Sex-Specific Expression of Heschl's Gyrus Functional and Structural Abnormalities in Paranoid Schizophrenia
Abstract
OBJECTIVE: Evidence supports abnormal temporal lobe structure and function in schizophrenia. Some abnormalities, particularly involving the auditory cortex, appear to be sex specific. These findings were extended to anatomical and physiological descriptors. METHOD: The authors quantified the volume, surface area, and three-dimensional location of Heschl's gyri on magnetic resonance imaging (MRI) images of 21 patients with paranoid schizophrenia and 24 healthy comparison subjects. Neuromagnetic localizations of the 100-msec latency auditory evoked field (M100) were compared with MRI-determined locations of Heschl's gyri, computed as the geometric center of mass of the volume. RESULTS: Volumetric measures revealed small Heschl's gyri only in male patients. Asymmetry was found in the location of the Heschl's gyrus centroid (more anterior on the right) across all groups. Male comparison subjects had M100 locations posterior to the Heschl's gyrus centroid in the left hemisphere and close to the Heschl's gyrus centroid on the right, while male patients had M100 sources anterior to the Heschl's gyrus centroid on the left. All women had M100 locations posterior to the Heschl's gyrus centroid on the left and anterior to it on the right. CONCLUSIONS: These results demonstrate that some temporal lobe abnormalities in schizophrenia are sex specific. They also suggest that the anomalous lateralization of the auditory evoked field cannot be explained by a shift in the underlying anatomy, since the anatomical substrate is lateralized in both comparison subjects and patients of both sexes. These findings may indicate a sex-specific functional reorganization in the auditory cortex in schizophrenia. (Am J Psychiatry 1997; 154:1655–1662)
The 100-msec latency auditory evoked field component, termed M100, has shown sex-specific anomalous asymmetry in actively psychotic patients with paranoid schizophrenia (1). In normal adults the neuroanatomical source of the M100 component is further anterior in the right hemisphere, but this asymmetry is less pronounced in women than in men (2–4). Actively psychotic men with DSM-III-R paranoid schizophrenia demonstrate less asymmetry (source appears both relatively more anterior in the left hemisphere and less anterior in the right hemisphere) (5, 6), whereas actively psychotic women appear to demonstrate greater asymmetry (source is further anterior in the right hemisphere) (1). The M100 component appears to be generated on or near the transverse gyrus of Heschl on the superior temporal gyrus (7–10), with possible contributions from auditory association areas surrounding Heschl's gyrus (11, 12). The M100 is considered by some a physiological index of echoic, or auditory, sensory memory (11, 13, 14).
A logical question, then, is does the anomalous asymmetry of M100 in schizophrenia reflect abnormal placement of Heschl's gyrus on the superior temporal gyrus, indicate abnormal placement of the superior temporal gyrus within the brain, or rather, suggest that cortical regions other than Heschl's gyrus produce this auditory evoked field component in patients with schizophrenia? Sensory cortical reorganization in schizophrenia has yet to be empirically demonstrated, although substantial evidence supports intracortical cytoarchitectural abnormalities (15–19). This question, as well as the prominence of auditory-system-related symptoms (i.e., hallucinations) in schizophrenia and the central relationship of Heschl's gyrus to the auditory system, led us to the present study.
This study was undertaken to clarify what abnormalities, if any, are present with respect to the volume, surface area, and location of Heschl's gyri in persons with schizophrenia and also to directly compare the anatomical findings with the functional information provided by magnetoencephalographic source localization of the M100 component. We therefore examined the location of Heschl's gyrus on the superior temporal gyrus and in relation to the whole brain in patients with schizophrenia. We also determined the superior temporal gyrus's position within the brains of the subjects, and we examined the location of the M100 generator source in relation to the aforementioned structures. We hypothesized that the sex-specific abnormalities in physiological function reflected in the M100 of patients with schizophrenia would not be reflected in the M100's anatomical substrate, as a preliminary test of our theoretical position concerning functional reorganization of the auditory cortex in schizophrenia (1).
METHOD
Subjects
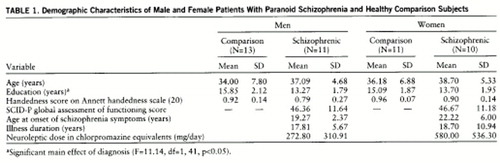
Forty-five subjects participated in this study. Twenty-one were diagnosed with paranoid schizophrenia (10 women), and 24 nonschizophrenic persons served as comparison subjects (11 women). All subjects were strongly right-handed; i.e., they had scores of 0.50 or higher on the Annett handedness inventory (20). Demographic data on the comparison and patient groups are provided in table 1.
The patients were volunteers recruited either from outpatient clinics in the Denver metropolitan area or from the Psychiatry Service at the Department of Veterans Affairs Medical Center in Denver. Patient diagnosis was made by DSM-III-R criteria on the basis of standardized coding of a Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P) (21) performed by one of us (M.R.) and a review of medical records. Each patient had a history of neuroleptic medication, and 18 were medicated at the time of the study. For the patients (16 of 18) taking medications for which phenothiazine equivalency has been established (22, 23), the mean chlorpromazine-equivalent dose was 360.60 mg/day (SD=392.47). The patients with schizophrenia were all actively psychotic, i.e., they had had active hallucinatory activity at least within the past month. The nonschizophrenic comparison subjects had never been mentally ill according to the Research Diagnostic Criteria (24). All subjects were given a complete description of the study, after which they signed informed consent statements. The project was approved by the Colorado Multiple Institutional Review Board human subjects committee.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was performed at the University of Colorado hospital by using a GE Signa 1.5-T system. Images of the head were acquired with a standard head coil by using a spoiled-gradient echo acquisition. This resulted in 1.7-mm-thick T1-weighted images with a TR of 40 msec, a TE of 5 msec, and a 40° flip angle. The 256×256 matrix acquisition resulted in 0.94×0.94×1.7-mm voxel dimensions. The entire head was encompassed within 124 slices. The MRI images were then transferred to a personal computer and stored on optical disks for further analyses.
Image Reconstruction and Definitions of Regions of Interest
With custom image-processing software routines designed in Interactive Data Language 3.1.1 (Research Systems, Boulder, Colo.), each set of brain images was segmented by an experienced investigator (J. Sheeder) who was blind to the diagnosis and sex of the subject. Segmentation was accomplished by using a combination of automated pixel thresholding and manual segmentation. From the segmented brain images, total brain volume was calculated. This volume-computation algorithm has been tested on regular and irregular phantoms of known volumes and found to be accurate to within ±1%. Manual segmentation and volume computations (by M.R., who was also blind to sex and diagnosis) were also performed for the superior temporal gyrus and Heschl's gyrus for both hemispheres of each brain. The superior temporal gyrus included all clearly visible slices of the superior temporal gyrus anterior to the fornix (inclusive). Total brain and superior temporal gyrus volumes for 40 of these subjects have been reported elsewhere (1) and are used in this report only for geometric comparisons with Heschl's gyri. We are unaware of any published volumetric criteria concerning Heschl's gyrus data and therefore present the method used in this project in more detail.
Heschl's gyrus traverses an anterior-lateral to posterior-medial course on the superior surface of the superior temporal gyrus. Its most anterior anterior-lateral boundary is indistinct and may fuse with the anterior superior temporal gyrus (4). Its posterior-medial course and junction with the insula are distinct. Scanning the entire coronal series, we first identified Heschl's gyrus at its prominent midsuperior temporal gyrus position, where its medial and lateral boundaries are unequivocal. We next moved anteriorly until the white matter stem, and the associated gray matter accentuation, merged indistinguishably with the anterior superior temporal gyrus. This defined the most anterior slice. We then moved posteriorly until Heschl's gyrus gray matter was no longer distinct from the insular origin at the circular sulcus. The slice anterior to this defined the posterior boundary of Heschl's gyrus. We have found that surface renderings of the superior temporal gyrus viewed from the superior perspective can be helpful in identifying useful landmarks for segmenting Heschl's gyrus images. Reconstructions of the superior temporal gyrus are adequate for this purpose. They allow for the identification of the anterior and posterior transverse temporal sulci (Heschl's sulcus), which are useful in conjunction with the segmentation criteria described.
It has been reported that many superior temporal gyri appear to contain two (or more) Heschl's gyri, on the basis of the dorsal surface view of the superior temporal gyrus. Galaburda and Sanides (25) suggested that only the most anterior transverse gyrus should be considered Heschl's gyrus, and any more-posterior transverse gyrus should be considered part of the temporal planum. This is supported by a study by Rademacher et al. (26), who reported that in cases where two Heschl's gyri were present, auditory koniocortex (area 41 of Brodmann [27]) was restricted to the more anterior gyrus. We also found additional transverse gyri, but in no case (in 96 hemispheres) did we find a second transverse gyrus extending the full anterior-lateral to posterior-medial extent of the superior temporal gyrus. There were cases in which a second (posterior) transverse gyrus merged with Heschl's gyrus (defined as described in the preceding) before the Heschl's gyrus merged with the insula. In those cases we included the volume of the second (posterior) transverse gyrus at the point where the two gyri merged to include a single common white matter stem, before it merged with the insula. The portion of the second (posterior) transverse gyrus lateral to the anterior transverse gyrus that was supported by a separate white matter stem was not included in the volume. Our segmented volumes for the Heschl's gyrus included both the white matter stem and gray matter of the gyrus.
To provide a basis for comparison with previous reports (e.g., Kulynych et al. [28]), surface areas were also determined for each of the Heschl's gyri. These were obtained by calculating the length of a line (in millimeters) traced on the interface of the gray matter and fluid of the gyrus in each coronal image, then multiplying the result by the slice thickness.
Reliability of MRI Measurements
The reliability of the measurements of total brain volume has been discussed in another paper (1). To determine the reliability of the measurements of Heschl's gyrus and surface area, 10 participants were randomly selected from the original 45, for a total of 20 Heschl's gyri for reliability analyses. The images of all 20 gyri were segmented by the first author (D.C.R.), who then calculated Heschl's gyrus volume and surface area as already described. Interrater reliability was determined by comparing these measurements with the volumetric measurements and surface area measurements produced by the last author (M.R.); the intraclass correlation coefficients for Heschl's gyrus volume and surface area were ICC=0.90 and ICC=0.72, respectively. Each volume was then redetermined by the first author 1 week from the original segmentation; the estimates of intrarater reliability were ICC=0.93 and ICC=0.94 for Heschl's gyrus volume and surface area, respectively.
Centroid Measurements
We also wished to determine the absolute three-dimensional location of Heschl's gyrus within the brain to permit direct comparison of the M100 source generator location and its gross anatomical substrate. We therefore calculated the centroid (geometric center of mass, with the assumption of uniform pixel density) of each slice in the regions of interest (total brain, Heschl's gyrus, and superior temporal gyrus) to assess the location of the region of interest within the skeletally based coordinate system. This required coregistration of magnetoencephalographic and MRI coordinate systems. For this purpose, before MRI was performed vitamin A capsules were attached to the nasion and two preauricular points. The skin-capsule interface points are small with respect to the capsule dimensions and are easily identified visually in the MRI. The MRI coordinates of the centroid units were converted into skeletal coordinates by a standard transformation (29). A geometric summation of the centroids of individual two-dimensional slices provided a single centroid measure for each Heschl's gyrus and each superior temporal gyrus in each hemisphere, as well as the geometric center of mass for the entire brain. Figure 1 illustrates the skeletally based coordinate system and resulting surface reconstructions of the superior temporal gyrus, Heschl's gyrus, and centroids for four representative subjects.
Neuromagnetic Recordings and M100 Source Localization
Data on M100 lateralization from 39 of the participants have been reported elsewhere (1) and will not be discussed in this paper. Data for six additional subjects were recorded for this project. Details of the recording procedures and equipment have been reported in several previous publications (1, 4, 10). Briefly, we recorded neuromagnetic activity from each hemisphere in response to 1-kHz tone pips, at a 90-dB sound pressure level, delivered to the contralateral ear with a random interstimulus interval between 1.2 and 1.8 sec. Recordings were obtained from an area sufficient to encompass both outgoing and ingoing magnetic extrema (approximately 35 locations per hemisphere). Auditory evoked field signals within a 20-msec poststimulus time window around the M100 peak latency (identified as the largest signal between 60 and 120 msec poststimulus) were used in an inverse solution algorithm (10) to estimate M100 source locations with reference to the skeletal coordinate system. M100 data for two subjects (one male comparison subject and one male patient) were excluded from further analyses because the equivalent current dipole locations were viewed as anatomically unreasonable (equivalent current dipole location ±95% spherical confidence region of 7-mm radius was not contained on the superior temporal gyrus), possibly because of digitization error or excessive noise in the magnetoencephalographic signals.
For both hemispheres in each subject, a set of x, y, and z difference vectors (X, Y, and Z) was computed as the distance from the three-dimensional location of the centroid of Heschl's gyrus to the three-dimensional M100 source location (e.g., Y=yHeschl's–yM100). These vectors provide measurements of the in-plane directional distance between functional source activity (the M100) and the putative neuroanatomical generator (Heschl's gyrus). We also computed the straight-line distance between the two points by taking the square root of the sums of the squares of the three vectors. We realize that there is no a priori reason to expect the M100 source to be absolutely co-located with the centroid of Heschl's gyrus, although we assume that the tonotopic representation of a 1-kHz sound on the auditory cortex has a regular, if yet undefined, relationship to the center of Heschl's gyrus. Therefore, the difference vectors provide only the distance from the center of the gyrus that represents the anatomical correlate of the primary auditory cortex to the source of neurophysiological activity putatively generated in that cytoarchitectural region. The same set of vector computations was also performed to ascertain the relationship between Heschl's gyrus and the superior temporal gyrus and the relationship of the superior temporal gyrus to the entire brain.
Statistical Analyses
Statistical analyses were performed by using Statistica 5.0 (StatSoft, Tulsa, Okla.). All analyses were two-tailed and evaluated for significance at the 0.05 alpha level. Univariate analysis of variance (ANOVA) sums of squares were computed with controls for partial correlations with other effects in the design (i.e., type III sums of squares).
To test for possible differences in demographic variables of interest, 2×2 ANOVAs (sex by diagnosis) were performed for handedness, education level, and age. Independent t tests of differences between male and female patients were performed for age at onset, illness duration, global assessment of functioning score from the SCID-P, and phenothiazine-equivalent doses. Subsequently, Pearson coefficients (r) were computed for the correlations between any demographic variable with a significant effect and all other dependent variables to ascertain potential confounding effects.
The volume of Heschl's gyrus was analyzed with a 2×2×2 (sex by diagnosis by hemisphere) mixed-design analysis of covariance (ANCOVA), with hemisphere treated as a within-subjects variable and total brain volume used as the single covariate. Total brain volume was used as a covariate in this analysis because we have previously reported a trend for the brains of women with schizophrenia to be smaller than those of healthy female comparison subjects (1). Therefore, the usual correction for interindividual differences in brain size would have been inappropriate, since Heschl's gyrus may have appeared abnormally large in the female schizophrenic patients given their smaller brain volumes. The surface area of Heschl's gyrus was also subjected to an equivalent ANOVA, uncorrected for brain size since there is no appropriate brain surface area metric to use in the correction. In addition, correlations between surface area measurements and volumetric measurements for Heschl's gyrus were computed.
To examine the relationships between the M100 source and Heschl's gyrus, between Heschl's gyrus and the superior temporal gyrus, and between the superior temporal gyrus and the total brain, the vector computations X, Y, and Z were used as dependent variables in separate 2×2×2 mixed ANOVAs (sex by diagnosis by hemisphere). The distances between the three-dimensional locations of the M100 and Heschl's gyrus were also analyzed this way.
RESULTS
Demographic Variables
No significant effects were observed in the ANOVAs for age and handedness. However, a significant group difference emerged for education (table 1), indicating that the comparison group had significantly more years of formal education than the patients with schizophrenia. There was no significant correlation between education and any of the other dependent variables reported in this paper, however, suggesting that this group difference did not affect any of the other analyses. Additionally, no significant sex differences in the patient groups emerged from the t tests of chlorpromazine-equivalent neuroleptic dose, age at illness onset, and illness duration.
Volumetric and Surface Area Analyses
The ANCOVA revealed a significant hemisphere main effect for Heschl's gyrus; the left hemisphere volumes (mean=2.45 ml, SD=0.72) were greater than those for the right hemisphere (mean=1.99 ml, SD=0.56) in both groups (F=11.90, df=1,41, p<0.05). There was also a significant sex-by-diagnosis interaction (F=8.56, df=1,41, p<0.05), indicating that while the male patients had smaller Heschl's gyri than the male comparison subjects, the female patients had slightly larger Heschl's gyri than the female comparison subjects (figure 2). The same two effects were obtained when we did not covary for total brain volume; however, the F value for the sex-by-diagnosis interaction was smaller (F=4.89, df=1,41, p<0.05). No significant main effects or interaction effects were obtained in the analysis of Heschl's gyrus surface area, although moderately sized significant correlations between Heschl's gyrus volumes and surface areas were obtained (left: r=0.64, N=45, p<0.05; right: r=0.45, N=45, p<0.05).
MRI Centroid Analyses
The centroids of the total brain volumes did not differ significantly between diagnostic groups or sexes in three-dimensional space. The same was true of the superior temporal gyrus centroids. Additionally, no hemispheric differences were observed for the superior temporal gyri. With respect to the location of Heschl's gyrus within the skeletal reference frame, the y axis locations of the right hemisphere centroids (mean=0.65 cm, SD=0.67) were significantly more anterior than in the left hemisphere (mean=0.31 cm, SD=0.64) (F=26.00, df=1,41, p<0.05). There was also a significant main effect of sex (F=5.09, df=1,41, p<0.05); the Heschl's gyrus centroid locations of the men (mean=0.68 cm, SD=0.47) were more anterior than those of the women (mean=0.27 cm, SD=0.75).
Comparisons of Magnetoencephalographic Source Locations and MRI Anatomical Data
Inspection of the data for coregistration of M100 sources and the MRI-determined Heschl's gyrus locations indicated that for the male comparison subjects the M100 (±95% confidence interval on localization provided by MEGEEG custom software [1]) was located within the volume boundaries of Heschl's gyrus in 20 out of 26 cases (left and right hemispheres included). For the female comparison subjects 14 out of 22 M100 sources were within the Heschl's gyrus boundaries. For the male patients 15 out of 22 M100 sources were within Heschl's gyrus, and for the female patients 14 of 20 were within the boundaries.
The analysis of the set of difference vectors X, Y, and Z yielded significant effects only in Y. For Y a significant hemisphere effect was obtained (F=26.85, df=1,39, p<0.05), indicating that the M100 generator tended to be anterior to the Heschl's gyrus centroid in the left hemisphere but posterior to the Heschl's gyrus centroid in the right. However, a significant sex-by-hemisphere interaction was also seen (F=9.59, df=1,39, p<0.05), indicating that the magnitude of the difference was greater in the women than in the men. A significant diagnosis-by-sex-by-hemisphere interaction was also obtained (F=5.13, df=1,39, p<0.05), suggesting that the men with schizophrenia had M100 sources slightly anterior to the centroid of Heschl's gyrus in the left hemisphere (figure 3; see also MRI surface renderings in figure 1). The analysis of the distance between centroid and M100 source revealed a significant diagnosis-by-hemisphere interaction (F=4.66, df=1,39, p<0.05), suggesting that although the distance between Heschl's gyrus and the M100 source is greater in the left hemisphere in normal subjects (left: mean=1.39 cm, SD=0.56; right: mean=1.03 cm, SD=0.33), in patients the distance is slightly greater in the right hemisphere (left: mean=1.34 cm, SD=43; right: mean=1.44 cm, SD=0.50).
DISCUSSION
Several interesting findings emerged from this study. First, volumetric abnormalities in Heschl's gyrus appear to be sex specific. In our study group, only the men with schizophrenia had smaller Heschl's gyri than comparison subjects of the same sex. These findings are consonant with other data from our laboratory on superior temporal gyrus volumes, indicating that male but not female patients with schizophrenia have smaller than normal superior temporal gyrus volumes (1). The observation that the women with schizophrenia had slightly larger Heschl's gyrus volumes than those of the female comparison subjects is unique but not without precedent. Larger than normal volumes in schizophrenia have previously been reported for the caudate nucleus and putamen (30).
We are not aware of previous studies that have addressed the volumes of Heschl's gyri in people with schizophrenia, although at least two studies of Heschl's gyrus surface area showed no group differences between persons with schizophrenia and comparison subjects (28, 31). Our surface area measurements were consistent with the ones from these previous reports, showing no difference between patients and comparison subjects. Several issues may relate to the disparity between the volumetric and surface area observations: a) the surface area measurements were not corrected for total brain surface area, complicating their interpretation in the face of interindividual differences in brain size; b) surface area would appear to primarily reflect gray matter, whereas our volume measure included both gray and white matter; and finally, c) surface area measures may imperfectly represent deeper gray matter, particularly between opposed gyri where the actual surface does not follow the apparent gray matter invagination (i.e., where the gyri appear to be in close apposition, with no real sulcus). This might tend to decrease the sensitivity of the measure in a manner akin to decreased signal-to-noise ratio.
Second, we found no evidence of a sex-specific abnormality in axial-plane location of Heschl's gyrus centroids in our subjects with schizophrenia. While the Heschl's gyrus centroids were more anterior in the right hemisphere, this effect was not significantly related to either sex or diagnosis. Thus, the previously reported anomalous asymmetry of M100 source location in schizophrenia does not appear to reflect an anatomical shift of Heschl's gyrus. This raises the possibility that the cortex involved in generating the M100 has a different relationship to Heschl's gyrus than is the case for persons without schizophrenia. Should later studies support this conclusion, two explanations might be entertained. First, this could reflect a developmental anomaly in which the auditory cytoarchitectural region supporting echoic memory (the M100 source) is diverted from its normal development with respect to Heschl's gyrus. Alternatively, the symptoms of schizophrenia (e.g., auditory hallucinatory activity) might induce a plastic reorganization of cortical functions whose effects are reflected in altered M100 location (e.g., see reference 1), such that a different auditory region generates the M100 in hallucinating men. Both possibilities have precedents in the literature. With respect to the first, magnetoencephalography has shown evidence of plastic reorganization of digit representation in musicians (32). Directly related to the second, Tiihonen et al. (33) found alteration in M100 latency in a single subject with schizophrenia who was recorded while hallucinating.
In addition, when we directly compared the location of the M100 auditory evoked field source to the anatomical location of Heschl's gyrus (by means of the difference vector), we found a slight reversal of the axial-plane relationship between structure and function specific to the left hemisphere of men with schizophrenia, suggesting that the functional displacement in men with the disorder may be restricted to the left hemisphere. This finding is consistent with our first report of the phenomenon (5) and with anatomical studies of the superior temporal gyrus that suggest left-hemisphere-specific low volume in men (34, 35). We have also recently reported neuromagnetic evidence suggesting that men with schizophrenia have a deficit in auditory short-term memory scanning and retrieval specific to the left hemisphere, possibly also reflecting this anomaly in cortical function (36).
Many neuroimaging studies have used only male patients diagnosed with the disease (28, 34, 35, 37, 38), and thus the generalizability of their findings may be limited. Others (31, 39) have studied male and female subjects combined in the same group, clouding any potential sex-related neuroanatomical differences. The increasingly likely possibility of sex-related differences in superior temporal gyrus anatomy (40, 41) and function (1) in schizophrenia is potentially critical to our understanding of the disorder, however. Although the potential for sex differences in neuroanatomical morphology has received relatively little attention in the schizophrenia literature (42), and findings are sometimes conflicting (as reviewed by Cowell et al. [43]), there are well-described differences in the clinical features of the disorder (44–46). Our finding of sex-specific anomalous representation of auditory cortical activity in the superior temporal gyrus in schizophrenia is therefore intriguing and may relate to the sex differences in clinical features of the disorder. The broader issue of whether relative preservation of brain morphology in women with schizophrenia relates to their overall better treatment response and outcome and whether this may reflect in part a neuroprotective effect of sex hormones on these structures awaits future study.
The results of this study may be somewhat limited in that we used a relatively homogeneous group of subjects with schizophrenia (i.e., those with primarily positive symptoms). Studies will need to be undertaken to ascertain to what extent, if any, these findings generalize to other schizophrenia subtypes. Finally, the scope of this study with respect to sex specificity is limited by lack of data concerning clinically observable sex differences in the study group. Future efforts will need to include comprehensive evaluation of symptoms, as well as brain structure and function, to determine the relevance of these sex-specific findings to the disorder.
Our findings are essentially consistent with the view that schizophrenia is a neurodevelopmental disorder (47, 48); the evidence points to different neuroanatomical expression in the two sexes. On the basis of these findings and those from other studies of auditory evoked fields (1, 5, 36), we believe it is possible that some functional reorganization of the primary auditory cortex has occurred in these patients, such that there is relative dissociation of that functional region from Heschl's gyrus. However, we cannot rule out the possibility that either medication or the time elapsed since the onset of the disorder is responsible for these results. To our knowledge, the effects of neuroleptic medication on the measures included in this study are not known, and they could theoretically be a considerable confound. Therefore, there is a still a need for studies of this type with first-episode or never-medicated patients with schizophrenia and for longitudinal studies of children at risk for developing the disorder.
 |
Presented in part at the 35th annual meeting of the American Congress of Neuropsychopharmacology, San Juan, Puerto Rico, Dec. 9–11, 1996. Received Oct. 31, 1996; revision received March 24, 1997; accepted May 6, 1997. From the Departments of Psychiatry, Radiology, and Neurology, University of Colorado Health Sciences Center, and the Psychiatry Service, Denver Department of Veterans Affairs Medical Center. Address reprint requests to Dr. Rojas, Department of Psychiatry, Box C268-68, University of Colorado Health Sciences C enter, 4200 East Ninth Ave., Denver, CO 80262; Don.Rojas@uchsc .edu (e-mail). Supported by NIMH grants MH-47476, MH-15442 (Dr. Rojas), and MH-46335 (Dr. Reite).
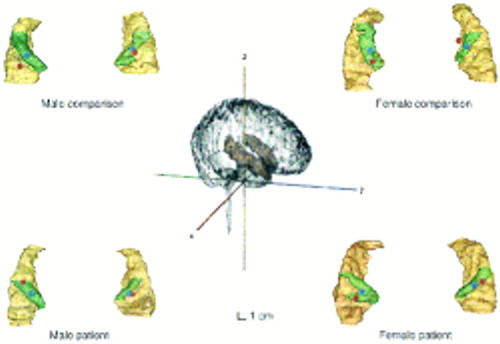
FIGURE 1. Surface Renderings of the Superior Temporal Gyrus and Heschl's Gyrus in Representative Male and Female Patients With Paranoid Schizophrenia and Healthy Comparison Subjectsa
aThe left and right superior temporal gyri are viewed from the superior perspective, with the image of the rest of the brain removed from view. The superior temporal gyrus is beige, and Heschl's gyrus is reconstructed in green. The source location of the M100 component is illustrated as a red dot on each superior temporal gyrus, and the centroid of Heschl's gyrus is shown as a blue dot. The scales and viewing angles for all superior temporal gyri are the same. The center of the figure illustrates all structures in our coordinate system and the relationship between the superior temporal gyrus and the brain (transparent in the figure). The brain is shown with the right hemisphere facing and slightly rotated toward the viewer. The superior temporal gyri are seen in pink embedded within the brain.
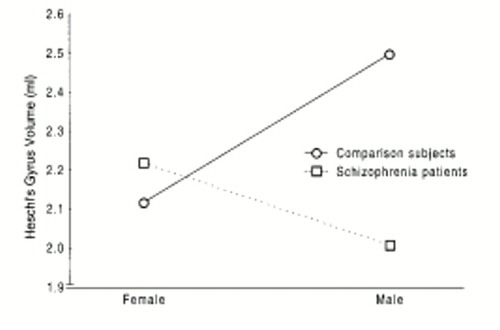
FIGURE 2. Mean Heschl's Gyrus Volume in Male and Female Patients With Paranoid Schizophrenia (N=21) and Healthy Comparison Subjects (N=24)a
aVolumes shown are unadjusted for total brain volume. Significant sex-by-diagnosis interaction (F=8.56, df=1,41, p<0.05).
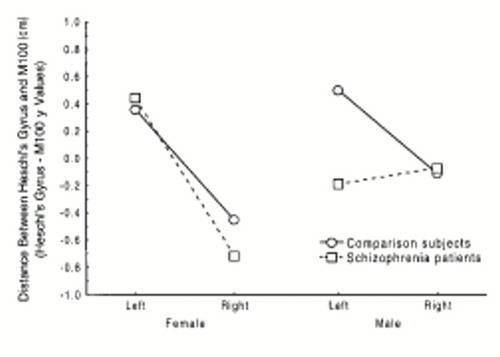
FIGURE 3. Mean Distance Between MRI-Determined Heschl's Gyrus Location and Source of M100 Auditory Evoked Field for Male and Female Patients With Paranoid Schizophrenia (N=20) and Healthy Comparison Subjects (N=23)a
aSignificant sex-by-hemisphere-by-diagnosis interaction (F=5.13, df=1,39, p<0.05).
1. Reite M, Sheeder J, Teale P, Adams M, Richardson D, Simon J, Jones RH, Rojas DC: Magnetic source imaging evidence of sex differences in cerebral lateralization in schizophrenia. Arch Gen Psychiatry 1997; 54:433–440Crossref, Medline, Google Scholar
2. Scheuneman D, Teale P, Linnville S, Goldstein L, Reite M: Magnetic auditory M100 source location in normal females. Brain Res Bull 1991; 26:747–751Crossref, Medline, Google Scholar
3. Baumann S, Rogers R, Guinto F, Saydjari C, Papanicolaou A, Eisenberg H: Gender differences in source location for the N100 auditory evoked magnetic field. Electroencephalogr Clin Neurophysiol 1991; 80:53–59Crossref, Medline, Google Scholar
4. Reite M, Sheeder J, Teale P, Richardson D, Adams M, Simon J: MEG based brain laterality: sex differences in normal adults. Neuropsychologia 1995; 33:1607–1616Google Scholar
5. Reite M, Teale P, Goldstein L, Whalen J, Linnville S: Late auditory sources may differ in the left hemisphere of schizophrenic patients: a preliminary report. Arch Gen Psychiatry 1989; 46:565–572Crossref, Medline, Google Scholar
6. Karhu J, Tiihonen J, Pekkonen E, Katila H, Houtilainen M, Virtanen J, Ilmoniemi R: Reversed hemispheric asymmetry of auditory N100m in schizophrenics, in Advances in Biomagnetism, vol 12. Edited by Aine C, Flynn E, Okada Y, Wood C. New York, Plenum (in press)Google Scholar
7. Pantev C, Hoke M, Lehnertz K, Lutkenhoner B, Anogianakis G, Wittkowski W: Tonotopic organization of the human auditory cortex revealed by transient auditory evoked magnetic fields. Electroencephalogr Clin Neurophysiol 1988; 69:160–170Crossref, Medline, Google Scholar
8. Pantev C, Hoke M, Lehnertz K, Lutkenhoner B, Fahrendorf G, Stober U: Identification of sources of brain neuronal activity with high spatiotemporal resolution through combination of neuromagnetic source localization (NMSL) and magnetic resonance imaging (MRI). Electroencephalogr Clin Neurophysiol 1990; 75:173–184Crossref, Medline, Google Scholar
9. Yamamoto T, Uemura T, Llinas R: Tonotopic organization of human auditory cortex revealed by multi-channel SQUID system. Acta Otolaryngol (Stockh) 1992; 112:201–204Crossref, Medline, Google Scholar
10. Reite M, Adams M, Simon J, Teale P, Sheeder J, Richardson D, Grabbe R: Auditory M100 component 1: relationship to Heschl's gyri. Brain Res Cogn Brain Res 1994; 2:13–20Crossref, Medline, Google Scholar
11. Lu Z-L, Williamson SJ, Kaufman L: Behavioral lifetime of human auditory sensory memory predicted by physiological measures. Science 1992; 258:1668–1670Google Scholar
12. Sams M, Hari R, Rif J, Knuutila J: The human auditory sensory memory trace persists about 10 sec: neuromagnetic evidence. J Cognitive Neurosci 1993; 5:363–370Crossref, Medline, Google Scholar
13. Makela J, Ahonen A, Hamalainen M, Hari R, Ilmoniemi R, Kajola M, Knuutila J, Lounasmaa O, McEvoy L, Salmelin R, Sams M, Simola J, Tesche C, Vasama J-P: Functional differences between auditory cortices of the two hemispheres revealed by whole-head neuromagnetic recordings. Human Brain Mapping 1993; 1:48–56Crossref, Google Scholar
14. Hari R: Human cortical functions revealed by magnetoencephalography, in Progress in Brain Research. Edited by Bloom F. New York, Elsevier, 1994, pp 163–168Google Scholar
15. Kovelman JA, Scheibel AB: A neurohistological correlate of schizophrenia. Biol Psychiatry 1984; 19:1601–1621Google Scholar
16. Benes F: Neurobiological investigations in cingulate cortex of schizophrenic brain. Schizophr Bull 1993; 19:537–549Crossref, Medline, Google Scholar
17. Benes FM, Bird ED: An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Arch Gen Psychiatry 1987; 44:608–616Crossref, Medline, Google Scholar
18. Jeste D, Lohr J: Hippocampal pathologic findings in schizophrenia. Arch Gen Psychiatry 1989; 46:1019–1024Google Scholar
19. Falkai P, Bogerts B: Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986; 236:154–161Crossref, Medline, Google Scholar
20. Annett M: Left, Right, Hand and Brain: The Right Shift Theory. Hillsdale, NJ, Lawrence Erlbaum Associates, 1985Google Scholar
21. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version 1.0 (SCID-P). Washington, DC, American Psychiatric Press, 1990Google Scholar
22. Davis J: Dose equivalence of the antipsychotic drugs. J Psychiatr Res 1974; 11:65–69Crossref, Medline, Google Scholar
23. Gelenberg AJ: The Practitioner's Guide to Psychoactive Drugs. New York, Plenum, 1991Google Scholar
24. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry 1978; 35:773–782Crossref, Medline, Google Scholar
25. Galaburda A, Sanides F: Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol 1980; 190:597–610Crossref, Medline, Google Scholar
26. Rademacher J, Caviness VS, Steinmetz H, Galaburda AM: Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex 1993; 3:313–329Crossref, Medline, Google Scholar
27. Brodmann K: Vergleichende Lokalisationslehre der Grosshirnrinde. Leipzig, Germany, Barth, 1909Google Scholar
28. Kulynych JJ, Vladar K, Jones DW, Weinberger DR: Superior temporal gyrus volume in schizophrenia: a study using MRI morphometry assisted by surface rendering. Am J Psychiatry 1996; 153:50–56Link, Google Scholar
29. Rezai AR, Hund M, Kronberg E, Zonenshayn M, Cappell J, Ribary U, Kall B, Llinas R, Kelly P: The interactive use of magnetoencephalography in stereotactic image-guided neurosurgery. Neurosurgery 1996; 39:92–102Crossref, Medline, Google Scholar
30. Swayze VW II, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC: Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 1992; 31:221–240Crossref, Medline, Google Scholar
31. Petty RG, Barta PE, Pearlson GD, McGilchrist IK, Lewis RW, Tien AY, Pulver A, Vaughn DD, Casanova MF, Powers RE: Reversal of asymmetry of the planum temporale in schizophrenia. Am J Psychiatry 1995; 152:715–721Link, Google Scholar
32. Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E: Increased cortical representation of the fingers of the left hand in string players. Science 1995; 270:305–307Crossref, Medline, Google Scholar
33. Tiihonen J, Hari R, Naukkarinen H, Rim¢n R, Jousmäki V, Kajola M: Modified activity of the human auditory cortex during auditory hallucinations. Am J Psychiatry 1992; 149:255–257Link, Google Scholar
34. Shenton M, Kikinis R, Jolsz FA, Pollak SD, Lemay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley R: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
35. Becker T, Elmer K, Schneider F, Schneider M, Grodd W, Bartels M, Heckers S, Beckmann H: Confirmation of reduced temporal limbic structural volume on magnetic resonance imaging in male patients with schizophrenia. Psychiatry Res Neuroimaging 1996; 67:135–143Crossref, Medline, Google Scholar
36. Reite M, Teale P, Sheeder J, Rojas DC, Schneider EE: Magnetoencephalographic evidence of abnormal early auditory memory function in schizophrenia. Biol Psychiatry 1996; 40:299–301Crossref, Medline, Google Scholar
37. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyrus volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Google Scholar
38. Kulynych JJ, Vladar K, Fantie BD, Jones DW, Weinberger DR: Normal asymmetry of the planum temporale in patients with schizophrenia: three-dimensional cortical morphometry with MRI. Br J Psychiatry 1995; 6:742–749Crossref, Google Scholar
39. Rossi A, Stratta L, D'Albenzio L, Tartaro A, Schiazza G, di Michele V, Bolino F, Casacchia M: Reduced temporal lobe areas in schizophrenia: preliminary evidences from a controlled multiplanar magnetic resonance imaging study. Biol Psychiatry 1990; 27:61–68Crossref, Medline, Google Scholar
40. Hoff AL, Riordan H, O'Donnell D, Stitzke P, Neale C, Boccio A, Anand AK, DeLisi LE: Anomalous lateral sulcus asymmetry and cognitive function in first-episode schizophrenia. Schizophr Bull 1992; 18:257–270Crossref, Medline, Google Scholar
41. Falkai P, Bogerts B, Schneider T, Greve B, Pfeiffer U, Pilz K, Gonsiorzcyk C, Majtenyi C, Ovary I: Disturbed planum temporale asymmetry in schizophrenia: a quantitative post-mortem study. Schizophr Res 1995; 14:161–176Crossref, Medline, Google Scholar
42. Pfefferbaum A, Marsh L: Structural brain imaging in schizophrenia. Clin Neurosci 1995; 3:105–111Medline, Google Scholar
43. Cowell PE, Kostianovsky DJ, Gur RC, Turetsky BI, Gur RE: Sex differences in neuroanatomical and clinical correlations in schizophrenia. Am J Psychiatry 1996; 153:799–805Link, Google Scholar
44. Goldstein JM, Link BG: Gender and the expression of schizophrenia. J Psychiatr Res 1988; 22:141–155Crossref, Medline, Google Scholar
45. Angermeyer MC, Kuhn L, Goldstein JM: Gender and the course of schizophrenia: differences in treated outcomes. Schizophr Bull 1990; 16:293–307Crossref, Medline, Google Scholar
46. Szymanski S, Lieberman JA, Alvir JM, Mayerhoff D, Loebel A, Geisler S, Chakos M, Koreen A, Jody D, Kane J, Woerner M, Cooper T: Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. Am J Psychiatry 1995; 152:698–703Link, Google Scholar
47. Bloom FE: Advancing a neurodevelopmental origin for schizophrenia. Arch Gen Psychiatry 1993; 50:224–227Crossref, Medline, Google Scholar
48. Lillrank SM, Lipska BK, Weinberger DR: Neurodevelopmental animal models of schizophrenia. Clin Neurosci 1995; 3:98–104Medline, Google Scholar



