Quantitative Morphology of the Cerebellum and Fourth Ventricle in Childhood-Onset Schizophrenia
Abstract
OBJECTIVE: Studies have suggested that the maldeveloped neural circuitry producing schizophrenic symptoms may include the cerebellum. The authors found further support for this hypothesis by examining cerebellar morphology in severely ill children and adolescents with childhood-onset schizophrenia. METHOD: Anatomic brain scans were acquired with a 1.5-T magnetic resonance imaging scanner for 24 patients (mean age=14.1 years, SD=2.2) with onset of schizophrenia by age 12 (mean age at onset=10.0 years, SD=1.9) and 52 healthy children. Volumes of the vermis, inferior posterior lobe, fourth ventricle, and total cerebellum and the midsagittal area of the vermis were measured manually. RESULTS: After adjustment for total cerebral volume, the volume of the vermis and the midsagittal area and volume of the inferior posterior lobe remained significantly smaller in the schizophrenic patients. There was no group difference in total cerebellar or fourth ventricle volume. CONCLUSIONS: These findings are consistent with observations of small vermal size in adult schizophrenia and provide further support for abnormal cerebellar function in childhood- and adult-onset schizophrenia. (Am J Psychiatry 1997; 154:1663–1669)
Neurodevelopmental hypotheses of schizophrenia have found increasing support (1, 2); however, the nature and site of neurodevelopmental abnormalities in this disorder remain subjects of debate. Childhood-onset schizophrenia is a rare and clinically severe form of schizophrenia and offers an important opportunity to examine abnormal neurodevelopment in this disorder. Studies of this population have demonstrated clinical continuity with later-onset schizophrenia (3, 4), along with more severe premorbid language impairments (5) and a more chronic course (6), possibly reflecting a more extensive developmental brain lesion. Quantitative brain magnetic resonance imaging (MRI) of patients in the National Institute of Mental Health (NIMH) study of childhood-onset schizophrenia have revealed significantly smaller than normal midsagittal thalamic area, larger than normal lateral ventricular volume, which appears to be progressive, and no abnormality in the size of medial temporal lobe structures (7–9).
In humans the cerebellum undergoes intense neuronal proliferation and migration during the first 1.5 to 2.0 years of life, rendering this structure more susceptible to injury from infection, cytotoxic agents, or radiation during this period (10). Studies have suggested that the maldeveloped neural circuitry producing schizophrenic symptoms may include the cerebellum. Many of the motor and eye-tracking abnormalities seen in schizophrenia and in children at risk for schizophrenia are characteristic of cerebellar disease (11). The cerebellum is involved in higher cortical functions (12–14), including working memory, which is impaired in schizophrenia (15), often in association with failure to activate frontal cortical regions normally during task performance (16). Cerebellar activation during working memory tasks may be mediated by neurons in the ventral dentate nuclei that project to the prefrontal cortex via the thalamus (17). Finally, abnormal cerebellar blood flow and metabolism have been demonstrated in both adult-onset (18, 19) and childhood-onset (20) schizophrenia.
The cerebellum has also been implicated in language processing (21). Some patients with autistic disorder, a syndrome that includes impaired development of language and social skills, have associated hypoplasia of the cerebellar hemispheres and posterior vermis (22, 23). In contrast, patients with Williams syndrome, who exhibit preservation of language and social skills in the context of global cognitive impairments (24), have preserved cerebellar size despite hypoplasia of the cerebrum (25).
Quantitative studies of cerebellar morphology in adult-onset schizophrenia have focused primarily on the midsagittal area of the vermis, with inconsistent results. Early computed tomography (CT) (26) and postmortem (27) studies yielded evidence of vermal hypoplasia in schizophrenic patients, but other CT studies (28) did not replicate this finding. MRI studies of the midsagittal vermis in schizophrenia have similarly shown no abnormalities (29, 30), larger than normal area (31), and smaller area in male than in female schizophrenic patients (32). Results from MRI examinations of cerebellar volume have included one finding of lower volume in female schizophrenic patients than in female comparison subjects (33), one preliminary report of significantly lower white matter volume of both cerebellar hemispheres and lower volume of lobules IX and X of the vermis in male schizophrenic patients relative to male comparison subjects (34), and another preliminary report of larger volume of a combined cerebellum/brainstem measure in male schizophrenic patients relative to male comparison subjects (35). With one exception (34), fourth ventricle size in schizophrenia has been estimated by using midsagittal area or maximum width; some studies have shown a larger than normal fourth ventricle (26, 30, 34), and others have indicated no abnormalities (29, 36–38). While some of these inconsistencies may reflect differences between study groups across studies, failure to use intrastructural landmarks in determining the midsagittal plane of the cerebellum, which frequently deviates from the midsagittal plane of the cerebrum (39), may also contribute to the lack of consensus.
In the present study midsagittal area of the vermis and volumes of the vermis, inferior posterior lobe, fourth ventricle, and total cerebellum were examined in the patients in the NIMH study of childhood-onset schizophrenia and were contrasted with those of a group of matched healthy children. Intrastructural landmarks were used to define the midsagittal plane. Given the evidence of cerebellar involvement in language development and processing (21, 22, 25), together with the severe premorbid language deficits in this group (5), we hypothesized that total cerebellar volume would be smaller than normal in subjects with childhood-onset schizophrenia. We further hypothesized that the vermis, and particularly the inferior posterior lobe of the vermis, would be smaller in schizophrenic patients, reflecting the role of these structures in oculomotor control (40, 41) and, hence, potentially in the eye movement abnormalities seen in this disorder.
METHOD
Subjects
Schizophrenic children and adolescents were recruited nationally for an ongoing study of childhood-onset schizophrenia (42). The inclusion criteria were a DSM-III-R diagnosis of schizophrenia with onset of psychotic symptoms by age 12, a premorbid full-scale IQ of at least 70, the absence of active medical or neurological disease, and a history of poor response to or inability to tolerate treatment with at least two different typical neuroleptics. The diagnosis was established by using previous records and clinical and structured interviews of the children and parents based on portions of the Schedule for Affective Disorders and Schizophrenia for School-Age Chidren—Epidemiologic Version (43) and of the Diagnostic Interview for Children and Adolescents (DSM-III-R version) (44).
The 24 patients with schizophrenia included 12 girls and 12 boys ranging in age from 9 to 18 years (mean=14.1, SD=2.2). The mean age at the onset of psychotic symptoms was 10.0 years (SD=1.9), and all of the patients had evidence of pubertal change upon admission to the study (mean Tanner score=3.9, SD=1.2). Most of these patients had substantial previous exposure to neuroleptics (mean exposure=25.0 months, SD=15.5) and hospitalization (mean=8.3 months, SD=13.0). There was no history of substance abuse, alcohol abuse, or ECT. The mean WISC-R (45) vocabulary subscale score for 19 patients was 7.1 (SD=3.6), and the mean block design subscale score for 21 patients was 7.6 (SD=3.3). Five patients were not testable because of the severity of their psychotic symptoms. The mean height for 23 patients and mean weight for 24 patients were 158.4 cm (SD=10.8) and 62.1 kg (SD=18.8), respectively. A previously described method (5) was used to review neuropsychological reports and school and medical records from the prepsychotic period of all patients, and this review indicated that nine patients (three girls, six boys) had been diagnosed with a language disorder during the prepsychotic period.
Fifty-two healthy children and adolescents, selected to be similar to the patients in age (mean=14.3, SD=2.0), sex (24 girls, 28 boys), and handedness, were recruited from the community. Screening included a telephone interview of the parents, completion of the Conners Preliminary Parent Report (46) and the Achenbach Child Behavior Checklist (47) by the parents, the Conners Teacher Preliminary School Report and Conners Teacher Questionnaire (46, 48), physical and neurologic examinations of the child, and structured interview of the child and parent with the Diagnostic Interview for Children and Adolescents (44). Psychiatric histories of all first- and second-degree relatives were obtained from the parents. Individuals with physical or neurologic abnormalities or lifetime histories of psychiatric problems and those for whom major psychiatric disorders were present in first-degree relatives or in more than 20% of second-degree relatives were excluded. The mean Tanner stage, height, and weight of the normal subjects were 3.6 (SD=1.4, N=51), 164.0 cm (SD=13.8), and 55.4 kg (SD=12.8), respectively, while their mean WISC-R vocabulary and block design subscale scores were 12.9 (SD=2.5, N=50) and 13.5 (SD=2.9, N=50), respectively.
Handedness was determined for both groups by using the 12 handedness items from the Revised Neurological Examination for Subtle Signs (49). Eighteen of the schizophrenic patients and 45 of the healthy subjects were right-handed. The mean age, Tanner stage, height, and weight of the subgroup of 27 healthy subjects for whom total cerebellar volume was measured were 14.6 years (SD=2.2), 3.7 (SD=1.4), 164.4 cm (SD=13.3), and 55.9 kg (SD=14.5), respectively. Thirteen individuals in this subgroup of healthy subjects were female, and 20 were right-handed.
The parents of all subjects provided written informed consent, and the subjects provided assent for participation in this study. This study was approved by the NIMH Institutional Review Board.
MRI Image Acquisition
All subjects were scanned on the same GE 1.5-T Signa magnetic resonance scanner, as described in detail elsewhere (50). We acquired 2-mm-thick contiguous slices in the coronal plane and 1.5-mm-thick contiguous slices in the axial plane, using three-dimensional spoiled gradient recalled echo in the steady state (TE=5 msec, TR=24 msec, flip angle=45°, acquisition matrix=192×256, number of excitations=1, and field of view=24 cm2).
Image Analysis
All scans were read by a clinical neuroradiologist, who noted enlargement of the left lateral ventricle in one patient scan and a focal area of increased signal in the left frontal white matter in another.
As previously described (50, 51), total cerebral volume was quantified by using an image analysis program that supplements characteristics of MRI signal intensity with a template based on a priori information about expected brain surface shape and location. The interclass correlation coefficient (ICC) for the interrater reliability of measurements of 10 brains by two raters was 0.99.
Midsagittal cerebellar areas and volumes of the vermis, inferior posterior lobe, fourth ventricle, and total cerebellum were measured by raters (L.K.J., P.C.B., A.L.K.) using NIH Image (52) while blind to subject identity. For the midsagittal area measurements, a midsagittal image was reconstructed from the axial data set in the plane, perpendicular to the axial orientation, that bisected the pyramid of the vermis, the fourth ventricle, and the pyramid of the medulla. The midsagittal areas of the anterior, superior posterior, and inferior posterior lobes of the vermis were measured by manual tracing on this slice. The anterior superior fissure and the fourth ventricle demarcated the anterior lobe (comprising lobules I–V), while the anterior superior and prepyramidal fissures demarcated the superior posterior lobe (comprising lobules VI–VII). The inferior posterior lobe (comprising lobules VIII–X) was defined as the area between the prepyramidal fissure and the ventral edge of the fourth ventricle (23, 53, 54). The cerebellar tonsils were not included in the measure of the cerebellar vermis. Ten brains were remeasured by a second rater to yield interrater reliabilities (ICC) of 0.83 for the anterior lobe, 0.85 for the superior posterior lobe, and 0.90 for the inferior posterior lobe.
The volumes of the vermis (lobules I–X) and inferior posterior lobe (lobules VIII–X) were measured by manually outlining these structures on all coronal slices in which they were visible for all subjects. Figure 1 provides an example of outlines of the vermis on coronal slices from the brain MRI of a subject with schizophrenia. Total cerebellar volume was measured by manually outlining the cerebellum on all axial slices in which it was visible (53, 54). The fourth ventricle and brainstem were excluded, while the cerebellar peduncles and vermis were included in this measure. The cerebellum was separated from the brainstem by drawing a straight line from the lateral point where the cerebellar hemispheres abut the pons to the sulcus limitans of the floor of the fourth ventricle (55). Each cerebellar slice was bisected at the midline for a measure of right and left cerebellum. Because of the time-consuming nature of manually measuring this large structure, total cerebellar volume was measured for each subject with schizophrenia and for a subset of 27 healthy subjects selected to match the schizophrenic group in age, sex, and handedness. Fourth ventricle volume was measured by using an operator-supervised thresholding technique available in NIH Image (52) for all slices in which this ventricle was visible. The volumes were calculated by multiplying the summed area by slice thickness. Ten cerebella were remeasured by a second rater, and the interrater reliability (ICC) was 0.94 for the vermis, 0.97 for the inferior posterior lobe, 0.91 for total cerebellar volume, 0.87 for the right cerebellum, 0.92 for the left cerebellum, and 0.97 for the fourth ventricle.
Statistical Analysis
Group differences in demographic measures and total cerebral volume were assessed with chi-square analyses and t tests for independent samples. The midsagittal vermal area and the volumes of the vermis, inferior posterior lobe, and fourth ventricle were examined by using repeated measures analysis of variance (ANOVA) with diagnosis and sex as between-subjects factors. Total cerebellar volume was examined by using an ANOVA with diagnosis and sex as between-subjects factors and side as a within-subjects factor. Because total cerebral volume was significantly correlated with the total, right, and left cerebellar volumes and with the vermis and inferior posterior lobe volumes in the patients, repeated measures analysis of covariance (ANCOVA) was also performed, with total cerebral volume used as a covariate.
We compared the effect size for the amount by which the midsagittal area of the inferior posterior lobe of the schizophrenic patients in the present study was smaller than that of the comparison subjects to the effect sizes in three previous MRI studies of adult schizophrenic patients for which means and standard deviations for this measure have been reported. The three studies of adults included, respectively, 36 schizophrenic patients and 51 healthy subjects (30), 23 schizophrenic patients and 16 healthy subjects (32), and 30 schizophrenic patients and 11 healthy subjects (31). Comparisons were performed by computing a z score weighted for group size for each study (56), subtracting the mean of these z scores from a z score derived from the present study, and dividing by the square root of 2 (the sum of the variances) to obtain a difference z score (57).
For the schizophrenic group, Pearson correlation coefficients were used to examine the relationship of the morphology of cerebellar structures showing diagnostic differences to WISC-R vocabulary, block design, and digit span subscale scores, total number of months of neuroleptic exposure, and duration of illness. The relationship between presence of prepsychotic language disorder and morphology of cerebellar structures showing diagnostic differences was also examined with Pearson correlation coefficients, to approximate the point biserial correlation (58). Statistical analyses were performed with SAS (59), except for the ANCOVA, which was performed with BMDP (60). All p values are two-tailed.
RESULTS
There were no significant differences between the schizophrenic group and either the total healthy comparison group or the subgroup of healthy subjects included in the assessment of total cerebellar volume in age, height, weight, Tanner stage, gender, handedness, or total cerebral volume. The mean total cerebral volume of the schizophrenic patients was 1076.8 cm3 (SD=129.8), and for the healthy subjects it was 1118.5 cm3 (SD=117.6) (t=1.39, df=74, p=0.17). The patients with schizophrenia had significantly lower scores on the WISC-R subscales for vocabulary (t=7.53, df=67, p<0.0001) and block design (t=7.61, df=69, p<0.0001) than the healthy comparison group. The differences in WISC-R scores for these variables between the schizophrenia group and the healthy subgroup involved in the analysis of cerebellar volume were similar in magnitude and were also significant.
Midsagittal Vermal Area
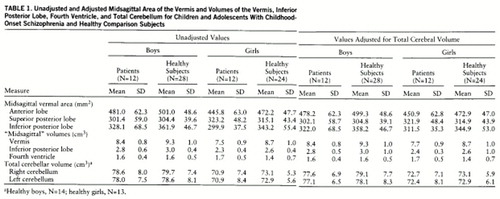
The mean midsagittal areas of the vermal lobes and the volumes of the vermis, inferior posterior lobe, fourth ventricle, and left and right cerebellum are shown in table 1. ANOVAs indicated that the midsagittal area of the inferior posterior lobe of the vermis was significantly smaller in the schizophrenic patients than in the comparison subjects (F=8.94, df=1,72, p=0.004), while the midsagittal area of the anterior lobe was smaller in the female subjects in each group (F=5.96, df=1,72, p=0.02). There were no significant diagnosis-by-sex interactions.
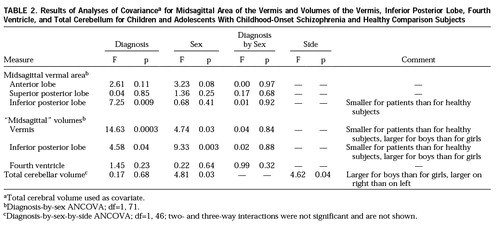
The results of the ANCOVAs for all structures are shown in table 2. The ANCOVA for the midsagittal vermal areas indicated that, after adjustment for total cerebral volume, all sex differences were lost while the area of the inferior posterior lobe remained significantly smaller in the schizophrenic patients than in the comparison subjects. Comparison of effect sizes to assess whether the difference in midsagittal inferior posterior lobe area between these patients with childhood-onset schizophrenia and the comparison subjects was greater than for patients with adult-onset schizophrenia (30–32) resulted in a difference z score of 2.38, which was significant at p=0.02 (two-tailed).
Volume of Total Cerebellum, Vermis, Inferior Posterior Lobe, and Fourth Ventricle
ANOVAs revealed no significant diagnostic differences for total, right, or left cerebellar volume. Female subjects had significantly smaller cerebellar volumes across sides and diagnostic groups (F=11.17, df=1,47, p=0.002), and this difference persisted after adjustment for total cerebral volume with ANCOVA. Although the mean volumes for left and right cerebellar hemispheres were similar across groups, a significant right-greater-than-left asymmetry was demonstrated for this structure. There were no significant interactions.
ANOVAs indicated that the vermis and inferior posterior lobe volumes were significantly smaller in the schizophrenic patients (F=16.66, df=1,72, p=0.0001, and F=5.91, df=1,72, p=0.02, respectively) and in the female subjects across groups (F=9.69, df=1,72, p=0.003, and F=18.52, df=1,72, p<0.0001). After adjustment for total cerebral volume, the vermal and inferior posterior lobe volumes remained significantly smaller in the schizophrenic patients and in the female subjects across groups. Because mean values for the volumes of these structures in adult schizophrenic patients and comparison subjects have not been published, comparisons of effect sizes could not be performed. ANOVA and ANCOVA revealed no significant diagnostic or sex differences and no significant diagnosis-by-sex interaction for the fourth ventricle.
Relationship Between Cerebellar Morphology and Clinical Variables
Within the schizophrenic group, no significant relationships were observed between volume of the vermis or volume or midsagittal area of the inferior posterior lobe and scores on WISC-R vocabulary, block design, or digit span subscales, number of months of neuroleptic exposure, duration of illness, or history of prepsychotic language disorder.
DISCUSSION
In what we believe to be the first study of cerebellar morphology in childhood-onset schizophrenia, predicted group differences were found for the volume of the vermis and the midsagittal area and volume of the inferior posterior lobe. Vermis volume was 11.7% smaller than normal in patients with childhood-onset schizophrenia, while midsagittal inferior posterior lobe area was 10.9% smaller and midsagittal inferior posterior lobe volume was 8.9% smaller. These findings are consistent with reports of small vermal size in adults with schizophrenia (26, 27, 32) and with evidence of abnormal cerebellar function in childhood-onset and adult-onset schizophrenia (18–20). The observation of a greater difference between schizophrenic and comparison subjects in midsagittal inferior posterior lobe area in the present study than has been observed in studies of adult schizophrenic patients suggests that persons with childhood-onset schizophrenia may sustain a more severe neurodevelopmental lesion at this structure.
While effects of previous neuroleptic medications on vermal or inferior posterior lobe size in the present study cannot be ruled out, the absence of significant correlations between measures of neuroleptic exposure and the size of these structures suggests that the observed diagnostic differences may not reflect medication effects. The absence of a diagnostic difference in fourth ventricle volume is consistent with some observations of adult-onset schizophrenia (29, 36–38).
Although our failure to observe significant diagnostic differences in total cerebellar volume may reflect lack of statistical power, the right and left cerebellar volumes were only 2.2% and 2.0% smaller in the patients with childhood-onset schizophrenia than in the comparison subjects. To detect differences of this magnitude as significant, by assuming a power of 80% and significance level of 0.05, groups containing more than 200 subjects would be required (61). The absence of a significant diagnostic difference in total cerebellar size does not preclude abnormalities of neural organization or function within the cerebellar hemispheres in schizophrenia. Subnormal activation of prefrontal-thalamic-cerebellar circuitry has been demonstrated in unmedicated adults with schizophrenia performing practiced and novel memory tasks, suggesting that “cognitive dysmetria,” or poor coordination of retrieval, processing, and expression of information, may be a fundamental deficit in schizophrenia (18). Cerebellar glucose metabolism in medicated adult schizophrenic patients at rest has been found to be lower than normal (19), while unmedicated adolescents with childhood-onset schizophrenia performing an auditory continuous performance task were found to have greater than normal cerebellar and thalamic glucose metabolism (20). These observations are consistent with animal studies demonstrating that cerebellar glucose metabolism decreases after administration of dopamine antagonists and increases after administration of dopamine agonists (62, 63), and they suggest that neuroleptics may attenuate abnormally activated cerebellar neurons in schizophrenia. Given the absence of dopamine receptors in the cerebellum, this effect of neuroleptic medication may occur through cortical-cerebellar neural circuits (64).
The lack of a relationship between block design, vocabulary, or digit span performance and the size of the vermis or inferior posterior lobe is consistent with observations of healthy children (Giedd et al., in preparation) and healthy adults (65), as is the persistence of sex differences in the size of the total cerebellum after adjustment for total cerebral volume (Giedd et al., in preparation). Our failure to observe a significant relationship between WISC-R vocabulary subtest score or presence of prepsychotic language disorder and the morphology of the vermis or inferior posterior lobe does not preclude the possibility that abnormal function in these brain regions contributes to impaired language function.
While the inclusion criterion of previous nonresponse to neuroleptic medication may have resulted in a nonrepresentative group of patients with childhood-onset schizophrenia, phenomenologic similarities between this study group and other groups of patients with childhood-onset schizophrenia described in the literature (3, 4, 66) suggest that the present findings are relevant to childhood-onset schizophrenia in general.
Preliminary data from a 2-year follow-up rescan study of 11 schizophrenic and 22 healthy subjects from the present study suggest that the cerebellar abnormalities in childhood-onset schizophrenia may not be progressive. However, ongoing longitudinal rescanning of these subjects will continue to more definitively address this question.
 |
 |
Received Dec. 10, 1996; revisions received March 5 and June 4, 1997; accepted June 19, 1997. From the Child Psychiatry Branch, NIMH. Address reprint requests to Dr. Jacobsen, Child Psychiatry Branch, NIMH, Rm. 6N240, Bldg. 10, 9000 Rockville Pike, Bethesda, MD 20892; [email protected] (e-mail). The authors thank Charles T. Gordon, M.D., Kathleen McKenna, M.D., Jean A. Frazier, M.D., and Paul Lee, B.A., for their assistance with this study.
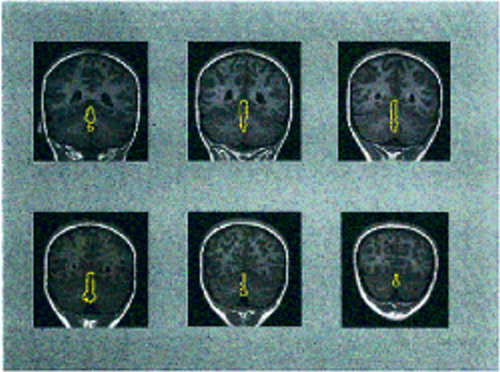
FIGURE 1. Coronal Slices From the Brain MRI of a 12-Year-Old Girl With Chronic Schizophrenia, From Least to Most Posterior (left to right, top to bottom)a
aThe vermis (lobules I–X) is outlined on each slice.
1. Bloom FE: Advancing a neurodevelopmental origin for schizophrenia. Arch Gen Psychiatry 1993; 50:224–227Crossref, Medline, Google Scholar
2. Weinberger DR: Schizophrenia as a neurodevelopmental disorder: a review of the concept, in Schizophrenia. Edited by Hirsch SR, Weinberger DR. London, Blackwood Press, 1995, pp 294–323Google Scholar
3. Russell AT: The clinical presentation of childhood-onset schizophrenia. Schizophr Bull 1994; 20:631–646Crossref, Medline, Google Scholar
4. Spencer EK, Campbell M: Children with schizophrenia: diagnosis, phenomenology, and pharmacotherapy. Schizophr Bull 1994; 20:713–725Crossref, Medline, Google Scholar
5. Alaghband-Rad J, McKenna K, Gordon CT, Albus KE, Hamburger SD, Rumsey JM, Frazier JA, Lenane MC, Rapoport JL: Childhood-onset schizophrenia: the severity of premorbid course. J Am Acad Child Adolesc Psychiatry 1995; 34:1273–1283Google Scholar
6. Gordon CT, Frazier JA, McKenna K, Giedd J, Zametkin A, Zahn T, Hommer D, Hong W, Kaysen D, Albus KE, Rapoport JL: Childhood-onset schizophrenia: an NIMH study in progress. Schizophr Bull 1994; 20:697–712Crossref, Medline, Google Scholar
7. Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, Vaituzis AC, Rajapakse JC, Lenane MC, McKenna K, Jacobsen LK, Gordon CT, Breier A, Rapoport JL: Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry 1996; 53:617–624Crossref, Medline, Google Scholar
8. Jacobsen LK, Giedd JN, Vaituzis AC, Hamburger SD, Rajapakse JC, Frazier JA, Kaysen D, Lenane MC, McKenna K, Gordon CT, Rapoport JL: Temporal lobe morphology in childhood-onset schizophrenia. Am J Psychiatry 1996; 153:355–361; correction, 153:851Link, Google Scholar
9. Rapoport JL, Giedd J, Kumra S, Jacobsen LK, Smith A, Lee P, Nelson J, Hamburger S: Childhood onset schizophrenia: progressive ventricular change during adolescence. Arch Gen Psychiatry (in press)Google Scholar
10. Jacobson M: Histogenesis and morphogenesis of cortical structures, in Developmental Neurobiology. New York, Plenum, 1991, pp 401–451Google Scholar
11. Taylor MA: The role of the cerebellum in the pathogenesis of schizophrenia. Neuropsychiatry, Neuropsychology, and Behavioral Neurology 1991; 4:251–280Google Scholar
12. Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT: Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science 1996; 272:545–547Crossref, Medline, Google Scholar
13. Mushiake H, Strick PL: Cerebellar and pallidal activity during instructed delay periods. Society for Neuroscience Abstracts 1995; 21:411Google Scholar
14. Kim SG, Ugurbil K, Strick PL: Activation of a cerebellar output nucleus during cognitive processing. Science 1994; 265:949–951Crossref, Medline, Google Scholar
15. Goldberg TE, Gold JM, Greenberg R, Griffin S, Schulz SC, Pickar D, Kleinman JE, Weinberger DR: Contrasts between patients with affective disorders and patients with schizophrenia on a neuropsychological test battery. Am J Psychiatry 1993; 150:1355–1362Google Scholar
16. Berman KF, Illowsky BP, Weinberger DR: Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, IV: further evidence for regional and behavioral specificity. Arch Gen Psychiatry 1988; 45:616–622Crossref, Medline, Google Scholar
17. Middleton FA, Strick PL: Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 1994; 266:458–461Crossref, Medline, Google Scholar
18. Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD: Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 1996; 93:9985–9990Google Scholar
19. Volkow ND, Levy A, Brodie JD, Wolf AP, Cancro R, Van Gelder P, Henn F: Low cerebellar metabolism in medicated patients with chronic schizophrenia. Am J Psychiatry 1992; 149:686–688Link, Google Scholar
20. Jacobsen LK, Hamburger SD, Van Horn JD, Vaituzis AC, McKenna K, Frazier JA, Gordon CT, Lenane MC, Rapoport JL, Zametkin AJ: Cerebral glucose metabolism in childhood onset schizophrenia. Psychiatry Res (in press)Google Scholar
21. Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME: Positron emission tomographic studies of the processing of single words. J Cognitive Neuroscience 1989; 1:153–170Crossref, Medline, Google Scholar
22. Murakami JW, Courchesne E, Press GA, Yeung-Courchesne R, Hesselink JR: Reduced cerebellar hemisphere size and its relationship to vermal hypoplasia in autism. Arch Neurol 1989; 46:689–694Crossref, Medline, Google Scholar
23. Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL: Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med 1988; 318:1349–1354Google Scholar
24. Bellugi U, Wang PP, Jernigan TL: Williams syndrome: an unusual neuropsychological profile, in Atypical Cognitive Deficits in Developmental Disorders: Implications for Brain Function. Edited by Broman SH, Grafman J. Hillsdale, NJ, Lawrence Erlbaum Associates, 1994, pp 23–56Google Scholar
25. Jernigan TL, Bellugi U, Sowell E, Doherty S, Hesselink JR: Cerebral morphologic distinctions between Williams and Down syndromes. Arch Neurol 1993; 50:186–191Crossref, Medline, Google Scholar
26. Dewan MJ, Pandurangi AK, Lee SH, Ramachandran T, Levy BF, Boucher M, Yozawitz A, Major L: Cerebellar morphology in chronic schizophrenic patients: a controlled computed tomography study. Psychiatry Res 1983; 10:97–103Crossref, Medline, Google Scholar
27. Weinberger DR, Kleinman JE, Luchins DJ, Bigelow LB, Wyatt RJ: Cerebellar pathology in schizophrenia: a controlled postmortem study. Am J Psychiatry 1980; 137:359–361Link, Google Scholar
28. Coffman JA, Mefferd J, Golden CJ, Bloch S, Graber B: Cerebellar atrophy in chronic schizophrenia (letter). Lancet 1981; 1:666Crossref, Medline, Google Scholar
29. Mathew RJ, Partain CL: Midsagittal sections of the cerebellar vermis and fourth ventricle obtained with magnetic resonance imaging of schizophrenic patients. Am J Psychiatry 1985; 142:970–971Link, Google Scholar
30. Aylward EH, Reiss A, Barta PE, Tien A, Han W, Lee J, Pearlson GD: Magnetic resonance imaging measurement of posterior fossa structures in schizophrenia. Am J Psychiatry 1994; 151:1448–1452Google Scholar
31. Nasrallah HA, Schwarzkopf SB, Olson SC, Coffman JA: Perinatal brain injury and cerebellar vermal lobules I–X in schizophrenia. Biol Psychiatry 1991; 29:567–574Crossref, Medline, Google Scholar
32. Rossi A, Stratta P, Mancini F, de Cataldo S, Casacchia M: Cerebellar vermal size in schizophrenia: a male effect. Biol Psychiatry 1993; 33:354–357Crossref, Medline, Google Scholar
33. Flaum M, Swayze VW II, O'Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC: Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 1995; 152:704–714Link, Google Scholar
34. Deshmukh A, Sullivan EV, Mathalon DH, Desmond JE, Matsumoto B, Lim KO, Pfefferbaum A: Cerebellar volume deficits in schizophrenia (abstract). Biol Psychiatry 1996; 39:600Crossref, Google Scholar
35. Levitt JJ, Donnino R, Shenton ME, Kikinis R, Jolesz FA, McCarley RW: A quantitative volumetric MRI study of the brainstem and cerebellum in schizophrenia (abstract). Biol Psychiatry 1996; 39:639Crossref, Google Scholar
36. Rossi A, Stratta P, D'Albenzio L, Di Michele V, Serio A, Giordano L, Petruzzi C, Casacchia M: Quantitative computed tomographic study in schizophrenia: cerebral density and ventricle measures. Psychol Med 1989; 19:337–342Crossref, Medline, Google Scholar
37. Rossi A, Stratta P, de Cataldo S, Di Michele V, Orfanelli G, Serio A, Petruzzi C, Casacchia M: Cortical and subcortical computed tomographic study in schizophrenia. J Psychiatr Res 1988; 22:99–105Crossref, Medline, Google Scholar
38. Uematsu M, Kaiya H: Midsagittal cortical pathomorphology of schizophrenia: a magnetic resonance imaging study. Psychiatry Res 1989; 30:11–20Crossref, Medline, Google Scholar
39. Courchesne E, Yeung-Courchesne R, Egaas B: Methodology in neuroanatomic measurement. Neurology 1994; 44:203–208Crossref, Medline, Google Scholar
40. Nagao S: Different roles of flocculus and ventral paraflocculus for oculomotor control in the primate. Neuroreport 1992; 3:13–16Crossref, Medline, Google Scholar
41. Lewis RF, Zee DS: Ocular motor disorders associated with cerebellar lesions: pathophysiology and topical localization. Rev Neurol 1993; 149:665–677Medline, Google Scholar
42. Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Hamburger SD, Smith AK, Albus KE, Alaghband-Rad J, Lenane MC, Rapoport JL: Childhood-onset schizophrenia: a double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry 1996; 53:1090–1097Google Scholar
43. Puig-Antich J, Orvaschel H, Tabrizi MA, Chambers W: The Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (Kiddie-SADS-E), 3rd ed. New York, New York State Psychiatric Institute and Yale University School of Medicine, 1980Google Scholar
44. Welner Z, Reich W, Herjanic B, Jung KG, Amado H: Reliability, validity, and parent-child agreement studies of the Diagnostic Interview for Children and Adolescents (DICA). J Am Acad Child Adolesc Psychiatry 1987; 26:649–653Crossref, Medline, Google Scholar
45. Wechsler D: Wechsler Intelligence Scale for Children—Revised. New York, Psychological Corp, 1974Google Scholar
46. Goyette CH, Conners CK, Ulrich RF: Normative data on revised Conners parent and teacher rating scales. J Abnorm Child Psychol 1978; 6:221–236Crossref, Medline, Google Scholar
47. Achenbach TM, Edelbrock CS: Manual for the Child Behavior Checklist and Revised Behavior Profile. Burlington, University of Vermont, 1983Google Scholar
48. Werry JS, Sprague RL, Cohen MN: Conners' Teacher Rating Scale for use in drug studies with children—an empirical study. J Abnorm Child Psychol 1975; 3:217–229Crossref, Medline, Google Scholar
49. Denckla MB: Revised Neurological Examination for Subtle Signs (1985). Psychopharmacol Bull 1985; 21:773–800Medline, Google Scholar
50. Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL: Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex 1996; 6:551–560Crossref, Medline, Google Scholar
51. Snell JW, Merickel MB, Ortega JM, Goble JC, Brookeman JR, Kassell NF: Boundary estimation of complex objects using hierarchical active surface templates. Pattern Recognition 1995; 28:1599–1609Google Scholar
52. Rasband W: Image 1.6. Bethesda, Md, National Institutes of Health, Research Services Branch, 1993Google Scholar
53. Duvernoy HM: The Human Brain Stem and Cerebellum: Surface, Structure, Vascularization, and Three-Dimensional Sectional Anatomy With MRI. New York, Springer-Verlag, 1995Google Scholar
54. Delmas A, Pertuiset B: Cranio-Cerebral Topometry in Man. Springfield, Ill, Charles C Thomas, 1959Google Scholar
55. Carpenter MB: Core Text of Neuroanatomy. Baltimore, Williams & Wilkins, 1991Google Scholar
56. Bahn AK: Basic Medical Statistics. London, Grune & Stratton, 1972, p 213Google Scholar
57. Glass GV, McGaw B, Smith ML: Meta-Analysis in Social Research. Beverly Hills, Calif, Sage Publications, 1981Google Scholar
58. Andrews FM, Klem L, Davidson TN, O'Malley PM, Rodgers WL: A Guide for Selecting Statistical Techniques for Analyzing Social Science Data. Ann Arbor, University of Michigan, 1981Google Scholar
59. SAS Language and Procedures: Usage, version 6.07. Cary, NC, SAS Institute, 1989Google Scholar
60. BMDP Statistical Software I: BMDP. Berkeley, Calif, University of California Press, 1990Google Scholar
61. Bartko JJ, Pulver AE, Carpenter WT: The power of analysis: statistical perspectives, part 2. Psychiatry Res 1988; 23:301–309Crossref, Medline, Google Scholar
62. Pizzolato G, Soncrant TT, Holloway HW, Rapoport SI: Reduced metabolic response of the aged rat brain to haloperidol. J Neurosci 1985; 5:2831–2838Google Scholar
63. McCulloch J, Savaki HE, McCulloch MC, Jehle J, Sokoloff L: The distribution of alterations in energy metabolism in the rat brain produced by apomorphine. Brain Res 1982; 243:67–80Crossref, Medline, Google Scholar
64. Houk JC, Wise SP: Distributed modular architectures linking basal ganglia, cerebellum and cerebral cortex: their role in planning and controlling action. Cereb Cortex 1995; 5:95–110Crossref, Medline, Google Scholar
65. Paradiso S, Robinson RG, O'Leary D, Arndt S, Woodworth CH, Andreasen NC: Cerebellar size correlates with memory functions in young normal humans. Society for Neuroscience Abstracts 1995; 21:1444Google Scholar
66. Green WH, Padron-Gayol M, Hardesty AS, Bassiri M: Schizophrenia with childhood-onset: a phenomenological study of 38 cases. J Am Acad Child Adolesc Psychiatry 1992; 31:968–976Crossref, Medline, Google Scholar



