Risk of Major Malformations in Infants Following First-Trimester Exposure to Quetiapine
Abstract
Objective:
Second-generation antipsychotics are commonly prescribed to reproductive-age women for the treatment of a spectrum of psychiatric disorders. Quetiapine is the most commonly prescribed medication in this class, and therefore a better understanding of its reproductive safety profile is critical. The goal of this study was to determine the risk of major malformations among infants exposed to quetiapine during pregnancy compared with a group of infants whose mothers had a history of psychiatric morbidity but who did not use a second-generation antipsychotic during pregnancy.
Method:
The National Pregnancy Registry for Atypical Antipsychotics interviews pregnant women ages 18–45 during pregnancy and the postpartum period. Obstetric, labor, and delivery medical records and pediatric medical records from the first 6 months of life were screened for evidence of major malformations, followed by adjudication by a blinded dysmorphologist. Women with first-trimester exposure to quetiapine were compared with control subjects without exposure to second-generation antipsychotics.
Results:
As of March 2017, 888 women had enrolled prospectively and 357 were eligible for analysis. Of these, 152 women with first-trimester exposure to quetiapine were compared with 205 control subjects without any second-generation antipsychotic exposure. For the 155 infants born to women in the exposed group (including three sets of twins), two major malformations were confirmed (1.3%), compared with three major malformations among the 210 infants born in the unexposed group (including five sets of twins) (1.4%). The unadjusted odds ratio for major malformations between infants with and without quetiapine exposure was 0.90 (95% CI=0.15, 5.46), which is consistent with the pooled estimate of the available controlled data on fetal exposure to quetiapine.
Conclusions:
These data regarding the safety of fetal exposure to quetiapine in a small sample of well-characterized participants are reassuring given that the confidence interval does not exceed a fivefold increased risk of major malformations relative to psychiatric control subjects. Future analyses based on ongoing data collection will produce more precise estimates.
The National Pregnancy Registry for Atypical Antipsychotics (NPRAA) at Massachusetts General Hospital was established in 2008 to increase the availability of systematically gathered reproductive safety data for second-generation antipsychotics. Over the past decade, use of second-generation antipsychotics has continued to increase, with a growing number of approved indications for their use and concurrent off-label use (1). The primary aim of the NPRAA is to delineate the risk of major malformations following in utero exposure to second-generation antipsychotics compared with a control group of women with psychiatric disorders who did not use agents in this drug class during pregnancy. Preliminary data from the NPRAA regarding the safety of second-generation antipsychotics as a class have been reported (2).
To date, available reproductive safety data do not suggest that second-generation antipsychotics as a class are major teratogens. In addition, no specific pattern of malformations has been identified following fetal exposure to this class of medication (3–6). Nonetheless, in contrast to the scope of use of second-generation antipsychotics by reproductive-age women, systematic, prospectively gathered data regarding the reproductive safety of this class of agents remain relatively limited. Analysis of rigorously collected reproductive safety data is timely given recent changes in U.S. Food and Drug Administration (FDA) regulations governing product labeling incorporated as part of the Pregnancy and Lactation Labeling Rule, which shifts from the previous system of category labeling to a more descriptive summary of outcomes of exposure to medications during pregnancy and lactation. Consistent with these new guidelines, reproductive safety data will now be included in the medication labeling, along with reference to existing pregnancy registries. This will be applicable for new medications coming to market and for approved products as their labeling is updated. The FDA recently instituted a major revision of the labeling documentation for pharmacologic treatments pertaining to pregnancy and lactation information (7).
Quetiapine is used across a wide range of psychiatric indications in the United States. Its current FDA indications include adjunctive therapy to antidepressants in major depressive disorder, acute depressive episodes associated with bipolar I disorder, manic or mixed episodes in bipolar disorder, maintenance treatment of bipolar disorder, and schizophrenia. In the United States and elsewhere, quetiapine is also frequently used off-label for conditions such as treatment-resistant anxiety and sleep dysregulation (8–11). The Harvard Program on Perinatal and Pediatric Pharmacoepidemiology, using data on publicly insured pregnant women from an administrative health care utilization database, recently described the use of second-generation antipsychotics (12) and noted that quetiapine and aripiprazole were the most commonly prescribed agents in this drug class, primarily for management of bipolar disorder. The study also found that quetiapine was commonly prescribed in conjunction with antidepressants, benzodiazepines, or other mood stabilizers.
The ability to delineate risk of malformations associated with particular second-generation antipsychotics has been limited. The existing data on malformations associated with quetiapine exposure is derived mainly from a small number of studies with differing methodologies and outcome measures. Researchers from the Harvard Program on Perinatal and Pediatric Pharmacoepidemiology, using the above-mentioned administrative health care utilization database, examined the reproductive safety of antipsychotics (5). The prevalence of birth defects was elevated in the 4,221 pregnancies exposed to quetiapine; however, after adjustment for confounding variables (e.g., psychiatric indication), the association attenuated. Three cohort studies (3, 13, 14) reported the absolute risk of birth defects in exposed babies to be similar to or slightly above estimates of the risk of birth defects in the general population; Haberman et al. (3) reported an absolute risk of 4.05%, Kulkarni et al. (13) 3.59%, and Sadowski et al. (14) 6.45%, based on a total of 306 women across the three studies. These three studies either included healthy control subjects or did not have a comparison group, which greatly limits the ability to distinguish the relative contributions of underlying maternal illness from exposure to medication. Six other prospective studies (15–20) included 10 or fewer live births with a history of fetal exposure to quetiapine; none of the offspring had a malformation. Despite a growing literature on risks associated with exposure to quetiapine during pregnancy, the need still exists for better reproductive safety data, derived from cohort studies that incorporate rigorous methodology, for this medication.
Our aim in this study was to quantify the relative risk of major malformations associated with first-trimester exposure to quetiapine compared with an unexposed control group of pregnant women who had psychiatric disorders but did not use quetiapine or any other second-generation antipsychotics during pregnancy.
Method
Data Collection
The NPRAA was established in 2008 to provide rigorously obtained postmarketing reproductive safety data on the risk of major malformations associated with second-generation antipsychotic use during pregnancy. Methods of data collection, assessment of outcomes, the governance structure of the registry, and guidelines for release of findings have been published elsewhere (21). In brief, any pregnant woman between the ages of 18 and 45 with a history of a psychiatric illness may enroll. The participants themselves (as opposed to the clinicians prescribing for them) enroll in the registry; they are often referred by a health care provider. After verbal ;consent is obtained, participants are prospectively interviewed across pregnancy and the early postpartum period by telephone, at enrollment, at 7 months’ gestation, and 12 weeks after delivery. Information systematically collected includes demographic data, obstetric history, medication use and dosages, psychiatric diagnoses, medical history, and family history of birth defects. Participants can also consent to collection of obstetric, labor, delivery, neonatal, and pediatric medical records. Pediatric records (and records from relevant specialists when indicated) are obtained from birth through 6 months after delivery. Approximately 83% of participants agree to release medical records. To maintain fidelity to the prospective nature of the study and to avoid biasing the results, women with already documented evidence of a birth defect via prenatal testing at the time of enrollment are excluded from analysis for the primary outcome. The study continues to recruit participants who use psychiatric medications during pregnancy.
The Scientific Advisory Board of the NPRAA determines a priori parameters for release of risk estimates of major malformations for the aggregate sample and individual medications. Factors that determine the timing of data release include 1) a minimum number of participants exposed to a medication of interest (particularly if no data are available in the literature regarding an agent that was released relatively recently and for which no reproductive safety data have been reported), and 2) appropriately stabilized confidence intervals, such that the data would, even if preliminary, be informative, particularly for clinicians.
Case Identification
Data collected from interviews and medical records are reviewed and abstracted using a standardized outcome template by a trained research assistant as well as a senior investigator (A.C.V.). If a major malformation is suspected, the records, which include information extending to 6 months after birth, are redacted and sent to a dysmorphologist for final blind adjudication. Chromosomal and single-gene abnormalities, as well as minor anomalies, are excluded by the dysmorphologist, who has specialty training in teratology and genetics.
Data Infrastructure
Study data were collected and managed using the REDCap (Research Electronic Data Capture) electronic data capture tools, hosted by Partners HealthCare Research Computing, Enterprise Research Infrastructure and Services group. REDCap is a secure web-based application designed to support data capture for research studies (22).
Statistical Analysis
Baseline demographic data and clinical characteristics were summarized and compared between the quetiapine-exposed and control groups to assess balance in risk factors for major malformations. For continuous variables, data were presented as means and standard deviations and groups were compared using unpaired t tests. Categorical variables were presented as absolute numbers and percentages and were compared by either chi-square or Fisher’s exact tests, as determined by cell frequencies. All statistical analyses were performed using Stata/SE, version 14.2 (StataCorp, College Station, Tex.).
The exposure of interest was defined as use of quetiapine during the first trimester of pregnancy (<13 weeks gestational age). Participants were deemed evaluable for this analysis if medical records had been obtained or if a postpartum interview had been conducted by the time of data extraction. The main comparison group consisted of women with psychiatric disorders who did not use second-generation antipsychotics during pregnancy (but many of whom were treated with other psychotropic medications, such as antidepressants, anxiolytics, and mood stabilizers such as anticonvulsants and lithium). The primary outcome was the presence of a major malformation identified within 6 months of birth. Cases of terminations or stillbirths where there was evidence of a major malformation were included for blind adjudication by the dysmorphologist. A major malformation was defined as a structural abnormality with surgical, medical, or cosmetic importance.
Unconditional logistic regression was used to estimate the odds ratio and 95% confidence interval for major malformations between groups. To determine the impact of potential confounders, each covariate was added individually to the unadjusted model and the change in odds ratio was examined to assess the magnitude and direction of the bias. A change ≥10% was used as a guide to identify confounders.
Results
A total of 888 women were prospectively enrolled in the registry from November 14, 2008, to March 14, 2017. For the analysis, 357 women had evaluable data and first-trimester exposure to a second-generation antipsychotic (Figure 1). Of the 357 eligible participants, 152 used quetiapine during the first trimester. Of the 152 women exposed to quetiapine during the first trimester, three gave birth to twins, resulting in 155 exposed live births. Among the 205 control women with evaluable data, five sets of twins were born, resulting in 210 live births in the control group. Medical records were obtained and reviewed for 87.8% of the participants with evaluable data.
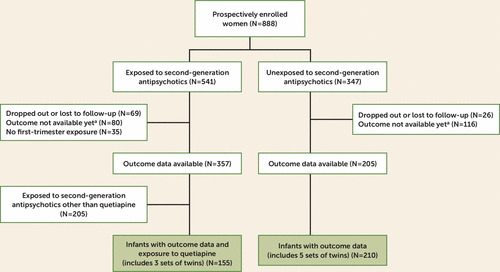
FIGURE 1. Study Enrollment and Selection of the Analytic Sample in a Study of the Risk of Major Malformations in Infants Following First-Trimester Exposure to Quetiapine
a Participants still actively enrolled.
The participants’ demographic and clinical characteristics are summarized in Table 1. Women exposed to quetiapine during the first trimester were more likely to have a primary psychiatric diagnosis of bipolar disorder compared with the control women, who were not exposed to second-generation antipsychotics (67.8% compared with 28%). Unexposed women were more likely to have a primary diagnosis of depression (26.8% compared with 13.8%) or an anxiety disorder (24.4% compared with 4%). Selective serotonin reuptake inhibitors were more commonly used in the control group, and anticonvulsants and anxiolytics were more commonly used in the exposed group.
| Measure | First-Trimester Exposure to Quetiapine (N=152) | Unexposed to Second-Generation Antipsychotics (N=205) | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | pa | |
| Psychiatric illness severity | |||||
| Age at onset of primary disorder (years) | 17 | 6.5 | 18 | 6.1 | 0.054 |
| Lifetime number of psychiatric hospitalizations | 5 | 5.4 | 2.84 | 3.6 | 0.06 |
| Chronicity (proportion of life, %) | 51 | 19.1 | 44 | 17.9 | 0.001 |
| Demographic characteristics | |||||
| Age (years) | 32.3 | 4.89 | 33.3 | 4.07 | 0.04 |
| Baseline BMI | 27.1 | 6.5 | 25.3 | 5.1 | 0.004 |
| N | % | N | % | pa | |
| White | 137 | 90.1 | 191 | 93.2 | 0.30 |
| College education | 112 | 73.7 | 175 | 85.4 | 0.006 |
| Married | 114 | 75.0 | 184 | 90.2 | <0.001 |
| Pregnancy history | |||||
| Planned pregnancy | 111 | 74.0 | 174 | 85.7 | 0.006 |
| >1 prior pregnancy | 116 | 76.3 | 144 | 70.2 | 0.20 |
| Prior miscarriage | 54 | 55.1 | 56 | 52.3 | 0.69 |
| History of postpartum depression or psychosis | 104 | 68.4 | 141 | 68.8 | 0.94 |
| First-trimester use | |||||
| Cigarettes | 32 | 21.1 | 11 | 5.4 | <0.001 |
| Alcohol | 30 | 19.7 | 64 | 31.7 | 0.012 |
| Illicit drugs | 11 | 7.3 | 5 | 2.5 | 0.031 |
| Prenatal vitamins | 112 | 73.7 | 173 | 84.8 | 0.009 |
| Primary psychiatric diagnosis | |||||
| Bipolar disorder | 103 | 67.8 | 51 | 28.0 | <0.001 |
| Schizophrenia | 2 | 1.3 | 0 | 0.0 | 0.10 |
| Depression | 21 | 13.8 | 55 | 26.8 | 0.003 |
| Anxiety disorder | 6 | 4.0 | 50 | 24.4 | <0.001 |
| First-trimester psychotropic medication use | |||||
| First-generation antipsychotic | 3 | 2.0 | 2 | 1.0 | 0.65 |
| SSRI | 48 | 31.6 | 103 | 50.2 | <0.001 |
| SNRI | 11 | 7.2 | 12 | 5.9 | 0.60 |
| Tricyclic antidepressant | 2 | 1.3 | 3 | 1.5 | 1.00 |
| Second-generation antidepressant | 19 | 12.5 | 31 | 15.1 | 0.48 |
| Lithium | 4 | 2.6 | 4 | 2.0 | 0.73 |
| Anticonvulsant | 70 | 46.1 | 41 | 20.0 | <0.001 |
| Anxiolytic | 44 | 29.0 | 40 | 19.5 | 0.044 |
| Sedative | 14 | 9.2 | 9 | 4.4 | 0.07 |
| Stimulant | 7 | 4.6 | 10 | 4.9 | 0.91 |
TABLE 1. Demographic and Clinical Characteristics of Women Exposed to Quetiapine or Unexposed to Antipsychotics During Pregnancya
Most of the women in the exposed group used quetiapine during the entire gestational period (N=126, 83%); 152 women were exposed to quetiapine during the first trimester, 150 during the second trimester, and 142 during the third trimester. Eleven women were exposed only during the first trimester, three women only during the second trimester, and six women only during the third trimester.
The prevalence of major malformations among infants with first-trimester exposure to quetiapine (N=155) was 1.29% (95% CI=0.16, 4.58) and among infants unexposed to second-generation antipsychotics (N=210), 1.43% (95% CI=0.30, 4.12). Two major malformations were reported among infants with exposure to quetiapine: one infant with transposition of the great arteries and one infant with pulmonary stenosis due to dysplastic pulmonary valve (Table 2). In the control group, three major malformations were reported: one infant with midshaft hypospadias requiring surgical repair, one infant with isolated cleft lip and palate, and one infant with a thickened pulmonary valve associated with mild pulmonary stenosis. The unadjusted odds ratio of major malformations for infants with first-trimester exposure to quetiapine compared with infants unexposed to second-generation antipsychotics was 0.90 (95% CI=0.15, 5.46; p=0.91).
| Malformation | Other First-Trimester Exposures |
|---|---|
| Infants exposed to quetiapine | |
| Transposition of the great arteries | Aripiprazole, bupropion, labetalol |
| Pulmonary stenosis due to dysplastic pulmonary valve | Duloxetine, valacyclovir, levothyroxine/liothyronine, metformin |
| Infants unexposed to second-generation antipsychotics | |
| Midshaft hypospadias requiring surgical repair | Lorazepam |
| Isolated cleft lip and palate | Fluoxetine |
| Thickened pulmonary valve associated with mild pulmonary stenosis | Citalopram, triamcinolone |
TABLE 2. Summary of Major Malformations (N=5) and Other First-Trimester Exposures Among Infants With First-Trimester Exposure to Quetiapine (N=155) and Infants Unexposed to Any Second-Generation Antipsychotics (N=210)
Maternal body mass index (BMI), having a college education, use of prenatal vitamins, marital status, first trimester use of cigarettes, use of anticonvulsants, and diagnosis of depression met the confounder criteria. Maternal BMI moved the odds ratio downward (odds ratio=0.77, 95% CI=0.12, 4.88), and use of anticonvulsants moved the odds ratio upward (odds ratio=1.36, 95% CI=0.22, 8.29). All other variables moved the odds ratio upward but below the null value of 1 (Figure 2). Potential confounding variables will continue to be examined as the sample continues to grow.
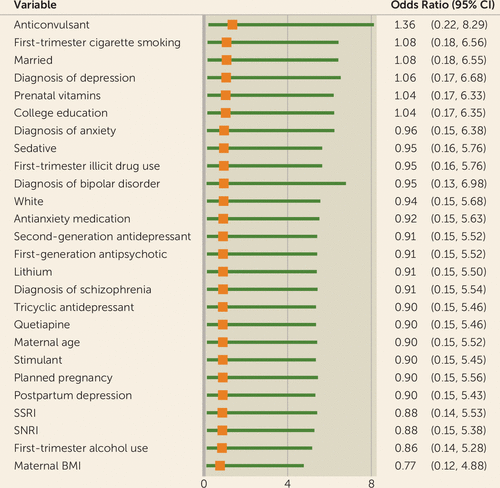
FIGURE 2. Odds of Major Malformations in Infants Following First-Trimester Exposure to Quetiapine Compared With Those Unexposed to Any Second-Generation Antipsychotics, After Adjustment for Potential Confoundersa
a BMI=body mass index; SSRI=selective serotonin reuptake inhibitor; SNRI=serotonin-norepinephrine reuptake inhibitor.
Discussion
In this study, we provide preliminary but systematically and prospectively obtained data regarding the prevalence of major congenital malformations following first-trimester exposure to quetiapine compared with an unexposed comparison group. Given the considerable use of quetiapine among women of reproductive age across multiple indications, it is critical to have better information regarding the potential risks of fetal exposure to this medication so that women can make informed treatment decisions consistent with their personal wishes and the severity of their underlying psychiatric disorder.
The study results suggest that quetiapine is not a major teratogen. However, it should be pointed out that given the uncertainty about the exact risk estimate (secondary in large part to the sample size), the results can rule out only an approximately fivefold increased risk of major malformations (which has been seen in medications such as valproate). That being said, the data from this analysis are consistent with the existing literature, which does not suggest a strong association between fetal exposure to second-generation antipsychotics and an increase in the rates of major malformations (23–25). Considering the accumulated evidence derived from controlled studies (N=4) (3, 5, 14) examining the relationship between fetal exposure to quetiapine and risk of malformations, the pooled risk estimate is more on the order of 1.03 (95% CI=0.89, 1.19) (Table 3), which suggests no increased risk and is reassuring.
| Study | Risk Ratio | 95% CI |
|---|---|---|
| Habermann et al. (3)b | 1.46 | 0.57, 3.75 |
| Sadowski et al. (14)b | 2.49 | 0.64, 9.71 |
| Huybrechts et al. (5)c | 1.01 | 0.88, 1.17 |
| Cohen et al. (this study)c | 0.90 | 0.15, 5.46 |
| Pooled risk ratiod | 1.03 | 0.89, 1.19 |
TABLE 3. Pooled Risk Ratio for Major Malformations in Infants Exposed In Utero to Quetiapinea
The risk of malformations must be considered in the context of a therapeutic risk-benefit balance. Notably, stopping medications for psychiatric disorders is associated with a considerable risk of relapse, including bipolar disorder (26, 27), which was the disorder for which most women in this study were treated with quetiapine. Psychiatric disorders tend to have onset prior to pregnancies, typically in childhood and adolescence (28, 29). Because these disorders are often chronic or recurrent, many women use maintenance pharmacotherapy to sustain euthymia throughout the reproductive years. In addition, some women will experience the onset of an index episode psychiatric disorder during pregnancy, which may require treatment.
The strengths of this study include its prospective design; inclusion of a control group with psychiatric diagnoses; careful collection of information regarding potential confounders, comorbidity, substance use, and use of concomitant medication; confirmation of outcomes with medical records; and verification of the primary outcome of major malformations by a dysmorphologist blind to medication exposures. Because of the existence of numerous potential confounding variables, attention to factors such as demographic characteristics, psychiatric diagnoses, use of concomitant medications and illicit substances, and other exposures must be carefully factored into study designs, and this is a clear strength of the data derived from the NPRAA.
The limitations of this study include the small number of participants specifically exposed to quetiapine. However, the sample size in this study is still relatively large considering the total number of exposures described in the literature. Also, confidence intervals remain wide, and it is anticipated that the relative risk estimate will stabilize over time as confidence intervals narrow with a growing sample size.
These findings represent preliminary yet important data with profound clinical implications for pregnant women and women of reproductive potential. The combination of data from rigorous analyses described in this report from the NPRAA and reproductive safety information from large administrative databases provides clinicians with a number of complementary tools to inform treatment paths for patients, taking into account individual clinical situations and patient wishes. It is imperative that research efforts continue to focus on the reproductive safety of psychiatric medications that are commonly used by women during their childbearing years.
1 Maglione M, Maher AR, Hu J, et al: Off-label use of atypical antipsychotics: an update. Rockville, Md, Agency for Healthcare Research and Quality, 2011Google Scholar
2 : Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry 2016; 173:263–270Link, Google Scholar
3 : Atypical antipsychotic drugs and pregnancy outcome: a prospective cohort study. J Clin Psychopharmacol 2013; 33:453–462Crossref, Medline, Google Scholar
4 : Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract 2009; 15:183–192Crossref, Medline, Google Scholar
5 : Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 2016; 73:938–946Crossref, Medline, Google Scholar
6 : Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry 2005; 66:444–449Crossref, Medline, Google Scholar
7 : Content and format of labeling for human prescription drug and biological products: requirements for pregnancy and lactation labeling: final rule. Fed Regist 2014; 79:72063–72103Medline, Google Scholar
8 : The antipsychotic agent quetiapine is increasingly not used as such: dispensed prescriptions in Norway, 2004–2015. Eur J Clin Pharmacol 2017; 73:1173–1179Crossref, Medline, Google Scholar
9 : Diagnoses associated with use of atypical antipsychotics in a commercial health plan: a claims database analysis. Clin Ther 2013; 35:1867–1875Crossref, Medline, Google Scholar
10 : Dispensed prescriptions for quetiapine and other second-generation antipsychotics in Canada from 2005 to 2012: a descriptive study. CMAJ Open 2014; 2:E225–E232Crossref, Medline, Google Scholar
11 : International trends in antipsychotic use: a study in 16 countries, 2005–2014. Eur Neuropsychopharmacol 2017; 27:1064–1076Crossref, Medline, Google Scholar
12 : Antipsychotic medication use among publicly insured pregnant women in the United States. Psychiatr Serv 2017; 68:1112–1119Link, Google Scholar
13 : A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One 2014; 9:e94788Crossref, Medline, Google Scholar
14 : Pregnancy outcomes following maternal exposure to second-generation antipsychotics given with other psychotropic drugs: a cohort study. BMJ Open 2013; 3:e003062Crossref, Medline, Google Scholar
15 : Use of polypharmacotherapy in pregnancy: a prospective outcome in a case. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28:603–605Crossref, Medline, Google Scholar
16 : Effects of prenatal exposure to atypical antipsychotics on postnatal development and growth of infants: a case-controlled, prospective study. Psychopharmacology (Berl) 2013; 228:577–584Crossref, Medline, Google Scholar
17 : Pregnancies exposed to the SSRI or atypical antipsychotic drugs: CZTIS small prospective study. Reprod Toxicol 2011; 31:262Crossref, Google Scholar
18 : Atypical antipsychotic use in pregnancy: a retrospective review. Arch Women Ment Health 2009; 12:53–57Crossref, Medline, Google Scholar
19 : The safety of quetiapine: results of a post-marketing surveillance study on 1728 patients in England. J Psychopharmacol 2007; 21:392–399Crossref, Medline, Google Scholar
20 : Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol 2008; 28:279–288Crossref, Medline, Google Scholar
21 : Establishment of the National Pregnancy Registry for Atypical Antipsychotics. J Clin Psychiatry 2015; 76:986–989Crossref, Medline, Google Scholar
22 : Research Electronic Data Capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381Crossref, Medline, Google Scholar
23 : Obstetric and neonatal outcomes after antipsychotic medication exposure in pregnancy. Obstet Gynecol 2015; 125:1224–1235Crossref, Medline, Google Scholar
24 : A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry 2017; 78:e477–e489Crossref, Medline, Google Scholar
25 : Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations: a systematic review. Basic Clin Pharmacol Toxicol 2015; 116:315–320Crossref, Medline, Google Scholar
26 : Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry 2000; 157:179–184Link, Google Scholar
27 : Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry 2007; 164:1817–1824Link, Google Scholar
28 : Psychiatric epidemiology: it’s not just about counting anymore. Arch Gen Psychiatry 2005; 62:590–592Crossref, Medline, Google Scholar
29 : Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:617–627Crossref, Medline, Google Scholar



