Antipsychotic Medication Use Among Publicly Insured Pregnant Women in the United States
Abstract
Objective:
Given the increasing use and broadening of indications for use of antipsychotic medications in the general population, as well as the paucity of information on the safety of this drug class during pregnancy, the study documented patterns of antipsychotic medication use among pregnant women.
Methods:
Medicaid Analytic eXtract data (2001–2010) from pregnant women who delivered live-born infants were used. Antipsychotic use at both the class and the individual drug level was defined based on dispensed outpatient prescriptions. Users’ characteristics, including mental disorder diagnoses, were described. Temporal trends in use, as well as discontinuation patterns and psychotropic polytherapy, during pregnancy were evaluated.
Results:
Among 1,522,247 pregnancies, the prevalence of use of second-generation antipsychotics at any time during pregnancy increased threefold, from .4% to 1.3%, over the ten-year period, while the use of first-generation antipsychotics remained stable at around .1%. The increased use of second-generation antipsychotics was largely driven by more frequent use among patients with bipolar disorder. Quetiapine and aripiprazole were the most frequently dispensed drugs, and polytherapy with antipsychotics and antidepressants (65.2%), benzodiazepines (24.9%), and other mood stabilizers (22.0%) was common. More than 50% of women receiving an antipsychotic in the three months prior to pregnancy discontinued the drug during pregnancy.
Conclusions:
A growing number of pregnant women in Medicaid are exposed to second-generation antipsychotics, frequently in combination with other psychotropic medications. This study highlights the importance of documenting the use and safety of these drugs during pregnancy to inform therapeutic decision making for pregnant women with psychiatric disorders.
Over the past two decades, the use of antipsychotic medications to treat psychiatric disorders has greatly expanded in the United States (1). Schizophrenia and other psychotic disorders have long been treated with both first-generation and second-generation antipsychotics. However, since 2000, a number of second-generation antipsychotics have received approval for broader indications, including irritability in autism, mood stabilization in bipolar disorder, and adjunct therapy for major depressive disorder. Increased off-label use of antipsychotics to treat attention-deficit hyperactivity disorder (ADHD) or behavioral disorders has also been reported in recent years (2–4).
For women, psychiatric disorders that are treated with antipsychotics often present during the childbearing years (5), and managing the risk-benefit trade-off of providing treatment during pregnancy is challenging. Although continuous treatment may be important to prevent symptomatic episodes or relapse (6), concerns exist about the effects of antipsychotic treatment on maternal and fetal safety. Case reports and studies with small samples have reported conflicting findings on the association between use of first-generation antipsychotics and the risk of congenital anomalies (7–9). There are few large, well-controlled studies examining the teratogenicity of second-generation antipsychotics (10,11), but the results from a recent large study with 9,258 women exposed to second-generation antipsychotics did not find increased risk of congenital malformation (12). Second-generation antipsychotics are known to cause weight gain and increase the risk of type 2 diabetes mellitus in the general population (13). Among pregnant women, these risks may translate into higher risks for diabetes-associated adverse pregnancy outcomes, such as fetal macrosomia or gestational diabetes and its attendant effects.
It is important to understand the extent and patterns of use of antipsychotics among pregnant women, in light of the changes that have occurred in antipsychotic prescribing in the past decade (1–3). During that time, there has been rising use of antipsychotics in the general population and broadening of the indications for antipsychotic treatment. At the same time, there is limited information on the safety profile of this drug class during pregnancy. Describing antipsychotic utilization trends in a publicly insured population is especially important because Medicaid covers the costs for approximately 80% of all antipsychotic prescriptions (14) and for close to 50% of all deliveries of babies in the United States (15). We used nationwide Medicaid data to investigate the patterns of antipsychotic use among publicly insured women in the United States.
Methods
Data Source and Study Population
We used Medicaid Analytic eXtract (MAX) data from 2001 to 2010 from the Centers for Medicare and Medicaid Services. MAX contains person-level information on demographic characteristics, hospitalizations, and outpatient visits as well as on medications dispensed by an outpatient pharmacy. We created a linked cohort of pregnant women and their live-born babies based on a process described in detail elsewhere (16). Linkage provided delivery dates that were used to estimate the last menstrual period (LMP) by using a previously validated algorithm (17). To ensure complete capture of health care use information, women were required to have continuous coverage from three months before the LMP to one month after delivery and to not have other private insurance or restricted benefits during pregnancy.
Antipsychotic Medication Use
To describe antipsychotic exposure and patient characteristics, the time period was divided into four windows: three months prior to the LMP (baseline), the LMP to gestational day 90 (first trimester), gestational day 91 to gestational day 180 (second trimester), and gestational day 181 to delivery (third trimester). Antipsychotic exposure was defined as filling at least one prescription during the respective window, based on dispensing dates in the outpatient pharmacy dispensing file. [The full list of antipsychotics considered in the study is available as an online supplement to this article.] Prochlorperazine and promethazine were excluded because they are more commonly used for nonpsychiatric conditions (18). We examined antipsychotic use at the class level (first or second generation) and at the generic level (specific drug) for the six most frequently used drugs (aripiprazole, olanzapine, quetiapine, risperidone, ziprasidone, and haloperidol). Most women (>99%) received oral medications, so we did not distinguish injectable forms. When a woman was dispensed more than one type of antipsychotic, each dispensation was counted separately toward each drug exposure category, given that the main purpose of this study was to describe the extent of antipsychotic use in this population.
Psychiatric Disorder Diagnoses
To document psychiatric disorders, we searched for the presence of ICD-9 codes for anxiety disorder, ADHD, bipolar disorder, depression, and schizophrenia or other psychotic disorder from 90 days prior to the LMP to delivery [see online supplement for the full list of diagnostic codes considered in the study]. Following the approach of Crystal and associates (1), we created mutually exclusive hierarchical diagnosis categories, given that multiple diagnoses often occur concurrently (5). Women were classified into the highest possible category from the following five categories, ranked from lowest to highest: ADHD only; anxiety with or without ADHD; depression with or without anxiety or ADHD; bipolar disorder with or without depression, anxiety, or ADHD; and schizophrenia or other psychotic disorder with or without bipolar disorder, depression, anxiety, or ADHD.
Analyses
Trends in use.
Temporal trends in antipsychotic use by delivery year were examined, and the p value for trend was reported. The prevalence of any antipsychotic use, stratified by age at time of delivery and race-ethnicity, was examined. To evaluate changes in drug use as a function of changes in diagnoses, we examined the yearly prevalence of each diagnosis by using the hierarchical definitions as well as the proportion of women with each diagnosis who were receiving any antipsychotic medication.
Characteristics of the study population.
We used descriptive statistics to characterize the population in terms of demographic variables; comorbid diagnoses, such as other mental disorders, pain disorders, hypertension, and diabetes; and use of other medications, such as anxiolytics, hypnotics, and opioids, during the baseline period and the first trimester. Additionally, we investigated concomitant treatment with major psychotropic medications (antidepressants, benzodiazepines, and other mood stabilizers [defined in this study as lithium, carbamazepine, lamotrigine, oxcarbazepine, topiramate, and valproate]) among women who received antipsychotic medication during pregnancy.
Discontinuation and switching.
To evaluate the potential impact of pregnancy on the decision to continue or to discontinue treatment at various time points during pregnancy, we investigated the patterns of use during pregnancy. Continuation was defined as filling a prescription during pregnancy for the same antipsychotic class (class level) or specific drug (generic level) used before pregnancy. Initiation was defined as filling a prescription for an antipsychotic during pregnancy and having no record of having used an antipsychotic during the three months before the start of pregnancy. Switching was defined as filling a prescription during the first trimester for a different antipsychotic (class or generic level) than the one used at baseline.
Results
Trends in Use
We identified 1,522,247 pregnancies between 2001 and 2010 meeting our inclusion criteria. Over this ten-year period, the number of women who filled at least one prescription for a second-generation antipsychotic during pregnancy increased from .4% (N=376) to 1.3% (N=2,044) (p<.001). The increasing trend was similar across the age and racial-ethnic groups considered. In all years, use was higher among women ages 30 and older compared with younger women and among whites compared with Hispanics and blacks [see online supplement]. The proportion of women who received a first-generation antipsychotic remained stable at around .1% over the entire study period.
We observed different trends at the individual drug level for each of the six antipsychotics considered (Figure 1). The proportion of women using quetiapine increased substantially from .1% in 2001 to .6% in 2010. Since its introduction to the market in 2002, aripiprazole became the second most frequently used antipsychotic by 2010, with .4% of all women filling a prescription during pregnancy. In contrast, we observed a decreasing trend, from .2% to .1%, in the proportion of olanzapine users.
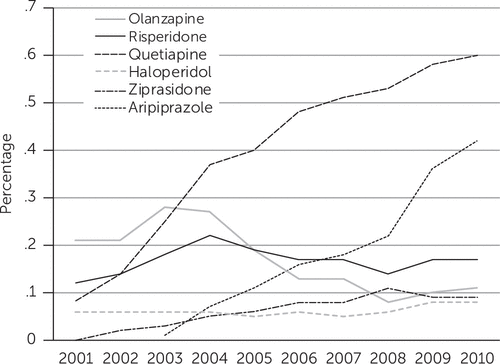
FIGURE 1. Proportion of women enrolled in Medicaid who received an outpatient prescription for an antipsychotic medication during pregnancy, 2001 to 2010, by drug
We observed a temporal increase both in the prevalence of the five psychiatric disorder diagnoses of interest and, for some diagnoses, in the proportion of women with the diagnosis who received antipsychotic medication during pregnancy (Figure 2). Most striking, the prevalence of bipolar disorder diagnosis increased more than threefold (from .7% to 2.5%) over ten years and the proportion of women with bipolar disorder who received antipsychotics increased from 13.6% in 2001 to 23.6% by 2010. Women with depression who did not have a diagnosis of schizophrenia or other psychotic disorders or bipolar disorder represented 6.7% of the study population in 2001 and 8.5% in 2010; the proportion treated with antipsychotics changed from 1.9% to 3.9%, representing about a twofold increase. The extent of antipsychotic use among women with apparent off-label indications, such as anxiety or ADHD, was small but increased over time.
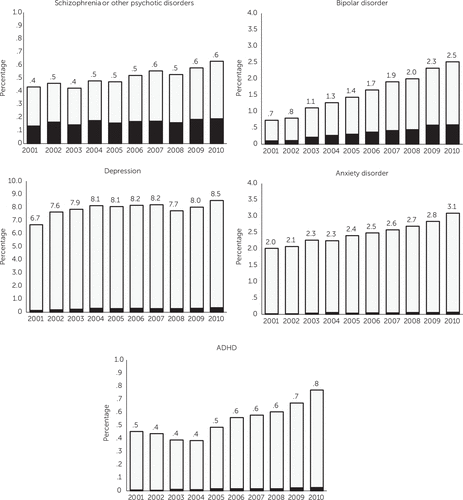
FIGURE 2. Proportion of pregnant women enrolled in Medicaid who received one or more outpatient prescriptions for an antipsychotic medication, 2001 to 2010, by diagnosisa
aEach bar indicates the proportion of pregnant women enrolled in Medicaid with that diagnosis. The black portion indicates the percentage of women with that diagnosis who were users of antipsychotics. Diagnoses were categorized by the highest possible category in the following hierarchy, from lowest to highest: ADHD only; anxiety with or without ADHD; depression with or without anxiety or ADHD; bipolar disorder with or without depression, anxiety, or ADHD; and schizophrenia or other psychotic disorder with or without bipolar disorder, depression, anxiety, or ADHD.
Demographic Characteristics
Compared with women who did not use antipsychotics during pregnancy, antipsychotic users were older; were disproportionately white; and had a higher prevalence of nonpsychiatric comorbidities, use of other medications, smoking, alcohol use, and recorded drug abuse or dependence (Table 1). Polytherapy with other psychotropic medications during pregnancy was common (Figure 3). Among the 15,196 women who used antipsychotics at any time during pregnancy, 65.2% also received antidepressants, 24.9% benzodiazepines, and 22.0% mood stabilizers. Five percent of women (N=765) received at least one prescription for all four drug classes at some point during pregnancy. Opioids were prescribed to more than 40% of women who received antipsychotics during pregnancy (Table 1).
| Users (N=15,196) | Nonusers (N=1,507,051) | ||||
|---|---|---|---|---|---|
| Characteristic | N | % | N | % | Standardized differencea |
| Demographic | |||||
| Age | |||||
| <20 | 2,888 | 19.0 | 364,962 | 24.2 | .13 |
| 20–29 | 8,246 | 54.3 | 880,000 | 58.4 | .08 |
| 30–39 | 3,745 | 24.6 | 242,338 | 16.1 | .21 |
| >40 | 317 | 2.1 | 19,751 | 1.3 | .06 |
| Race-ethnicity | |||||
| White | 9,144 | 60.2 | 602,772 | 40.0 | .41 |
| Black/African American | 3,766 | 24.8 | 504,884 | 33.5 | .19 |
| Hispanic/Latino | 827 | 5.4 | 225,390 | 15.0 | .32 |
| Asian/other Pacific Islander | 267 | 1.8 | 53,318 | 3.5 | .11 |
| Other | 1,192 | 7.8 | 120,687 | 8.0 | .01 |
| Mental disorder | |||||
| Schizophrenia or other psychotic disorder | 2,526 | 16.6 | 5,273 | .3 | .61 |
| Bipolar disorder | 6,488 | 42.7 | 20,383 | 1.4 | 1.15 |
| Depression | 7,910 | 52.1 | 127,273 | 8.4 | 1.08 |
| Anxiety disorder | 4,470 | 29.4 | 65,747 | 4.4 | .71 |
| ADHD | 1,374 | 9.0 | 15,433 | 1.0 | .37 |
| Other comorbid condition | |||||
| Personality disorder | 589 | 3.9 | 2,724 | .2 | .26 |
| Adjustment disorder | 329 | 2.2 | 8,249 | .5 | .14 |
| Sleep disorder | 598 | 3.9 | 9,639 | .6 | .22 |
| Epilepsy | 774 | 5.1 | 17,378 | 1.2 | .23 |
| Neuropathic pain | 603 | 4.0 | 18,462 | 1.2 | .17 |
| Fibromyalgia | 431 | 2.8 | 12,541 | .8 | .15 |
| Other pain disorder | 433 | 2.8 | 12,984 | .9 | .15 |
| Migraine/headache | 2,378 | 15.6 | 105,806 | 7.0 | .28 |
| Hypertension | 761 | 5.0 | 32,717 | 2.2 | .15 |
| Diabetes | 506 | 3.3 | 23,043 | 1.5 | .12 |
| Tobacco use | 1,446 | 9.5 | 45,623 | 3.0 | .27 |
| Alcohol use | 603 | 4.0 | 7,780 | .5 | .24 |
| Other drug abuse or dependence (2001–2006) | 942 | 12.8 | 13,418 | 1.5 | .45 |
| N of outpatient visits (median; IQR)b | 10; 6–17 | 4; 2–8 | .73 | ||
| Other medication usec | |||||
| Anxiolytic | 644 | 4.2 | 4,641 | .3 | .27 |
| Barbiturate | 695 | 4.6 | 24,574 | 1.6 | .17 |
| Other hypnoticd | 3,633 | 23.9 | 94,835 | 6.3 | .51 |
| Opioid | 6,112 | 40.2 | 30,6000 | 20.3 | .44 |
TABLE 1. Characteristics of pregnant women enrolled in Medicaid, 2001 to 2010, by use of antipsychotic medication
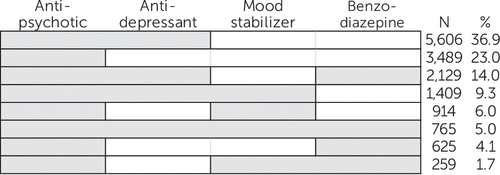
FIGURE 3. Treatment patterns in use of other psychotropic medications among women receiving antipsychotics during pregnancya
a The shaded cells indicate the use of each medication during baseline (three months prior to the last menstrual period) to delivery. Each row represents various combinations of psychotropic drug use patterns but does not necessarily indicate concurrent use of drugs. Mood stabilizers include lithium, carbamazepine, lamotrigine, oxcarbazepine, topiramate, and valproate.
Discontinuation and Switching
Of the 16,608 women (1.1%) who filled a prescription for a second-generation antipsychotic during the three months before the LMP, about half (50.2%) did not fill any additional prescription from LMP until delivery, and 15.4% continued using the same drug until the third trimester (Figure 4). Of the 1,505,639 women who did not use second-generation antipsychotics during the baseline period, 5,583 (.4%) initiated a prescription during pregnancy—.2% (N=3,641) in the first trimester, .07% (N=1,123) in the second trimester, and .05% (N=819) in the third trimester. For women who used first-generation antipsychotics prior to the LMP (N=774), the discontinuation rate was similar (51.7%). Regardless of the baseline use, 6,469 (.4%) women filled a prescription for a second-generation antipsychotic and 1,197 (.08%) women filled a prescription for a first-generation antipsychotic in the second or third trimester, when most women would be aware that they are pregnant.
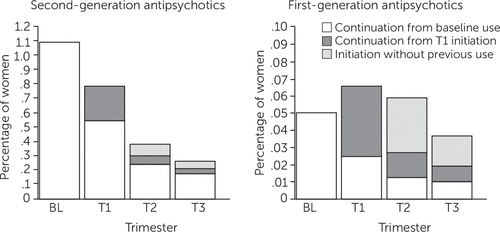
FIGURE 4. Continuation and initiation of first- and second-generation antipsychotic medication during pregnancy among women enrolled in Medicaid, 2001 to 2010, by medication classa
aBL, baseline (three months prior to the last menstrual period); T1, first trimester; T2, second trimester; T3, third trimester
The patterns of antipsychotic discontinuation observed at the generic level were similar to those at the class level [see online supplement]. Depending on the drug, 49.6% to 59.6% of women exposed to individual second-generation agents discontinued treatment during pregnancy, while 42.8% of women who received haloperidol in the baseline period discontinued the medication during pregnancy. Among baseline antipsychotic users, switching to a different antipsychotic in the first trimester was infrequent, and most women who switched who switched in the first trimester did so to quetiapine [see online supplement].
Discussion
In a nationwide sample of publicly insured pregnant women in the United States, the use of second-generation antipsychotics rose from .4% in 2001 to 1.3% in 2010, a threefold increase. The trend was largely driven by an increase in the use of two second-generation antipsychotics, aripiprazole and quetiapine. The characteristics of women who use antipsychotics during pregnancy have changed over time, with a notable increase in diagnosis of bipolar disorders. Use of antipsychotics for treatment of bipolar disorder among pregnant women has also increased significantly. Among the women who used antipsychotics during pregnancy, a large proportion were treated concomitantly with other psychotropic medications. More than 50% of women who used second-generation antipsychotics during the three months before LMP discontinued the medications in the first trimester. Among women who switched medications during the first trimester, the majority switched to quetiapine.
The prevalence of women on antipsychotic treatment during pregnancy was higher in our study compared with findings from other populations during partly overlapping periods (18–22). In European cohorts, the prevalence of women on antipsychotic treatment during pregnancy ranged from .1% to .3% (19–22). Among privately insured women in the United States, .7% used second-generation antipsychotics (18). That is not surprising because the prevalence of mental illness is known to be higher for the Medicaid-insured population compared with a privately insured population, due partly to the Medicaid population’s lower socioeconomic status and higher proportion of persons from racial-ethnic minority groups (23).
The increase in bipolar disorder diagnoses in our study population is consistent with the increase observed for the general population, including children and adolescents (24,25). It is not entirely clear why there has been such a rapid increase in bipolar disorder diagnoses, but plausible explanations include improved classification of persons who were previously misdiagnosed as having unipolar depression and overdiagnosis of this condition (25,26). We also observed a small increase in the use of antipsychotics for women with anxiety or ADHD, consistent with previous studies reporting frequent off-label use among nonpregnant populations with either disorder (1,4). Given that both anxiety and ADHD often co-occur with other psychiatric disorders, it is also possible that we did not capture the diagnoses in the claims for which the antipsychotics were truly used (27).
The utilization trends over time were different for the various antipsychotic agents. The greater increase in the use of aripiprazole and quetiapine may be due in part to the expansion of the drugs’ indications in 2007 (aripiprazole) and 2009 (quetiapine) for treatment of major depressive disorder. However, an increase in use of these drugs was observed during the years preceding the expansion of indications. Quetiapine has a wide range of off-label uses, which may partly explain why its use increased during the years preceding the expansion of indications. It is also perceived as relatively safe because of prior evidence showing a lower rate of placental passage for quetiapine compared with the other antipsychotics (28,29). The decrease in the use of olanzapine may be explained by the known risk of metabolic side effects, including weight gain (30). Risperidone was the first second-generation antipsychotic to be approved, but its potential to cause hyperprolactinemia may explain why it is used less commonly among pregnant women compared with the general population (31).
The discontinuation patterns observed in this study were similar to the results from previous studies in the United Kingdom. Close to 50% of women in those studies had discontinued second-generation antipsychotics by six weeks of pregnancy, and 62% to 72.3% had discontinued second-generation antipsychotics by the third trimester (19,22). Interestingly, a greater number of women filled a prescription for a first-generation antipsychotic after LMP compared with the baseline period; a similar trend was not seen for second-generation antipsychotics. This might be due to the fact that physicians have more experience prescribing first-generation antipsychotics during pregnancy and therefore feel more comfortable doing so. In particular, concerns about the risk of metabolic side effects may deter physicians from prescribing second-generation antipsychotics during pregnancy. A small number of women appeared to initiate treatment during pregnancy. Although some might be true initiators, it should be acknowledged that for some of these women it might be a continuation of treatment from the prepregnancy period prior to our three-month baseline period.
Polytherapy with other psychotropic medications was very common, given that antipsychotics are often indicated as an adjunct agent (32). Potential drug interactions between antipsychotics and other psychotropics are largely unknown, particularly in pregnancy; given the high frequency of such use, this is an important priority for future research. It is also notable that opioid use during pregnancy was remarkably high among pregnant women taking antipsychotics, which may be due to a higher prevalence of mental illness among Medicaid recipients compared with a privately insured population and a strong link between mental illness and physical pain (33). There are potential harms associated with opioid use in pregnancy, such as neonatal abstinence syndrome (34), and the risk of other negative maternal and fetal outcomes is largely unknown. More attention to appropriate use of opioids in this population is needed.
This study had strengths and limitations. We used a nationwide sample of more than 1.5 million pregnancies covered by Medicaid. Because Medicaid pays for close to 50% of all deliveries of babies in the United States, the results reflect the real-world utilization of antipsychotics in a large proportion of pregnant women in the U.S. population. In addition, our study population was racially diverse—58% of the population was from nonwhite racial-ethnic minority groups compared with 39% in the general nonelderly U.S. population. The population was also young—the mean age was 24.1 compared with 25.4 in the general U.S. population (35,36). We were able to study trends by racial groups, which can be a proxy for differential access to mental health care (37).
Our study also had some limitations. The study findings about prevalence of use are not applicable to women with private insurance, given that the use of psychotropic medication is higher in Medicaid (14), but other trends in use may still be generalizable. Antipsychotic exposure was defined based on filling a prescription, which does not guarantee the actual intake. But claims from automated dispensing records are considered to be more accurate for defining drug exposures compared with other methods, such as patient recall or medical records (38,39). The date of LMP was estimated, so some misclassification of exposure timing is possible. But the algorithm we used estimated 75% of preterm and 99% of term deliveries within two weeks of the clinical gestational age at birth (17).
We defined psychiatric morbidity by using ICD-9 diagnostic codes that have imperfect sensitivity (40), and as a result we could have underestimated the prevalence of each psychiatric diagnosis in the study. However, because pregnant women have frequent contact with health services and because we required continuous coverage over the pregnancy period, concerns about incomplete capture of diagnoses are reduced. Also a number of women had more than one psychiatric diagnosis recorded, which implies that different diagnoses were recorded over time. We were not able to ascertain the specific diagnosis for which antipsychotics may have been prescribed. The lower than expected prevalence of smoking and alcohol problems in this cohort of women with psychiatric disorders (22,41) is attributed to the underrecording of lifestyle variables in claims data. Last, some of the trends in use might have been affected by changes in each state’s Medicaid program at various points in time, such as implementation of prior authorization or coverage expansion (42). We were not able to disentangle the impact of changes in policies versus changes in clinician preference. Nevertheless, this study provides insight into the observed patterns of antipsychotic medication use over time at a national level.
Conclusions
A growing number of pregnant women enrolled in Medicaid are exposed to antipsychotic medications during pregnancy, reaching 1.3% by the year 2010, but there is still limited evidence regarding the safety of antipsychotic medication during pregnancy. Polytherapy with other psychotropic medications, common in this population, deserves more attention with regard to fetal safety. The high rate of discontinuation observed in this population suggests that clinicians and patients have concerns about the safety of the use of these medications during pregnancy. However, discontinuation of these medications may have implications for maternal mental health. To help clinicians and patients make informed treatment decisions, there is an urgent need for further studies in this area to examine adverse pregnancy outcomes associated with maternal use of antipsychotics, in monotherapy or polytherapy, as well as studies examining comparative effectiveness of specific antipsychotic agents among pregnant women.
1 : Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Affairs 28:w770–w781, 2009Crossref, Medline, Google Scholar
2 : Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiology and Drug Safety 20:177–184, 2011Crossref, Medline, Google Scholar
3 : Prevalence of atypical antipsychotic use in psychiatric outpatients: comparison of women of childbearing age with men. Archives of Women’s Mental Health 17:583–586, 2014Crossref, Medline, Google Scholar
4 : Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry 72:867–874, 2015Crossref, Medline, Google Scholar
5 : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 62:593–602, 2005Crossref, Medline, Google Scholar
6 : A review of the use of psychotropic medication in pregnancy. Current Opinion in Obstetrics and Gynecology 23:408–414, 2011Crossref, Medline, Google Scholar
7 : Limb malformations following maternal use of haloperidol. JAMA 231:62–64, 1975Crossref, Medline, Google Scholar
8 : Haloperidol and limb deformity. JAMA 231:26, 1975Crossref, Medline, Google Scholar
9 : Safety of haloperidol and penfluridol in pregnancy: a multicenter, prospective, controlled study. Journal of Clinical Psychiatry 66:317–322, 2005Crossref, Medline, Google Scholar
10 : Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. Journal of Clinical Psychopharmacology 33:453–462, 2013Crossref, Medline, Google Scholar
11 : Atypical antipsychotic use in pregnancy: a retrospective review. Archives of Women’s Mental Health 12:53–57, 2009Crossref, Medline, Google Scholar
12 : Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry 73:938–946, 2016Crossref, Medline, Google Scholar
13 : Antipsychotic therapy during early and late pregnancy: a systematic review. Schizophrenia Bulletin 36:518–544, 2010Crossref, Medline, Google Scholar
14 : Mental health policy and psychotropic drugs. Milbank Quarterly 83:271–298, 2005Crossref, Medline, Google Scholar
15 Pandit S: Issue Brief. 2012 Maternal and Child Health Update. Washington, DC, National Governors Association, 2013. https://www.nga.org/files/live/sites/NGA/files/pdf/MCHUPDATE2012.PDFGoogle Scholar
16 : Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PLoS One 8:e67405, 2013Crossref, Medline, Google Scholar
17 : Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiology and Drug Safety 22:16–24, 2013Crossref, Medline, Google Scholar
18 : Prevalence and trends in the use of antipsychotic medications during pregnancy in the US, 2001–2007: a population-based study of 585,615 deliveries. Archives of Women’s Mental Health 16:149–157, 2013Crossref, Medline, Google Scholar
19 : Patterns of prescription of antidepressants and antipsychotics across and within pregnancies in a population-based UK cohort. Maternal and Child Health Journal 18:1742–1752, 2014Crossref, Medline, Google Scholar
20 : Maternal use of antipsychotics in early pregnancy and delivery outcome. Journal of Clinical Psychopharmacology 28:279–288, 2008Crossref, Medline, Google Scholar
21 : Antipsychotics during pregnancy: relation to fetal and maternal metabolic effects. Archives of General Psychiatry 69:715–721, 2012Crossref, Medline, Google Scholar
22 : Discontinuation of antipsychotic medication in pregnancy: a cohort study. Schizophrenia Research 159:218–225, 2014Crossref, Medline, Google Scholar
23 : Medicaid and mental health: be careful what you ask for. Health Affairs 22:101–113, 2003Crossref, Google Scholar
24 : National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Archives of General Psychiatry 64:1032–1039, 2007Crossref, Medline, Google Scholar
25 : Increased rates of bipolar disorder diagnoses among US child, adolescent, and adult inpatients, 1996–2004. Biological Psychiatry 62:107–114, 2007Crossref, Medline, Google Scholar
26 : Is bipolar disorder overdiagnosed? Journal of Clinical Psychiatry 69:935–940, 2008Crossref, Medline, Google Scholar
27 : The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. American Journal of Psychiatry 163:716–723, 2006Link, Google Scholar
28 : Prescribing of antipsychotic medication in a Medicaid population: use of polytherapy and off-label dosages. Journal of Managed Care Pharmacy 11:17–24, 2005Crossref, Medline, Google Scholar
29 : Atypical antipsychotic administration during late pregnancy: placental passage and obstetrical outcomes. American Journal of Psychiatry 164:1214–1220, 2007Link, Google Scholar
30 : Almost all antipsychotics result in weight gain: a meta-analysis. PLoS One 9:e94112, 2014Crossref, Medline, Google Scholar
31 : The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs 28:421–453, 2014Medline, Google Scholar
32 Drugs@FDA: FDA Approved Drug Products. Silver Spring, MD, US Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed July 27, 2016Google Scholar
33 : The association between chronic widespread pain and mental disorder: a population-based study. Arthritis and Rheumatism 43:561–567, 2000Crossref, Medline, Google Scholar
34 : Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ 350:h2102, 2015Crossref, Medline, Google Scholar
35 Health Coverage by Race and Ethnicity: The Potential Impact of the Affordable Care Act. Washington, DC, Kaiser Family Foundation, 2013. https://kaiserfamilyfoundation.files.wordpress.com/2014/07/8423-health-coverage-by-race-and-ethnicity.pdf%5Cnhttp://kff.org/disparities-policy/issue-brief/health-coverage-by-race-and-ethnicity-the-potential-impact-of-the-affordable-care-actGoogle Scholar
36 Martin JA, Hamilton BE, Ventura SJ, et al: Births: Final Data for 2010. National Vital Statistics Reports, vol 61, no 1. Hyattsville, MD, National Center for Health Statistics, 2012Google Scholar
37 : Persistence of racial disparities in prescription of first-generation antipsychotics in the USA. Pharmacoepidemiology and Drug Safety 24:1197–1206, 2015Crossref, Medline, Google Scholar
38 : Recall accuracy for prescription medications: self-report compared with database information. American Journal of Epidemiology 142:1103–1112, 1995Crossref, Medline, Google Scholar
39 : Completeness of prescription recording in outpatient medical records from a health maintenance organization. Journal of Clinical Epidemiology 47:165–171, 1994Crossref, Medline, Google Scholar
40 : Use of administrative data to identify off-label use of second-generation antipsychotics in a Medicaid population. Psychiatric Services 64:1236–1242, 2013Link, Google Scholar
41 : Risks and benefits of psychotropic medication in pregnancy: cohort studies based on UK electronic primary care health records. Health Technology Assessment 20:1–176, 2016Crossref, Medline, Google Scholar
42 : A new Medicaid program. Health Affairs (suppl Web exclusives) W3:426–439, 2003Crossref, Google Scholar



