Sleep Onset Problems and Subcortical Development in Infants Later Diagnosed With Autism Spectrum Disorder
Abstract
Objective:
Sleep patterns in children with autism spectrum disorder (ASD) appear to diverge from typical development in the second or third year of life. Little is known, however, about the occurrence of sleep problems in infants who later develop ASD and possible effects on early brain development. In a longitudinal neuroimaging study of infants at familial high or low risk for ASD, parent-reported sleep onset problems were examined in relation to subcortical brain volumes in the first 2 years of life.
Methods:
A total of 432 infants were included across three study groups: infants at high risk who developed ASD (N=71), infants at high risk who did not develop ASD (N=234), and infants at low risk (N=127). Sleep onset problem scores (derived from an infant temperament measure) were evaluated in relation to longitudinal high-resolution T1 and T2 structural imaging data acquired at 6, 12, and 24 months of age.
Results:
Sleep onset problems were more common at 6–12 months among infants who later developed ASD. Infant sleep onset problems were related to hippocampal volume trajectories from 6 to 24 months only for infants at high risk who developed ASD. Brain-sleep relationships were specific to the hippocampus; no significant relationships were found with volume trajectories of other subcortical structures examined (the amygdala, caudate, globus pallidus, putamen, and thalamus).
Conclusions:
These findings provide initial evidence that sleep onset problems in the first year of life precede ASD diagnosis and are associated with altered neurodevelopmental trajectories in infants at high familial risk who go on to develop ASD. If replicated, these findings could provide new insights into a potential role of sleep difficulties in the development of ASD.
The majority of the first 12 months of life is spent asleep (1). Critical brain maturation processes are thought to occur during sleep in early development (2, 3)—for example, development of the visual cortex relies on both sensory stimulation during wakefulness and endogenous stimulation during sleep, which together guide neuronal differentiation and developmentally regulated synaptic plasticity (4). Children with autism spectrum disorder (ASD) are 2–3 times more likely to have difficulties with initiating or sustaining sleep than typically developing children (5). The impact of inadequate sleep on child cognitive development, behavior, and family functioning in ASD is evident in the behavioral literature (6–8). However, to our knowledge, no research has examined the effect of poor sleep on brain development in this population.
Reduced sleep duration in children with ASD compared with typically developing children has been reported as early as 30 months of age (9) and can persist through adulthood (7). Sleep patterns in ASD may diverge even earlier. A recent study of more than 1,000 children found that the number of night awakenings at 12 months of age was associated with ASD screening scores at age 24 months (10). Emerging evidence suggests that preclinical brain and behavior differences exist during infancy in children later diagnosed with ASD. Atypical sensorimotor development (11) and accelerated brain growth in the first year of life have been shown to precede the consolidation of ASD symptoms (12, 13). Given that a substantial portion of an infant’s time is spent asleep, it is possible that some of the preclinical differences seen in early neurodevelopment in ASD could be related to atypical sleep.
Our aim in this study was to characterize altered sleep patterns and associations with brain development in a sample of infants at high familial risk (i.e., having an older sibling with ASD) or low familial risk for ASD (i.e., no family history of ASD in first-degree relatives). This sample provided an opportunity to characterize sleep difficulties in infants who later develop ASD and between those at high and low risk. We examined the relationship between sleep during infancy and subcortical volume during early development. We chose to focus on subcortical development because altered volume in these structures (i.e., the hippocampus, amygdala, thalamus, and basal ganglia) has been associated with ASD in numerous studies (reviewed in 13–15) and also with sleep problems. Measures of insomnia severity in adults (i.e., arousal level, sleep latency, quality, and fragmentation) have been associated with decreased hippocampal (16–18) and putamen volumes (18). The literature on brain correlates of pediatric sleep problems is limited. Sleep duration during weekdays in typically developing children has been found to be positively associated with bilateral hippocampal volume, after adjusting for age, sex, and intracranial volume (19). A recent prospective neuroimaging study found that trajectories of sleep disturbance from age 2 months to 6 years were associated with smaller total brain volumes by age 7; however, subcortical volumes were not significantly different after correcting for total volume (20). Therefore, whether sleep disturbance in early childhood is associated with morphometric changes in subcortical structures remains an open question—one well suited to investigation in the context of ASD.
We examined associations between sleep difficulties and developmental trajectories in six subcortical structures that are altered in ASD: the amygdala, hippocampus, caudate, putamen, globus pallidus, and thalamus (13–15). Based on available evidence of early sleep differences associated with ASD screening scores (10), we hypothesized that sleep difficulties in the first 12 months of life would occur more often among high-risk infants who go on to develop ASD (high-risk ASD), least often among low-risk infants, and at an intermediate level among high-risk infants who do not develop ASD (high-risk non-ASD) (21). We further hypothesized that poor sleep would be related to alterations in subcortical brain morphometry, a hypothesis that was nondirectional, given the paucity of data regarding the impact of sleep difficulties on brain morphometry in early development. Additional follow-up analyses were conducted to determine whether hemispheric differences, cognitive ability, infant temperament, and the timing of sleep measurement were significant contributors to the sleep-brain relationships we observed.
Methods
Participants
Data for 432 high- and low-risk infants collected across four clinical sites were included for analyses. Parents provided informed consent for their infant to participate, and all study procedures were approved by the institutional review boards at each site. Infants were assessed at 6, 12, and 24 months with MRI scans, parent-report measures, and standardized measures of cognitive and adaptive functioning. The 24-month visit also included a diagnostic assessment for ASD, which yielded three outcome groups: infants at high risk who met criteria for ASD (N=71), infants at high risk who did not meet criteria for ASD (N=234), and infants at low risk who did not meet criteria for ASD (N=127). The demographic and clinical characteristics of the study participants are summarized by group in Table 1. Unequal group sizes were anticipated given previous evidence from infant sibling studies of a 20% conversion rate for high-risk siblings. Our analysis approach enabled us to accommodate unequal group variances, and thus a balanced design was not required (see the Statistical Analysis section). Full inclusion and exclusion criteria, as well as details of the behavioral assessment, are presented in the online supplement. While there is general consensus that ASD can be reliably diagnosed by 24 months, approximately half of the infants in this sample participated in a second diagnostic visit at 36 months; those who met diagnostic criteria at either time point were included in the ASD group (see Table S1 in the online supplement), given previous evidence that high-risk siblings with higher cognitive ability and more subtle ASD symptoms may move between diagnostic categories throughout early childhood.
| Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | High-Risk ASD (71) | High-Risk Non-ASD (234) | Low-Risk (127) | Group Comparison | |||||
| N | % | N | % | N | % | χ2 | df | p | |
| Included scans | 6.3 | 4 | 0.2 | ||||||
| 6 months | 54 | 36 | 164 | 33 | 110 | 39 | |||
| 12 months | 44 | 29 | 178 | 35 | 95 | 34 | |||
| 24 months | 52 | 35 | 161 | 32 | 74 | 27 | |||
| Sex | 17 | 2 | 0.0002 | ||||||
| Male | 59 | 83 | 133 | 57 | 73 | 57 | |||
| Female | 12 | 17 | 101 | 43 | 54 | 43 | |||
| Mean | SD | Mean | SD | Mean | SD | F | df | p | |
| Age (months) | |||||||||
| MRI | |||||||||
| First | 6.5 | 0.6 | 6.6 | 0.7 | 6.7 | 0.7 | 1.1 | 2, 325 | 0.3 |
| Second | 12.7 | 0.7 | 12.6 | 0.7 | 12.7 | 0.7 | 0.6 | 2, 314 | 0.6 |
| Third | 24.6 | 0.7 | 24.7 | 0.9 | 24.7 | 0.8 | 0.6 | 2, 284 | 0.5 |
| Autism Diagnostic Observation Schedule (24 months)a | |||||||||
| Restricted/repetitive | 6.0 | 2.6 | 3.0 | 2.3 | 2.2 | 2.0 | 68.1 | 2, 416 | <0.0001 |
| Social affect | 5.6 | 2.1 | 1.8 | 1.1 | 1.7 | 1.0 | 251.2 | 2, 416 | <0.0001 |
| Mullen scales (24 months)b | 81.7 | 16.6 | 103.5 | 15.8 | 110.9 | 16.5 | 74.3 | 2, 418 | <0.0001 |
TABLE 1. Demographic and clinical characteristics of the study participants by diagnostic groupa
Measurement of Sleep Onset Problems in Infancy
Although the overall study did not include a standardized assessment of sleep, infant development was characterized at 6 and 12 months of age with the Infant Behavior Questionnaire–Revised (IBQ-R) (22), a measure of temperament with five items related to settling and sleep initiation (IBQ-R items 21–25; see Box 1). IBQ-R items were rated for frequency on a 7-point scale ranging from “never” to “always.” After reverse scoring, an average was taken across all five items to generate an Infant Sleep Onset Problems (ISOP) score; higher ISOP scores indicate more difficulty with sleep initiation and longer sleep onset latencies.
BOX 1. Questions from the Infant Behavior Questionnaire–Revised (IBQ-R) used to develop the Infant Sleep Onset Problems (ISOP) scorea
When going to sleep at night, how often did your baby:
21. Fall asleep within 10 minutes? (reverse scored)
22. Have a hard time settling down to sleep?
23. Settle down to sleep easily? (reverse scored)
When your baby awoke at night, how often did s/he:
24. Have a hard time going back to sleep?
25. Go back to sleep immediately? (reverse scored)
a Items on the IBQ-R were scored for frequency on a 7-point scale ranging from “never” to “always.” Some items were reversed-scored so that a higher total score was indicative of more sleep onset difficulties.
ISOP scores were available for 432 infants from 6 to 12 months old; infants included in the analyses had data from the 6-month visit only (N=46), the 12-month visit only (N=78), or both (N=308). ISOP scores from 6 to 12 months were significantly correlated, with the strongest stability across time demonstrated for the high-risk ASD group (r=0.54, p<0.0001), followed by the low-risk group (r=0.41, p<0.0001) and the high-risk non-ASD group (r=0.33, p<0.0001). To examine the relationship between sleep onset problems during infancy and subcortical development in the full sample of children, ISOP scores from the 6-month and 12-month assessments were averaged to create a single ISOP score for each participant. Selected analyses were also repeated using ISOP scores from the 6-month and 12-month assessments only.
Validation of the ISOP Score
A subgroup of participants in the study was given the parent-report Brief Infant Sleep Questionnaire (BISQ) (23) as part of a substudy conducted at one of the clinical sites (Children’s Hospital of Philadelphia). Sixty-seven participants (high-risk ASD group, N=10; high-risk non-ASD group, N=41; low-risk group, N=16) had both ISOP and BISQ scores available between ages 6 months and 12 months. This enabled us to empirically evaluate the IBQ-derived ISOP score for convergent validity by comparing it against a validated infant sleep measure (23). Average sleep latency from the BISQ (time taken to fall asleep at night, in hours) was chosen for comparison to the ISOP score, which measures difficulty settling to sleep at the beginning or in the middle of the night. The IBQ-based ISOP score was significantly correlated with average sleep latency as measured on the BISQ during the same developmental period (Pearson’s r=0.44, p=0.0002) (Figure 1). In contrast, it was not correlated with other aspects of sleep measured on the BISQ (e.g., average duration of nocturnal wakefulness; Pearson’s r=0.04 p=0.72). Finally, the five IBQ-R sleep items that form the ISOP were internally consistent at both 6 months (Cronbach’s alpha=0.76, 95% CI=0.74, 0.78) and 12 months (Cronbach’s alpha=0.76, 95% CI=0.74, 78). On the basis of this evidence of high internal consistency and convergent and divergent validity with a previously validated measure of sleep latency in infancy, we determined that the IBQ-derived ISOP score had sufficient construct validity for the purposes of this study.
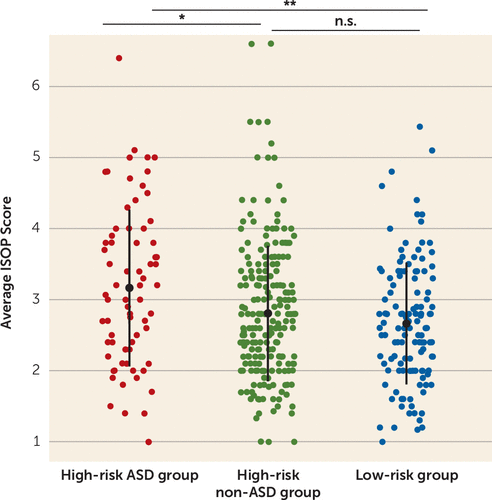
FIGURE 1. Average Infant Sleep Onset Problems (ISOP) score at 6–12 months by outcome groupa
a A significant difference was found across groups, with infants who developed autism spectrum disorder (ASD; high-risk ASD group) showing more parent-reported sleep onset problems at 6–12 months compared with infants who did not develop ASD (with and without familial risk). Vertical bars indicate standard deviations for each group. n.s.=not significant.
*p<0.05. **p<0.01.
MRI Acquisition and Processing
Imaging data were collected during natural sleep at 6, 12, and 24 months. High-resolution T1- and T2-weighted imaging data (1-mm3 voxels) were acquired on identical 3-T Siemens TIM Trio scanners equipped with standard 12-channel head coils across all sites. Geometry phantoms were scanned monthly, and human phantoms (two adult study subjects) were scanned annually to monitor scanner stability at each site across the study period.
T1- and T2-weighted images that passed visual inspection for quality control (99% and 98% of scans, respectively) underwent distortion correction, mutual registration, transformation to stereotactic space, and CSF/brain tissue segmentation. A graph-based, multi-atlas method developed by investigators in the Infant Brain Imaging Study network was employed to segment the subcortical structures (see the online supplement). Atlas templates were derived from 16 cases at each time point (6, 12, and 24 months), which were manually segmented by a single experimenter, used as training images in the multi-atlas segmentation, and then applied to all 6-, 12-, and 24-month data sets. All segmentations underwent visual quality inspection by one of two trained experimenters (blind to diagnosis, risk status, sex, and scan site). More than 98% of scans met quality inspection criteria for segmentation, with no difference in the segmentation pass rate between groups. A total of 932 scans, across three groups and three time points, were included in the present analysis (high-risk ASD group, N=150; high-risk non-ASD group, N=503; low-risk group, N=279) (Table 1).
Statistical Analysis
Categorical differences in ISOP scores were tested via analysis of variance, and Pearson correlation coefficients were calculated between ISOP scores and continuous measures of child functioning. Linear mixed-effects models were used to predict bilateral volume trajectories for six subcortical structures (the hippocampus, amygdala, caudate, globus pallidus, putamen, and thalamus). Volumes were summed across hemispheres to reduce the number of comparisons, consistent with previous work showing no laterality effects in subcortical volumes in this sample (24). Individual intercepts were included as a random effect. ISOP score, age, quadratic effect of age (age-squared), sex, and group interactions with each were included as fixed effects. Total cerebral volume and scan site were also included as covariates. All tests were two-tailed with alpha set at 0.05, and a false discovery rate procedure was used to correct for multiple comparisons.
Any subcortical structure associated with ISOP scores was subjected to additional follow-up analyses to determine the strength and specificity of the finding (see the online supplement). Specifically, we tested whether sleep-brain associations were present in both hemispheres, persisted when controlling for cognitive ability, were specific to IBQ-R sleep items (rather than infant temperament more broadly), and persisted when including ISOP scores from 6 months or 12 months only.
Results
ISOP Scores Across Diagnostic Groups
ISOP scores were compared across the three diagnostic groups and differed significantly (F=6.41, df=2, 429, p=0.002) (Figure 2). A post hoc Tukey test revealed greater sleep onset problems in the high-risk ASD group compared with the high-risk non-ASD group (difference, 0.36; 95% CI=0.06, 0.66, p=0.02) and the low-risk group (difference, −0.50; 95% CI=−0.83, −0.17, p=0.001). ISOP scores did not differ significantly between the high-risk non-ASD and low-risk groups (difference, −0.14; 95% CI=−0.39, 0.10, p=0.36). A disorder-specific effect was also found when examining ISOP scores from the 6-month time point only (high-risk ASD group compared with high-risk non-ASD group: t=2.72, df=82.7, p=0.008; high-risk ASD group compared with low-risk group: t=3.0, df=89.0, p=0.003; high-risk non-ASD group compared with low-risk group: t=0.53, df=272.3, p=0.60) and the 12-month time point only (high-risk ASD group compared with high-risk non-ASD group; t=2.00, df=90.9, p=0.04; high-risk ASD group compared with low-risk group: t=3.0, df=108.4, p=0.004; high-risk non-ASD group compared with low-risk group: t=1.62, df=229.4, p=0.11). It is noteworthy that there was no difference in ISOP scores between male and female infants in the study sample (F=0.10, df=1, 430, p=0.75).
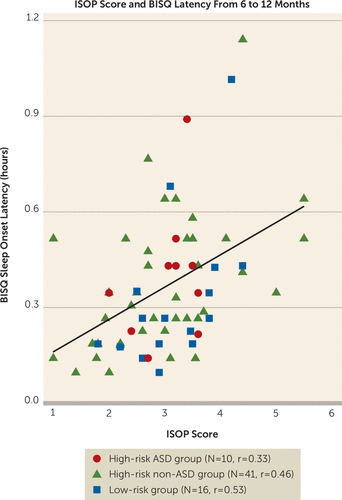
FIGURE 2. Validation of the Infant Sleep Onset Problems (ISOP) score against the Brief Infant Sleep Questionnaire (BISQ) score
Given the finding of categorical differences in ISOP scores, we explored associations between ISOP score and continuous measures of cognitive and adaptive development and ASD symptoms. Correlation results are presented in Table S2 in the online supplement. At each time point (6 months and 12 months), ISOP score correlated significantly with expressive language scores on the Mullen Scales of Early Learning (6 months, r=−0.14, p=0.01; 12 months, r=−0.11, p=0.03) but not with any of the other behavioral measures. By 24 months, average ISOP scores were significantly correlated with Autism Diagnostic Observation Schedule social affect severity scores (r=0.14, p=0.004), Mullen expressive language scores (r=−0.11, p=0.03), Vineland Adaptive Behavior Scale communication scores (r=−0.13, p=0.01), and Vineland socialization scores (r=–0.15, p=0.003). Across the entire sample, infants with worse sleep from 6 to 12 months showed weaker social communication skills by 24 months.
Sleep Onset Problems in Relation to Brain Volume Trajectories From 6 to 24 Months
Separate linear mixed-effects models were conducted for each subcortical structure (see Table S3 in the online supplement). Only hippocampal trajectory was significantly predicted by ISOP score (β=101.0, t=3.2, p=0.008), and group moderated the association between ISOP score and hippocampal trajectory in infancy (Figure 3). Examination of group-specific parameter estimates revealed that sleep onset difficulties in infancy were associated with increased hippocampal volume from 6 to 24 months only for high-risk siblings who went on to develop ASD (β=104.7; t=2.8, p=0.006); no relationship between sleep and hippocampal trajectory was found in the high-risk non-ASD group (β=−11.5; t=0.6, p=0.6) and the low-risk group (β=−4.9; t=0.2, p=0.9).
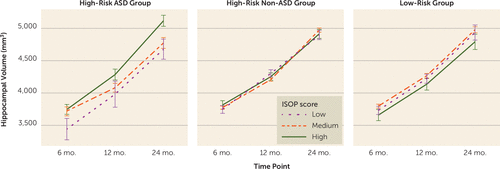
FIGURE 3. Hippocampal volume trajectories from 6 to 24 monthsa
a Categorical differences in Infant Sleep Onset Problems (ISOP) score are shown for visualization purposes only; all analyses treated ISOP score as a continuous variable. Hippocampal volumes for infants with low (1 standard deviation <mean), medium, and high (1 standard deviation >mean) ISOP scores are shown by outcome group. Higher ISOP scores indicate more sleep onset difficulties.
Hemispheric Differences
Examining associations in the left and right hemispheres separately revealed that ISOP score was significantly associated with trajectories of both left and right hippocampal volume. Specifically, there was a significant main effect of ISOP score (left: β=47.9, t=3.0, p=0.002; right: β=53.1, t=3.1, p=0.002) and significant group-by-ISOP interactions for each hemisphere (left: high-risk ASD group compared with high-risk non-ASD group: β=−49.9, t=2.7, p=0.007; high-risk ASD group compared with low-risk group: β=−54.5, t=2.5, p=0.012; right: high-risk ASD group compared with high-risk non-ASD group: β=−59.0, t=2.9, p=0.003; high-risk ASD group compared with low-risk group: β=−54.3, t=2.3, p=0.02).
Hippocampal Trajectory and Sleep Onset Problems, Controlling for Cognitive Ability
The association between ISOP score and hippocampal trajectory persisted after adding 24-month cognitive ability (Mullen early learning composite) scores to the model. Specifically, there was a significant main effect of ISOP score (β=100.0, t=3.1, p=0.002) and a significant group-by-ISOP interaction (high-risk ASD group compared with high-risk non-ASD group: β=−106.7, t=2.9, p=0.005; high-risk ASD group compared with low-risk group: β=−111.7, t=2.5, p=0.012). Twenty-four-month scores on the Mullen scales did not predict additional variance in hippocampal trajectory (β=−0.8, t=0.9, p=0.37).
Hippocampal Trajectory and Other IBQ-R Subscales
The IBQ-R has 14 subscales (see reference 22). The five sleep items that comprise the ISOP are part of the falling reactivity/rate of recovery from distress scale. To examine the specificity of the sleep-hippocampal finding, the same full linear mixed model predicting bilateral hippocampal trajectory was repeated, replacing the ISOP score with scores from each IBQ-R subscale (including the full 13-item falling reactivity/rate of recovery from distress scale, each averaged from 6 to 12 months). None of the other IBQ-R subscales were significantly related to hippocampal volume (see Table S4 in the online supplement), including the full falling reactivity/rate of recovery from distress scale from which the sleep items were derived. This suggests that the relationship with hippocampal trajectory may be specific to the five IBQ-R items comprising the ISOP score and does not represent a more general relationship with infant behavior or temperament.
Hippocampal Trajectory and Sleep Onset Problems Measured at 6 and 12 Months
The final follow-up analyses investigated whether the associations between ISOP score and trajectories of hippocampal volume from 6 to 24 months would persist if restricted to sleep measured at a single time point in development (either 6 or 12 months). Models were run substituting 6-month and 12-month ISOP scores for the averaged score and including cognitive ability (Mullen early learning composite score) measured at that same time point (6 or 12 months).
The association between hippocampal volume trajectory and sleep persisted when we used ISOP scores measured only at a single time point (6 or 12 months). Specifically, in the 6-month model, there was a significant main effect of 6-month ISOP score (β=75.9, t=2.3, p=0.02) and a significant group-by-6-month ISOP score interaction (high-risk ASD group compared with high-risk non-ASD group: β=−78.4, t=−2.1, p=0.04; high-risk ASD group compared with low-risk group: β=−109.8, t=2.6, p=0.008). Six-month scores on the Mullen scales did not predict additional variance in hippocampal trajectory (β=0.8, t=0.6, p=0.6).
Similarly, in the 12-month model, there was a significant main effect of 12-month ISOP score (β=74.5, t=2.4, p=0.02) and a significant group-by-12-month ISOP score interaction for the contrast of the high-risk ASD group compared with the high-risk non-ASD group (β=−86.9, t=−2.4, p=0.02). With the 12-month ISOP score, the contrast of the high-risk ASD group compared with the low-risk group did not reach statistical significance (β=−43.9, t=−1.1, p=0.3). Twelve-month scores on the Mullen scales did not predict additional variance in hippocampal trajectory (β=−0.22, t=0.20, p=0.8).
Discussion
In a large sample of infants at high and low familial risk for ASD, we found that sleep onset problems were more prevalent at 6–12 months among infants who went on to develop ASD. Difficulties with sleep onset during infancy were associated with weaker social communication skills by 24 months and were related to hippocampal volume trajectory from 6 months to 24 months only for the high-risk ASD group. Infants in the high-risk ASD group who had sleep onset difficulties at 6–12 months exhibited increased hippocampal volume trajectories compared with those in the high-risk ASD group who had better sleep. The association with sleep onset problems in infancy was specific to the hippocampus; no significant sleep-brain associations were found for the other subcortical structures examined (the amygdala, caudate, globus pallidus, putamen, and thalamus). Additional analyses revealed that the observed association between sleep onset problems and hippocampal trajectories in the high-risk ASD group existed in each hemisphere and persisted after controlling for cognitive ability at 24 months. In addition, the association was specific to sleep onset problems and did not generalize to other aspects of infant temperament measured on the IBQ-R. Finally, sleep-hippocampal trajectory associations persisted in models that included sleep and cognitive ability measured at a single time point (6 or 12 months).
Our finding that early sleep onset problems are specific to ASD is in line with findings from previous infant sibling studies. Sleep patterns in typically developing children who have a sibling with ASD are similar to those with no family history of ASD (21, 25). Associations with hippocampal trajectories were also specific to the high-risk ASD group. In contrast to previous studies with typically developing older children, with adults with insomnia, and with sleep-deprived animals (18, 19, 26), sleep onset problems in infants at high risk who developed ASD were associated with increased (rather than decreased) hippocampal volume across early development. The reason for this disorder-specific effect and its directionality is unclear, but it may be related to a disrupted coupling of hippocampal and brain sizes that has been observed in other ASD samples (27). Overgrowth of some structures, but not others, at different stages of development has been frequently reported in ASD (12, 13, 28). Indeed, in the same infant sibling sample as the present study, early cortical overgrowth was associated with later social deficits (12), larger corpus callosum volumes were associated with later restricted or repetitive behavior (29), and larger thalamus, caudate, and amygdala volumes were associated with abnormal language profiles (24). In a departure from this previous work, however, we show evidence of a brain-behavior relationship during infancy that precedes the onset of ASD symptoms. If replicated, these findings suggest that an individual difference in infant behavior (i.e., sleep onset difficulties) could help predict which infants will show abnormal trajectories of hippocampal growth before the onset of ASD symptoms.
The demonstration that our findings are specific to the hippocampus is consistent with a large body of non-ASD research linking sleep to morphometric changes (16–18) and accumulation of metabolic by-products in the hippocampus (e.g., beta-amyloid [30]). Sleep also plays a role in hippocampus-mediated cognitive processes, including spatial and declarative learning and consolidation (31). Adequate sleep may be required for typical hippocampal maturation: young (but not adolescent) mice that were deprived of REM sleep showed reduced long-term potentiation stability and lower expression of glutamatergic signaling proteins, both suggestive of altered hippocampal development (32). Thus, the hippocampus appears to be a brain region that is particularly sensitive to the effects of disturbed or inadequate sleep. However, it is important to recognize that the sleep-hippocampus associations we observed do not reveal a causal direction. Inadequate sleep may cause changes in the hippocampus that confer vulnerability to disorders of neurodevelopment or neurodegeneration. Alternatively, preexisting neurobiological or genetic differences may result in both hippocampal changes and sleep disturbance during the early stages of a disorder.
A variety of critical neurobiological processes occur during sleep and could underlie our findings. One potential mechanism involves neuroinflammatory processes that are modulated during sleep. Sleep deprivation has been shown to increase neuroinflammation, which affects synaptic plasticity in the hippocampus (33). Sleep disturbance has been associated with inflammatory responses in the hippocampus, but not the cortex, in adult mice and impaired subsequent performance on a hippocampus-dependent learning and memory task (34). In humans, the data on associations between sleep and inflammation are mixed. A recent meta-analysis found that increased levels of inflammatory markers were associated with sleep disturbance and long sleep duration (>8 hours per night) but not short sleep duration (<7 hours per night [35]). However, evidence of potential involvement of inflammatory responses in the pathogenesis of ASD (36) suggests that investigating sleep and neuroinflammation longitudinally in studies of early brain development (such as the present study) is a promising direction for future research.
This study has several limitations, including the use of a novel measure of sleep during infancy. Although our measure of sleep onset problems (derived from five items on the IBQ-R) revealed strong correspondence with BISQ scores and strong internal consistency in a subsample of infants, it was based on parent report and has not (to our knowledge) been validated against objective measures of sleep in infancy (i.e., actigraphy or polysomnography). In addition, these five items measured only one aspect of sleep (sleep onset latency) and did not provide information about sleep fragmentation, quality, or overall duration. It will be important in future studies to conduct more comprehensive sleep assessments to determine which characteristics of sleep during infancy are most relevant to brain and behavior development. Another important area for future research will be to investigate associations between infant sleep and cortical development, because altered cortical morphometry has been associated with ASD in high-risk siblings (12) and chronic sleep disturbance in typically developing children (20). Given the early stages of this study, and the absence of previous work examining sleep and neurodevelopment in infants at high risk for ASD, we believe these preliminary findings are of interest and warrant extension to other brain regions and replication with alternative measures of sleep.
If replicated, these observations could provide new insights into a potential role for disturbed sleep in the development of ASD. The high prevalence of ASD and comorbid sleep difficulties in some rare genetic syndromes (37) has led to the suggestion that ASD and sleep problems may have shared etiology (38). Impairments in circadian timing (39), sleep-wake regulation (8), sensory processing (38), and affective functioning (7) may underlie both sleep difficulties and core symptoms of ASD. The efficacy of both pharmacologic (e.g., melatonin) and behavioral sleep interventions for some children with ASD suggests that both neurobiological and psychosocial factors should be considered. Our findings provide initial evidence that sleep difficulties in the first year of life may precede ASD diagnosis and are associated with altered neurodevelopmental trajectories in high-risk siblings who go on to develop ASD. We expect future work to reveal the implications of these results for understanding neurodevelopment in ASD and for developing early, targeted interventions for sleep difficulties in infants at high risk for ASD.
1 : Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics 2003; 111:302–307Crossref, Medline, Google Scholar
2 : Sleep and development in genetically tractable model organisms. Genetics 2016; 203:21–33Crossref, Medline, Google Scholar
3 : Relations between physiological and cognitive regulatory systems: infant sleep regulation and subsequent executive functioning. Child Dev 2010; 81:1739–1752Crossref, Medline, Google Scholar
4 : Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience 2002; 110:431–443Crossref, Medline, Google Scholar
5 : Sleep problems in 2- to 5-year-olds with autism spectrum disorder and other developmental delays. Pediatrics 2019; 143:e20180492Crossref, Medline, Google Scholar
6 : The relationship between sleep and behavior in autism spectrum disorder (ASD): a review. J Neurodev Disord 2014; 6:44Crossref, Medline, Google Scholar
7 : Sleep problems in autism spectrum disorders: prevalence, nature, and possible biopsychosocial aetiologies. Sleep Med Rev 2009; 13:403–411Crossref, Medline, Google Scholar
8 : Sleep in children with autistic spectrum disorder. Sleep Med 2010; 11:659–664Crossref, Medline, Google Scholar
9 : Sleep patterns in children with autistic spectrum disorders: a prospective cohort study. Arch Dis Child 2014; 99:114–118Crossref, Medline, Google Scholar
10 : Prospective associations between infant sleep at 12 months and autism spectrum disorder screening scores at 24 months in a community-based birth cohort. J Clin Psychiatry 2018; 79(1) pii: 16m11127Crossref, Google Scholar
11 : Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord 2015; 7:24Crossref, Medline, Google Scholar
12 : Early brain development in infants at high risk for autism spectrum disorder. Nature 2017; 542:348–351Crossref, Medline, Google Scholar
13 : The neurodevelopment of autism from infancy through toddlerhood. Neuroimaging Clin N Am 2020; 30:97–114Crossref, Medline, Google Scholar
14 : Neuroanatomy of autism. Trends Neurosci 2008; 31:137–145Crossref, Medline, Google Scholar
15 : The neuroanatomy of autism spectrum disorder: an overview of structural neuroimaging findings and their translatability to the clinical setting. Autism 2017; 21:18–28Crossref, Medline, Google Scholar
16 : The relationship between hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol 2012; 8:130–138Crossref, Medline, Google Scholar
17 : Insomnia severity is associated with a decreased volume of the CA3/dentate gyrus hippocampal subfield. Biol Psychiatry 2010; 68:494–496Crossref, Medline, Google Scholar
18 : Changes in subcortical shape and cognitive function in patients with chronic insomnia. Sleep Med 2017; 35:23–26Crossref, Medline, Google Scholar
19 : Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. Neuroimage 2012; 60:471–475Crossref, Medline, Google Scholar
20 : The developmental course of sleep disturbances across childhood relates to brain morphology at age 7: the Generation R Study. Sleep (Basel) 2017; 40:40Google Scholar
21 : Behavior and sleep problems in children with a family history of autism. Autism Res 2013; 6:169–176Crossref, Medline, Google Scholar
22 : Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behav Dev 2003; 26:64–86Crossref, Google Scholar
23 : A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics 2004; 113:e570–e577Crossref, Medline, Google Scholar
24 : Subcortical brain and behavior phenotypes differentiate infants with autism versus language delay. Biol Psychiatry Cogn Neurosci Neuroimaging 2017; 2:664–672Crossref, Medline, Google Scholar
25 : Sibling sleep: what can it tell us about parental sleep reports in the context of autism? Clin Pract Pediatr Psychol 2016; 4:137–152Crossref, Medline, Google Scholar
26 : Sleep deprivation reduces proliferation of cells in the dentate gyrus of the hippocampus in rats. J Physiol 2003; 549:563–571Crossref, Medline, Google Scholar
27 : Understanding hippocampal development in young children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry (Epub ahead of print August 23, 2019)Google Scholar
28 : When is the brain enlarged in autism? a meta-analysis of all brain size reports. Biol Psychiatry 2005; 58:1–9Crossref, Medline, Google Scholar
29 : Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain 2015; 138:2046–2058Crossref, Medline, Google Scholar
30 : β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci USA 2018; 115:4483–4488Crossref, Medline, Google Scholar
31 : Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience 2015; 309:173–190Crossref, Medline, Google Scholar
32 : Rapid eye movement sleep deprivation decreases long-term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience 2008; 153:44–53Crossref, Medline, Google Scholar
33 : Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis 2013; 52:229–236Crossref, Medline, Google Scholar
34 : Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis 2012; 48:348–355Crossref, Medline, Google Scholar
35 : Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 2016; 80:40–52Crossref, Medline, Google Scholar
36 : Neuroinflammation in preterm babies and autism spectrum disorders. Pediatr Res 2019; 85:155–165Crossref, Medline, Google Scholar
37 : Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014; 158:263–276Crossref, Medline, Google Scholar
38 : The relationship between sleep problems, neurobiological alterations, core symptoms of autism spectrum disorder, and psychiatric comorbidities. J Clin Med 2018; 7:102Crossref, Google Scholar
39 : Circadian rhythms and sleep in children with autism. Neurosci Biobehav Rev 2010; 34:755–768Crossref, Medline, Google Scholar
40 : A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics 2012; 130(Suppl 2):S106–S124Crossref, Medline, Google Scholar



