Revised were corrected for age on the basis of published norms before data analysis. All analyses were performed with SPSS software (25). Analyses of variance (ANOVAs) were used to examine the main effects of group membership on neuropsychological test scores. Where questions remained regarding the effects of age and/or sex, analyses of covariance were used to confirm the results. For confabulations on the Paragraph Recall Test, where many subjects showed no confabulations and the data were not normally distributed, a nonparametric Kruskal-Wallis test was also applied.
Results
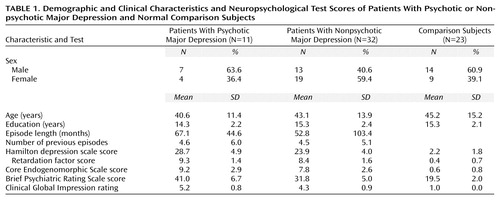
The subjects’ demographic data and Hamilton depression scale scores are presented in Table 1. There were no significant group differences in mean age, sex distribution, or level of education. The total Hamilton depression scale, Brief Psychiatric Rating Scale, and Clinical Global Impression scores were all higher in the patients with psychotic major depression than in those with nonpsychotic major depression, who, in turn, had higher scores than healthy comparison subjects. The comparison subjects had lower Hamilton depression scale retardation factor and Core Endogenomorphic Scale scores than patients with nonpsychotic and psychotic major depression, who did not differ from each other. There were no significant patient group differences in duration of present episode or number of previous episodes of major depression.
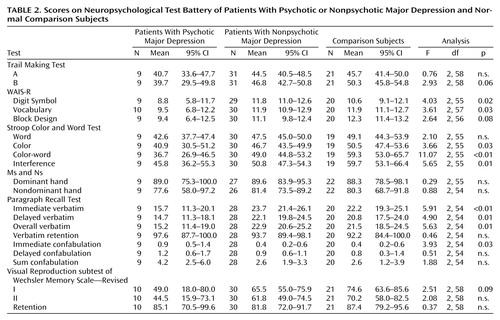
In this study, we wished to test the primary hypothesis that patients with psychotic major depression demonstrate significant impairment on tests of attention and response inhibition (Stroop Color and Word Test) and verbal declarative memory (Paragraph Recall Test). The Stroop Color and Word Test data indicate that attention and response inhibition were impaired in the patients with psychotic major depression. The groups did not differ on the Stroop word subscale (Table 2 and Table 3), where subjects are asked to read color nouns (red, green, or blue), all of which are printed in black ink. On the second component, patients with psychotic major depression performed significantly more poorly than the comparison group on the Stroop color naming portion (where the subject is asked to name the color ink in which the letter x is printed as quickly as possible). This requires attention and speedy responding. More importantly, the groups also differed on the next component, the Stroop color-word subscale, which is an index of the degree to which an overlearned verbal response (reading a word) interferes with the ability to name the color of the ink in which a word is printed. On this measure, which controls for the speed of response on the color trial, the patients with psychotic major depression were again particularly impaired.
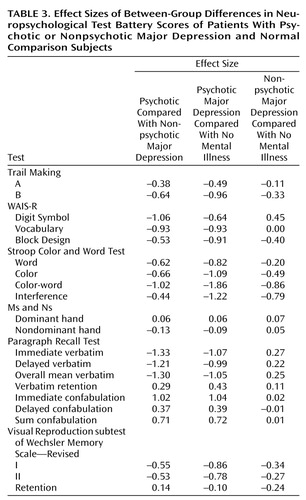
Effect sizes for patients with psychotic compared with nonpsychotic major depression and for patients with psychotic major depression compared with normal subjects were both greater than 1.00 (Table 3). Although the patients with nonpsychotic major depression did significantly worse than the comparison subjects, the mean score for patients with nonpsychotic major depression was close to the expected norm.
We also calculated interference scores, which represent a composite ratio of the Stroop Color and Word Test components and have been used to determine relative “freedom from distractibility.” The patients with nonpsychotic major depression achieved interference scores that were in the average range relative to normative data for this subscore. However, the patients with psychotic major depression and the patients with nonpsychotic major depression both demonstrated significantly lower scores than did comparison subjects. The score for patients with nonpsychotic major depression, again, was at the average normative value.
On the Paragraph Recall Test, patients with psychotic major depression recalled significantly fewer verbatim bits of information than did the other groups, under both the immediate and delayed recall conditions, as well as significantly more confabulations in the immediate recall condition. (These findings endured in separate analyses that covaried for education.) Effect sizes for the comparison of patients with psychotic major depression with each of the other groups on these measures were high (each greater than 1.00) (Table 3). Nonparametric analyses of confabulation revealed similar effects. These data suggest that patients with psychotic major depression have deficient memory for semantically organized information that is independent of their attentional deficit. The groups did not differ on an index of the percentage of learned information that they retained across the delay interval. Together, the data indicate that the patients with psychotic major depression are inefficient at learning new information but are able to retain learned material normally.
Secondary analyses were performed on the other measures. As indicated in Table 2, the three groups did not differ on the Trail Making Test A, a measure of psychomotor speed. On the Trail Making Test B, which combines speed and sequencing (alternating between numbers and letters), patients with psychotic major depression performed more poorly than did the other two groups, with moderate to large effect sizes. Patients with psychotic major depression performed significantly more poorly on the WAIS-R Digit Symbol subtest than patients with nonpsychotic major depression and comparison subjects, although their mean age-corrected score of 8.8 still fell within the low average range (normative sample mean=10, SD=3).
In terms of psychomotor skills, there were no significant group differences on the Ms and Ns test. On the Visual Reproduction subtest of the Wechsler Memory Scale—Revised, differences in both the immediate and delayed conditions were not significant. Again, scores for patients with psychotic major depression were close to the expected norm. On the WAIS-R Vocabulary subtest, patients with psychotic major depression performed more poorly than did the other two groups (F=3.61, df=2, 63, p=0.03); however, after covarying for years of education, this difference did not remain significant (p=0.10). The mean score of 9.50 of the patients with psychotic major depression was well within the average clinical range but was lower than the mean scores of the other two groups, whose performance was above average.
We examined effect sizes for comparisons of patients with nonpsychotic major depression and healthy comparison subjects. Other than the Stroop color-word and interference subscale scores (effect sizes=–0.86 and –0.79, respectively), effect sizes were generally below 0.40 (Table 3). On the Stroop color-word subscale, patients with nonpsychotic major depression scored at the expected normative range, but our comparison subjects scored well above average (Table 2).
Discussion
These data suggest that patients with psychotic major depression demonstrate significant impairment in attention and response inhibition and in verbal declarative memory. The low number of bits of information recalled by the patients with psychotic major depression could be due to an attentional problem. The score on another possible measure of aspects of attention (the WAIS-R Digit Symbol) was also somewhat low in patients with psychotic major depression. These attentional and memory deficits appear to separate patients with psychotic major depression from those with nonpsychotic major depression and from normal comparison subjects and suggest impairment in prefrontal and hippocampal function. Of interest is that retention appears intact in patients with psychotic major depression, as does visuospatial memory.
In this study, patients with psychotic and nonpsychotic major depression were required to have minimal scores on the Core Endogenomorphic Scale to ensure comparable endogenous status. Our findings thus support previous reports that differences between psychotic and nonpsychotic major depression in neuropsychological testing are not due to a greater severity of depression in psychotic major depression. To further assess the possible effects of motivation, interest, and concentration, differences between patients with psychotic or nonpsychotic major depression on the Stroop color-word subscale and Paragraph Recall Test were assessed covarying for scores on the retardation factor of the Hamilton depression scale. Significant differences were still observed on both tests, indicating that differences between groups were not due to differences in psychomotor retardation, concentration, and/or interest level.
Several factors could contribute to the immediate and delayed verbal memory deficits of the patients with psychotic major depression, including attention, semantic analysis, verbal encoding, and verbal retrieval. Two studies have identified sustained attentional deficits in patients with psychotic major depression as measured by Continuous Performance Test (3, 4). We are aware of no published studies on semantic analysis functions in psychotic major depression, although many studies have reported verbal retrieval deficits in patients with lesions in the prefrontal cortex (26–28). These studies suggest that verbal retrieval and semantic analysis deficits originating in prefrontal circuits may underlie our results in patients with psychotic major depression and that both factors should be assessed explicitly in subsequent studies on cognitive deficits in psychotic major depression.
It is unclear from this study whether patients with psychotic major depression may also suffer from verbal encoding deficits in the hippocampal formation, which are distinct from semantic processing and retrieval operations in the prefrontal cortex. However, our data do suggest that patients with psychotic major depression are normal in nonverbal encoding and retrieval, as measured by the Wechsler Memory Scale—Revised.
As indicated, excessive HPA axis activity has been reported in many studies of psychotic major depression. Glucocorticoids bind to receptors in various regions of the brain, and these receptors are well represented in both the frontal cortex and hippocampus. Recently, our group reported that administration of cortisol to squirrel monkeys for 28 days results in impairment in object retrieval (29)—a test of prefrontal cortical functions, including response inhibition. Impaired performance on this test has been reported in nonhuman primates by the administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, which destroys dopamine neurons, and by phencyclidine, which decreases dopamine utilization in the prefrontal cortex (30, 31). Our group has also reported that 7-day administration of corticosterone to rats results in a decrease in dopamine turnover in the medial prefrontal cortex (32). These data suggest that the attentional response inhibition deficit in psychotic major depression may be caused by glucocorticoid-induced decreases in dopamine function prefrontally. Of note is a recent report by Kim et al. (5) of prefrontal cortical atrophy on magnetic resonance imaging in patients with psychotic major depression compared with patients with nonpsychotic major depression.
Glucocorticoids have also been reported to result in impairment in declarative-type memory. Wolkowitz et al. (14) have reported that prednisone administered to control subjects over 7 days results in increases in errors of commission on the Wallach Memory Test. In this test, subjects are first shown a list of words and, after a delay, a second, larger list that includes all words from the first list as well as a set of new words. They are then asked to identify as many words as possible from the first list. Prednisone administration results in misidentifying words in the second list as having come from the first, so-called errors of commission. Similarly, Newcomer et al. (15, 16) reported that low doses of dexamethasone or high doses of hydrocortisone administration for 4 consecutive days results in impairment on the Paragraph Recall Test, as evidenced by reduced accurate bits of recall and increased total errors. They argued that administration of exogenous glucocorticoids results in impairment of hippocampal function, particularly in the encoding of information. Thus, the poorer performance on the Paragraph Recall Test we observed in psychotic major depression could reflect an effect of excessive cortisol activity on encoding.
We did not test whether cueing might help performance on the Paragraph Recall Test. In our study, patients with psychotic major depression made more confabulatory errors in the immediate condition than did the other two groups, and these errors appear similar to the errors of commission seen in the study of Wolkowitz et al. (14). It is possible that aspects of attention may have accounted for some of the poor performance on the Paragraph Recall Test. As indicated, Jetter et al. (26) reported that frontal lobe damage could be associated with poor performance on the Paragraph Recall Test. In addition, Lupien et al. (17) reported that high doses of hydrocortisone administered over several days to healthy subjects resulted in impairment in working but not verbal memory. Thus, differences in performance on the Paragraph Recall Test between subjects with psychotic and nonpsychotic major depression in our study could in part reflect problems in attention to semantic material. Our psychotic major depression data, therefore, could be interpreted as being consistent with functional impairment in prefrontal cortical and mediotemporal hippocampal tasks. We are following up on these observations using functional imaging in conjunction with specific neuropsychological tests.
The findings of Jeste et al. (3) and Nelson et al. (4) indicate that the performance of patients with psychotic major depression is similar to that of patients with schizophrenia on a variety of neuropsychological tests. Further studies are needed to determine whether the neuropsychological deficits and the neurochemical causes are indeed similar in the two disorders. Moreover, for patients with psychotic major depression, further research is required to determine if these deficits persist even after remission.
There are several strengths to this study, including particularly well-characterized patients and comparison subjects studied under drug-free conditions. There are, however, some limitations. For one, data on a larger number of subjects are required to determine if our results are representative. Moreover, many of the patients in this study were chronically ill; further research on more acutely ill subjects is also needed. Such studies are under way in our laboratory.
 |
 |
 |
Received Jan. 20, 1999; revision received Nov. 29, 1999; accepted Feb. 14, 2000. From Stanford University; Harvard Medical School (Brigham and Women’s Hospital), Boston; and Washington University, St. Louis, Mo. Address reprint requests to Dr. Schatzberg, Stanford University Medical Center, Department of Psychiatry, Stanford, CA 94305-5717; [email protected] (e-mail). Supported by NIMH grant MH-50604 and by National Institutes of Health grants RR-00070, RR-00036, and RR-02635. The authors thank John Newcomer, M.D., for this assistance on the Paragraph Recall Test and Thomas Jordan, Ph.D., for his helpful comments.
1. Schatzberg AF, Rothschild AJ: Psychotic (delusional) major depression: should it be included as a distinct syndrome in DSM-IV? Am J Psychiatry 1992; 149:733–745Google Scholar
2. Rothschild AJ, Benes F, Hebben N, Wood B, Lucianna M, Bakanas E, Samson JA, Schatzberg AF: Relationships between brain CT scan findings and cortisol in psychotic and nonpsychotic depressed patients. Biol Psychiatry 1989; 26:565–575Crossref, Medline, Google Scholar
3. Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris MJ, Heaton RK: Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry 1996; 153:490–496Link, Google Scholar
4. Nelson EB, Sax KW, Strakowski SM: Attentional performance in patients with psychotic and nonpsychotic major depression and schizophrenia. Am J Psychiatry 1998; 155:137–139Link, Google Scholar
5. Kim DK, Kim BL, Sohn SE, Lim S-W, Na DG, Paik CH, Krishnan RR, Carroll BJ: Candidate neuroanatomic substrates of psychosis in old-aged depression. Prog Neuropsychopharmacol 1999; 23:793–807Crossref, Google Scholar
6. Anton RF: Urinary free cortisol in psychotic depression. Biol Psychiatry 1987; 2:24–34Crossref, Google Scholar
7. Mendlewicz J, Charles G, Franckson JM: The dexamethasone suppression test in affective disorder: relationship to clinical and genetic subgroups. Br J Psychiatry 1982; 141:464–470Crossref, Medline, Google Scholar
8. Rothschild AJ, Schatzberg AF, Rosenbaum AH, Stahl JB, Cole JO: The dexamethasone suppression test as a discriminator among subtypes of psychotic patients. Br J Psychiatry 1982; 141:471–474Crossref, Medline, Google Scholar
9. Rudorfer MV, Hwu H-G, Clayton PJ: Dexamethasone suppression test in primary depression: significance of family history and psychosis. Biol Psychiatry 1982; 17:41–48Medline, Google Scholar
10. Evans DL, Burnett GB, Nemeroff CB: The dexamethasone suppression test in the clinical setting. Am J Psychiatry 1983; 140:586–589Link, Google Scholar
11. Schatzberg AF, Rothschild AJ, Stahl JB, Bond TC, Rosenbaum AH, Lofgren SB, MacLaughlin RA, Sullivan MA, Cole JO: The dexamethasone suppression test: identification of subtypes of depression. Am J Psychiatry 1983; 140:88–91Link, Google Scholar
12. Starkman MN, Schteingart OE: Neuropsychiatric manifestations of patients with Cushing’s syndrome: relationship to cortisol and adrenocorticotropic hormone levels. Arch Intern Med 1981; 141:215–219Crossref, Medline, Google Scholar
13. Rubinow DR, Post RM, Savard R, Gold PW: Cortisol hypersecretion and cognitive impairment in depression. Arch Gen Psychiatry 1984; 41:279–283Crossref, Medline, Google Scholar
14. Wolkowitz OM, Reus VI, Weingartner H, Thompson K, Breier A, Doran A, Rubinow D, Pickar D: Cognitive effects of corticosteroids. Am J Psychiatry 1990; 147:1297–1303Google Scholar
15. Newcomer JW, Craft S, Hershey T, Askins K, Mardgett ME: Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci 1994; 14:2047–2053Google Scholar
16. Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Alderson AL: Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry 1999; 56:527–533Crossref, Medline, Google Scholar
17. Lupien SJ, Gillin C, Hauger RL: The forgotten one: prefrontal cortex modulation of the acute effects of corticosteroids on human cognition, in Abstracts of the 24th Annual Meeting of the International Society of Psychoneuroendocrinology. Trier, Germany, ISPNE, 1998, p. 9Google Scholar
18. Thase ME, Hersen M, Bellack AS, Himmelhoch JM, Kupfer DJ: Validation of a Hamilton subscale for endogenomorphic depression. J Affect Disord 1983; 5:267–278Crossref, Medline, Google Scholar
19. Spitzer RL, Williams JBW, Gibbon M, First MB: User’s Guide for the Structured Clinical Interview for DSM-III-R (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
20. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia (SADS), 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1979Google Scholar
21. Wechsler D: WAIS-R Manual. New York, Psychological Corp, 1981Google Scholar
22. Golden CJ: Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Wood Dale, Ill, Stoelting Co, 1978Google Scholar
23. Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd ed. Tucson, Ariz, Neuropsychology Press, 1993Google Scholar
24. Wechsler D: Wechsler Memory Scale—Revised Manual. San Antonio, Tex, Psychological Corp, 1987Google Scholar
25. Norusis JJ: SPSS for Windows, Release 7.5.2. Chicago, SPSS, 1997Google Scholar
26. Jetter W, Poser U, Freeman RB Jr, Markowitsch HJ: A verbal long term memory deficit in frontal lobe damaged patients. Cortex 1986; 22:229–242Crossref, Medline, Google Scholar
27. Incisa della Rocchetta A, Milner B: Strategic search and retrieval inhibition: the role of the frontal lobes. Neuropsychologia 1993; 31:503–524Crossref, Medline, Google Scholar
28. Pillon B, Deweer B, Michon A, Malapani C, Agid Y, Dubois B: Are explicit memory disorders of progressive supranuclear palsy related to damage to striatofrontal circuits? comparison with Alzheimer’s, Parkinson’s, and Huntington’s diseases. Neurology 1994; 44:1264–1270Google Scholar
29. Lyons DM, Lopez J, Lindley SE, Schatzberg AF: Corticosteroids, catecholamines, and cognitive control on an object retrieval task. Society for Neuroscience Abstracts 1998; 24:682Google Scholar
30. Taylor JR, Elsworth JD, Roth RH, Collier TJ, Sladek JR Jr, Redmond DE Jr: Improvements in MPTP-induced object retrieval deficits and behavioral deficits after fetal nigral grafting in monkeys. Prog Brain Res 1990; 82:543–559Crossref, Medline, Google Scholar
31. Jentsch JD, Redmond DE Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH: Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 1997; 277:953–955Crossref, Medline, Google Scholar
32. Lindley SE, Bengoeschea TG, Schatzberg AF, Wong DL: Glucocorticoid effects on mesotelencephalic dopamine neurotransmission. Neuropsychopharmacology 1999; 21:399–407Crossref, Medline, Google Scholar



