Double-Blind Randomized Clinical Trial of Prazosin for Alcohol Use Disorder
Abstract
Objective:
Current medications for alcohol use disorder do not target brain noradrenergic pathways. Theoretical and preclinical evidence suggests that noradrenergic circuits may be involved in alcohol reinforcement and relapse. After a positive pilot study, the authors tested the α-1 adrenergic receptor antagonist prazosin to treat alcohol use disorder in a larger sample.
Method:
Ninety-two participants with alcohol use disorder but without posttraumatic stress disorder were randomly assigned to receive prazosin or placebo in a 12-week double-blind study. Medication was titrated to a target dosing schedule of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime by the end of week 2. The behavioral platform was medical management. Participants provided daily data on alcohol consumption. Generalized linear mixed-effects models were used to examine the impact of prazosin compared with placebo on number of drinks per week, number of drinking days per week, and number of heavy drinking days per week.
Results:
Eighty participants completed the titration period and were included in the primary analyses. There was a significant interaction between condition and week for both number of drinks and number of heavy drinking days, such that the rate of drinking and the probability of heavy drinking showed a greater decrease over time for participants in the prazosin condition compared with those in the placebo condition. Participants in the prazosin condition were more likely to report drowsiness and edema than participants in the placebo condition.
Conclusions:
Prazosin holds promise as a harm-reduction pharmacologic treatment for alcohol use disorder and deserves further evaluation by independent research groups.
Evidence suggests that elevated brain noradrenergic activity appears to be involved in the initiation and maintenance of alcohol use disorder (1, 2). A clinically feasible approach to reducing brain noradrenergic activity is to reduce activation by norepinephrine at the postsynaptic α-1 adrenoceptor. Prazosin is a clinically available lipid-soluble α-1 adrenoceptor antagonist that reduces brain α-1 adrenoceptor–mediated signaling when administered peripherally (3). In rodents, prazosin has been shown to decrease withdrawal-induced alcohol intake (4), alcohol drinking by alcohol-preferring (P) rats (2), and stress-induced alcohol seeking (5), and it has been shown to block yohimbine-induced reinstatement of alcohol seeking (6). In human alcohol use disorder studies, prazosin has been shown to reduce reactivity to stress and to result in reduced craving (7), reduced drinks per week (8, 9), and reduced drinking days per week (8). In persons with DSM-IV alcohol dependence and comorbid posttraumatic stress disorder (PTSD), one study found that prazosin reduced drinking but not PTSD outcomes (10), and another study found no prazosin effect on either outcome (11).
Doxazosin, another α-1 adrenoceptor antagonist, did not outperform placebo on drinking outcomes in a study of alcohol treatment seekers, but among those with a high family history density of alcohol problems, the active medication was associated with improved drinking outcomes (12). Across the entire sample, alcohol treatment seekers with higher standing diastolic blood pressure receiving active medication had better outcomes than those receiving placebo (13).
After obtaining positive results in a pilot study (8), we conducted a 12-week randomized controlled trial comparing prazosin and matched placebo in 92 participants who met diagnostic criteria for alcohol use disorder but not PTSD. Individuals with PTSD were excluded because there is evidence that prazosin reduces symptoms of PTSD (14), and we were interested in isolating the effects of prazosin on drinking alone in light of evidence linking excessive drinking to stress and the adrenergic system. Both treatment arms included medical management (15), and daily symptoms were monitored via a telephone-based interactive voice response system to obtain close to real-time data regarding alcohol consumption. Our primary hypotheses were that prazosin would lead to a decreased likelihood over time of any drinking and of heavy drinking (i.e., ≥4 drinks for women, ≥5 drinks for men) as well as a decrease in number of drinks consumed.
Method
Participants
Of 601 people who contacted the study, 151 provided informed consent, and 19 women and 73 men (N=92) underwent random assignment to a treatment condition (Figure 1). To be included in the study, participants had to have a current DSM-IV diagnosis of alcohol dependence (16), consumption during 4 consecutive weeks in the past 90 days, and a goal of abstaining from alcohol; alcohol consumption had to be ≥14 drinks per week for women or ≥21 drinks per week for men and had to include two occasions of heavy drinking.
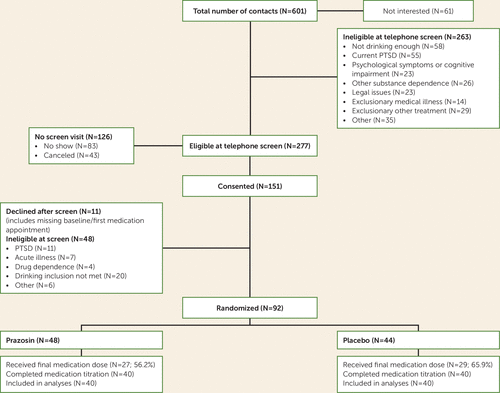
FIGURE 1. CONSORT Chart for a Placebo-Controlled Trial of Prazosin for Alcohol Use Disorder
Exclusion criteria were a current DSM-IV diagnosis of PTSD; an uncontrolled psychiatric disorder with psychotic symptoms or cognitive impairment; unstable psychiatric medication dosing in the past month; the use of alcohol abstinence medications (e.g., disulfiram, acamprosate, naltrexone) within the past month; current opioid dependence, use of opioids during the past month, or a positive urine screen for opioids, benzodiazepines, or sedative-hypnotics; significant acute or chronic illness; systolic blood pressure <100 mmHg or orthostatic hypotension; prazosin sensitivity or use of prazosin in the past 30 days; use of trazodone, tadalafil, or vardenafil in males; or participation in a drug or addiction study in the past month. Women of childbearing age could participate if they reported use of effective contraception. Participants could not receive behavioral or medication treatment for alcohol use disorder outside the study except through Alcoholics Anonymous and supportive counseling.
Procedures
Study design.
This was a 12-week outpatient double-blind randomized controlled trial, with two groups, comparing prazosin with matched placebo. Two weeks of titration were followed by 10 weeks of stable dosing. Participants were asked to complete 4- to 5-minute interactive voice response calls daily to report the previous day’s drinking, cravings, and study medication doses consumed. Participants were reimbursed for all study visits and calls.
Recruitment and screening.
Approval was granted by the VA Puget Sound Health Care System institutional review board. Participants were recruited from January 2008 to May 2014 through clinical referrals, flyers, and advertisements in newspapers and on Craigslist.
Participants who passed the screening evaluation returned for a baseline visit approximately 1 week later, at which time they received their randomized treatment assignment, received medication, and completed further assessments. Participants were not required to be abstinent prior to initiating medication.
Randomization.
A research pharmacist used randomization tables to assign participants to prazosin or placebo. All other personnel remained blind to participant condition. Randomization was stratified by gender, veteran status, and drinking frequency (drinking on <10 days or ≥10 days in the past 30 days).
Study visits.
Participants attended twice-weekly study visits during weeks 1 and 2 (titration), and then weekly study visits during weeks 3–12. Each visit included concomitant medication assessment, urine collection, adverse event checks, and orthostatic vital sign checks. Participants received brief medical management counseling weekly. To assess medication adherence, pills were counted and a riboflavin trace was added to the study medication; urine specimens were examined at each study visit via ultraviolet light to determine the presence of riboflavin.
Measures
Mental health diagnoses and symptoms.
Participants completed sections of the Structured Clinical Interview for DSM-IV Axis I Disorders (17) to confirm alcohol dependence and to detect exclusionary disorders. If participants scored 42 or greater on the PTSD Checklist–Civilian Version (18), the PTSD portion of the interview was completed to exclude individuals with PTSD.
Substance use.
At the baseline and final medication visits, the Form 90 (19) was used to assess alcohol and drug use for the preceding 90-day period.
Interactive voice response daily monitoring.
Participants were instructed to call a toll-free number daily starting on study day 2 to report daily alcohol consumption, craving (four items), and medication adherence (one item) in the previous 24 hours.
Study Treatments
Medications.
The target dosing of 4 mg in the morning, 4 mg in the afternoon, and 8 mg at bedtime was reached at the end of the 2-week titration period. Participants continued at this or their highest tolerated dosage for an additional 10 weeks. Dosing was held at 1 mg at bedtime for the first 2 nights to minimize the risk of first-dose syncope. Participants’ titration was stopped if they experienced symptomatic orthostatic hypotension or other intolerable side effects, and the medication dosing was then decreased to the prior tolerated medication dose. Dosing was targeted for three times per day.
Medical management.
During the weekly medical management visits, the study clinician emphasized medication adherence and encouraged attendance at self-help meetings (15).
Statistical Analysis
Analyses were performed using R, version 3.4.3 (20), and the packages lme4 (21), glmmTMB (22), and EnvStats (23). Descriptive statistics for demographic and baseline data included means and standard deviations for continuous variables (group differences analyzed using t tests) and percentages for categorical variables (group differences analyzed using chi-square tests).
Daily interactive voice response data.
Drinking measures were aggregated to the weekly level. Primary outcomes were number of drinks per week, number of drinking days per week, and number of heavy drinking days per week. Exploratory plots included observed group means and confidence intervals by week for each drinking measure. Following Kranzler et al. (24), we used generalized linear mixed-effects models to examine group differences in outcome changes during treatment. For number of drinks per week, we assumed a negative binomial distribution with a log link, and for number of drinking days and number of heavy drinking days per week, we assumed a binomial distribution with a logit link. Models included fixed effects for condition, week (treated as continuous), and a condition-by-week interaction, along with the covariates of sex, veteran status, age, proportion of weekend days for which drinking data were reported for that week, and baseline average number of drinks per day. Subjects were treated as a random effect, and we allowed for random intercepts but not random slopes (24). Primary analyses included all randomized subjects who provided data for the period after titration was completed. Model results were summarized by adjusted marginal means. The likelihood ratio test and Wald approximation were used for tests and confidence intervals for fixed effects (21), and confidence intervals for adjusted marginal means were computed using nonparametric case bootstrapping (25) for number of drinks per week and parametric bootstrapping (21) for number of drinking days and heavy drinking days per week.
As a secondary outcome, we used the mean of four daily craving items, each rated on a scale from 0 to 8: thinking about drinking, strength of craving, difficulty resisting drinking, and self-reported average craving. As with the primary outcomes, craving was aggregated to the weekly level. We used a linear mixed-effects model with the same terms as for the primary outcomes.
Sensitivity analyses.
We performed four sensitivity analyses for the primary outcomes: 1) using all randomized subjects and all available interactive voice response data for the entire medication period; 2) using data for the period after titration was completed and including only participants who attended at least 70% of all protocol visits and for whom the riboflavin trace was present in urine; 3) using models that included both random intercepts and random slopes, because of the potential for fixed-slope models to have an inflated type I error (26, 27); and 4) using only the last week for which daily data were reported and analyzing those data with generalized linear models that included a term for condition and the covariates of sex, age, veteran status, and baseline average number of drinks per day.
Exploratory analyses.
We also performed exploratory analyses to look at change in standing systolic and diastolic blood pressure over time using linear mixed-effects models with blood pressure as the outcome and fixed effects for condition, week, and a condition-by-week interaction, along with the covariates of sex, veteran status, and age. We looked at both fixed-slope and random-slope models. Following Raskind et al. (28), we also looked at whether baseline blood pressure was associated with change from baseline in any of the primary outcomes by adding main effect and interaction terms for baseline blood pressure (treated as continuous) to the generalized linear mixed-effects models for the primary outcomes.
Results
Twelve (13.0%) of the 92 participants (eight in the prazosin group and four in the placebo group) dropped out during the initial 2-week titration period and were excluded from the primary analyses. Table 1 summarizes demographic and baseline drinking information by condition for the entire sample (N=92) as well as drinking data from the last week of medication for the sample subset included in the primary analyses (N=80). There were no significant differences on demographic or baseline drinking variables between the participants who were included and those who were excluded (all p values >0.26).
| Characteristic | Placebo Group (N=44) | Prazosin Group (N=48) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 49.1 | 9.5 | 47.3 | 9.8 |
| N | % | N | % | |
| Male | 36 | 81.8 | 37 | 77.1 |
| Ethnicity | ||||
| Non-Hispanic white | 23 | 53.5 | 29 | 59.6 |
| Black | 13 | 30.2 | 15 | 31.9 |
| Hispanic | 5 | 11.6 | 2 | 4.3 |
| Other | 3 | 4.7 | 2 | 4.3 |
| Veteran | 9 | 20.5 | 9 | 18.8 |
| Marital status | ||||
| Never married | 20 | 46.2 | 33 | 68.2 |
| Married | 8 | 19.2 | 4 | 9.1 |
| Separated or divorced | 14 | 30.8 | 9 | 18.2 |
| Widowed or other | 2 | 3.8 | 2 | 4.5 |
| Employed (part-time or full-time) | 39.5 | 44.4 | ||
| Mean | SD | Mean | SD | |
| Baseline drinking information for the preceding 90-day perioda | ||||
| Total standard drink units | 815 | 547 | 824 | 439 |
| Percent days drinking | 76.6 | 22.4 | 76.8 | 27.5 |
| Percent heavy drinking days | 66.5 | 26.0 | 71.8 | 29.1 |
| Average drinks per day | 9.1 | 6.1 | 9.2 | 4.9 |
| Average drinks per drinking day | 12.1 | 7.2 | 12.8 | 6.8 |
| Drinking across the last week of medication useb | ||||
| Percent days drinking | 35.5 | 39.7 | 43.0 | 44.0 |
| Percent days heavy drinking | 22.6 | 34.1 | 11.4 | 22.8 |
| Average drinks per day | 2.4 | 3.8 | 2.0 | 3.0 |
| Average drinks per drinking dayc | 6.3 | 5.7 | 4.6 | 4.0 |
TABLE 1. Demographic Characteristics and Drinking Measures for Participants in a Placebo-Controlled Trial of Prazosin for Alcohol Use Disorder
Of the 80 participants who completed the titration phase, 70 reached the full titration dosage (35 each in the prazosin and placebo groups), and 30 participants in the placebo group (75%) and 26 in the prazosin group (65%) completed all 12 weeks. The mean duration of participation in the study was 10.5 weeks (SD=3.0) for the placebo group and 10.4 weeks (SD=2.7) for the prazosin group. (Figure S1 in the online supplement plots the numbers of participants who provided data for each monitoring day, by condition, in the posttitration period, and Figure S2 plots the numbers of reported drinks by monitoring day for each participant.)
Primary Outcomes: Effect of Prazosin and Time on Daily Drinking Outcomes
Figure 2 plots the observed group means by week for the three primary outcomes, and Table 2 summarizes the results of the mixed-effects models; Figure S3 in the online supplement plots individual trajectories and adjusted marginal means by condition and week. There was no difference between conditions in response to treatment over time for number of drinking days. For number of heavy drinking days, there was a significant interaction between condition and week (χ2=6.55, df=1, p=0.01), with days of heavy drinking decreasing more rapidly from the first posttitration week (week 3) to week 12 in the prazosin group than in the placebo group: 0.8 days and 0.3 days, respectively (95% CI for difference: 0.0, 0.8). The odds of heavy drinking for the prazosin group were 0.85 (95% CI=0.80, 0.91) times the odds of heavy drinking the previous week, whereas the odds of heavy drinking for the placebo group were 0.95 (95% CI=0.90, 1.0) times the odds of heavy drinking the previous week (odds ratio=0.90, 95% CI=0.82, 0.98). However, the number of heavy drinking days at week 12 did not differ between the two groups (1.0 days for the prazosin group and 1.2 days for the placebo group; p=0.56) (see Table 2; see also Figure S3C in the online supplement). Similarly, for number of drinks, there was a significant interaction between condition and week (χ2=4.50, df=1, p=0.03), with number of drinks per week decreasing more rapidly in the prazosin group than the placebo group: from week 3 to week 12, the prazosin group reduced number of drinks per week by 8.0 (95% CI=1.8, 19.5), compared with 1.5 (95% CI=−3.4, 6.8) for the placebo group, or a drink-per-week difference of 6.5 (95% CI=−2.4, 19.1). The rate of drinking decreased by 5% (95% CI=2, 8) each week for the prazosin group, compared with 1% (95% CI=−1, 4) for the placebo group (difference=4%, 95% CI=0.3, 7.7). Number of drinks per week at week 12 did not differ between conditions (13.3 for the prazosin group and 13.1 for the placebo group; p=0.98) (see Table 2; see also Figure S3A in the online supplement).
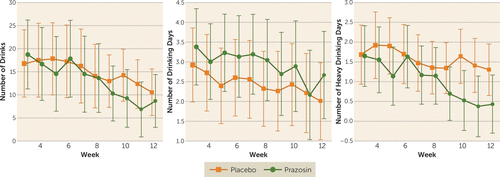
FIGURE 2. Observed Mean Values for Outcome Measures for the Posttitration Period, by Week and Condition, in a Placebo-Controlled Trial of Prazosin for Alcohol Use Disordera
a Error bars indicate 95% confidence interval. Symbol sizes are proportional to number of subjects with data for that week (see Figure S1 in the online supplement). Results of generalized mixed-effects fixed-slope models: for number of drinks per week, the interaction of condition by week was significant (p=0.03), but the main effect of condition at week 12 was not significant (p=0.98). For number of drinking days per week, the interaction of condition by week was not significant (p=0.94), and the main effect of condition at week 12 was not significant (p=0.47), but the main effect of week was significant (p=0.002). For number of heavy drinking days per week, the interaction of condition by week was significant (p=0.01), but the main effect of condition at week 12 was not significant (p=0.56).
| Prazosin Group (N=40) | Placebo Group (N=40) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 3 | Week 12 | Changeb | Week 3 | Week 12 | Changeb | Difference in Changeb | ||||||
| Drinking Outcome | Mean | Mean | Mean | 95% CI | Mean | Mean | Mean | 95% CI | Mean | 95% CI | χ2 | p |
| Number of drinks per week | 21.3 | 13.3 | 8.0 | 1.8, 19.5 | 14.6 | 13.1 | 1.5 | –3.4, 6.8 | 6.5 | –2.4, 19.1 | 4.50 | 0.03 |
| Number of drinking days per week | 3.2 | 2.8 | 0.4 | 0.1, 0.8 | 2.8 | 2.3 | 0.5 | 0.1, 0.7 | 0.0 | –0.4, 0.5 | 0.006 | 0.94 |
| Number of heavy drinking days per week | 1.8 | 1.0 | 0.8 | 0.3, 1.0 | 1.5 | 1.2 | 0.3 | 0.0, 0.5 | 0.5 | 0.0, 0.8 | 6.55 | 0.01 |
TABLE 2. Adjusted Marginal Means and Differences in Weekly Drinking Outcomes After Titration and at End of Study in a Placebo-Controlled Trial of Prazosin for Alcohol Use Disordera
Secondary Outcome, Sensitivity Analyses, and Exploratory Analyses
Although average craving decreased over time in both the prazosin and placebo groups, there was no difference between groups in change in craving over time.
Sensitivity analyses using intent-to-treat models that included all randomized participants and all available data for the entire medication phase, models that included only participants with 70% or better visit and medication adherence, and models that used only the last week of medication use yielded results similar to those of the primary analyses (see the online supplement). However, none of the condition-by-week interactions was significant for the random-slopes models (all p values ≥0.25).
Systolic blood pressure decreased in the prazosin group by a mean of 3.5 mmHg across the 12-week treatment period but increased in the placebo group by a mean of 3.1 mmHg, a difference of 6.6 mmHg (95% CI=1.4, 11.9) between conditions (condition-by-week interaction, χ2=6.1, df=1, p=0.01). There was no significant change in diastolic blood pressure for either group (p>0.11), and there was no significant difference in change by condition (p>0.26). Neither systolic nor diastolic blood pressure was a significant effect modifier in the difference in improvement in total number of drinks or heavy drinking days by condition (three-way blood pressure-by-condition-by-week interactions, all p values >0.26). Details of the sensitivity, secondary outcome, and exploratory analyses are provided in the online supplement.
Adherence
In the overall sample (N=92), more participants in the placebo group (65.9%) than in the prazosin group (56.2%) received the final 2 weeks of medication (χ2=0.54, df=1, n.s.). Among participants who completed titration (N=80), the average maximum study day on which daily data were provided was 71.6 (SD=20.6) for the prazosin group and 73.6 (SD=21.7) for the placebo group (t=0.43, df=78, n.s.) (Table 3). When missing daily data were coded as missed medication days, posttitration prazosin and placebo participants reported having taken one or more doses on 64.8% (SD=31.0) and 75.6% (SD=31.8) of days, respectively (t=1.54, df=78, n.s.). The riboflavin trace was detected at 64.1% (SD=26.0) and 71.3% (SD=28.6) of visits for the prazosin and placebo groups, respectively (t=1.2, df=78, n.s.). Prazosin and placebo participants reported having taken all three doses of medication on 54.7% of days (SD=33.0) and 69.7% of days (SD=31.1), respectively (t=2.10, df=78, p=0.04). Of the 40 placebo and 40 prazosin participants who completed titration, 25 placebo (62.5%) and 20 prazosin (50%) participants attended at least 70% of all protocol visits and had riboflavin trace present in urine at all visits.
| Prazosin Group (N=40) | Placebo Group (N=40) | |||
|---|---|---|---|---|
| Measure | Mean | SD | Mean | SD |
| Percent daily adherence to daily interactive voice response questions | 68.8 | 29.7 | 76.6 | 31.4 |
| Number of days in study | 71.6 | 20.6 | 73.6 | 21.7 |
| Percent days reported taking any study medicationa | 64.8 | 31.0 | 75.6 | 31.8 |
| Visits with positive riboflavin tracea | 64.1 | 26.0 | 71.3 | 28.6 |
TABLE 3. Study Adherence Measures in a Placebo-Controlled Trial of Prazosin for Alcohol Use Disorder
Safety Findings
Adverse events.
There were five serious adverse events, none of which were judged by the institutional review board to be related to study involvement. One participant in the placebo group committed suicide after completing treatment; two participants were hospitalized for medical reasons (an infected wisdom tooth in a participant in the prazosin group and acute pancreatitis in a participant in the placebo group); and two participants in the prazosin group were hospitalized because of withdrawal from alcohol.
Side effects.
A significantly greater proportion of participants in the prazosin group reported drowsiness and edema than in the placebo group (Table 4). The groups did not differ on other side effects. In addition to the 12 participants who did not progress past the titration period (eight in the prazosin group and four in the placebo group), four participants in the prazosin group (and none in the placebo group) opted midway through the study to discontinue study medication and continue as intent-to-treat, completing daily calls, study visits, and assessments (they are included in the primary analyses). One participant assigned to the prazosin condition and five assigned to the placebo condition did not reach the full target titration dose. Symptomatic orthostatic hypotension was noted in one participant in the prazosin group and one in the placebo group. Rates of asymptomatic orthostatic hypotension (i.e., a decrease of 20 mmHg or more in systolic blood pressure from sitting to standing) did not differ between groups.
| Prazosin Group (N=44) | Placebo Group (N=48) | ||||
|---|---|---|---|---|---|
| Event or Side Effect | Mean | SD | Mean | SD | p |
| Drowsiness | 28 | 58.3 | 15 | 34.1 | 0.020 |
| Edema | 9 | 18.8 | 2 | 4.6 | 0.036 |
| Dizziness | 20 | 41.7 | 14 | 31.8 | 0.328 |
| Light-headedness | 23 | 47.9 | 17 | 38.6 | 0.370 |
| Headache | 17 | 35.4 | 17 | 38.6 | 0.749 |
| Lacking energy | 23 | 47.9 | 16 | 36.4 | 0.263 |
| Weakness | 10 | 20.8 | 6 | 13.6 | 0.363 |
| Palpitations | 11 | 22.9 | 5 | 11.4 | 0.144 |
| Nausea | 18 | 37.5 | 11 | 25.0 | 0.197 |
| Change in urination | 9 | 18.8 | 5 | 11.4 | 0.324 |
TABLE 4. Adverse Events and Side Effects Reported for the Entire Sample in a Placebo-Controlled Trial of Prazosin for Alcohol Use Disorder
Discussion
These results indicate that prazosin has the potential to reduce the likelihood of heavy drinking and number of drinks per week over time but not the number of drinking days per week. They suggest that prazosin may be most useful in reducing heavy drinking associated with negative consequences (29), which is consistent with a harm reduction approach characterized by safer consumption rather than full abstinence.
Early research demonstrating that cocaine blocked norepinephrine reuptake suggested that norepinephrine may be involved in addiction (30). Involvement of norepinephrine in reward circuitry has been demonstrated by the inability to induce conditioned place preference (a key animal model indicating pharmacologic reinforcement) with rewarding substances in animals that have the noradrenergic α-1 receptor knocked out (31) or that have norepinephrine depletion (32). In addition to reducing rodent self-administration of alcohol (33), prazosin compared with vehicle has also been shown to reduce self-administration of cocaine (34), heroin (35), and nicotine (36). In humans, the previous positive pilot studies of prazosin for alcohol use disorder (8, 10) and the present study provide preliminary support for an effect of prazosin on heavy drinking and number of drinks per week. Another α-1 antagonist, doxazosin, has shown a signal for reducing drinking in alcohol-dependent individuals who have a positive family history of alcohol problems (12). Doxazosin has also been found to reduce cocaine use in cocaine-dependent individuals compared with placebo (37). These accruing lines of evidence point to the importance of further investigation of α-1 adrenoceptor antagonists as a pharmacotherapeutic intervention for substance use disorders, either alone or in combination with medications targeting other systems.
Similar significant but moderate effect size reductions in heavy drinking have been observed in other studies of pharmacotherapy for alcohol use disorder (38), including medications approved by the U.S. Food and Drug Administration for this indication, such as the opioid receptor antagonist naltrexone (39). This raises the possibility that combination regimens of drugs with different mechanisms of action may result in larger reductions of drinking than single-drug regimens. A drug regimen combining prazosin with naltrexone in a study of alcohol-preferring P rats decreased alcohol drinking more consistently than either drug administered alone (40). In male P rats with protracted alcohol dependence and repeated withdrawal designed to resemble severe human alcohol use disorder, the combined prazosin and naltrexone regimen was highly effective in suppressing postdeprivation alcohol drinking and more effective than either drug administered alone (41). A randomized controlled trial of this combination drug regimen in persons with alcohol use disorder is under way (ClinicalTrials.gov identifier, NCT02322047).
Limitations of the present study include lack of knowledge of optimal dosing for prazosin for this population, a relatively short titration period, and the lack of a placebo washout period. Although we excluded people with PTSD to avoid the possibility that prazosin’s effects on alcohol use are mediated through alterations in PTSD symptomatology, we did not assess or exclude individuals with other anxiety disorders. Medication adherence was also suboptimal (9), particularly among participants in the prazosin condition. We did find, however, that the intent-to-treat models had the same pattern of findings as the models that included only participants who attended at least 70% of all protocol visits and had positive riboflavin traces at all visits, suggesting that even partial adherence to prazosin improved drinking outcomes. Additionally, the study participants varied widely in both alcohol use disorder severity and recent drinking patterns, which may have contributed to the modest findings. Further research evaluating whether alcohol use disorder severity is a moderator of treatment response would be especially useful. A final important limitation is that unlike the fixed-slope models, random-slope models showed no differences between the prazosin and placebo groups. This is not surprising given the large variability between individual trajectories in both treatment groups. To our knowledge, few, if any, past studies of pharmacological treatment for alcohol use disorder used random-slope models, but recent methodological research (26, 27) suggests that random-slope models should always be considered.
This study supports the growing body of work suggesting that α-1 noradrenergic antagonists that cross the blood-brain barrier may help people limit unsafe heavy drinking. Replication by independent research groups is warranted, and future research should also establish optimal dosing regimens and evaluate which subgroups may especially benefit from prazosin.
Patient Perspective
“Mr. A” was a 57-year-old divorced African-American man who worked part-time as a hot dog vendor at the baseball stadium. Mr. A typically consumed 12 beers a day, which he supplemented with 2 to 4 shots of whiskey a few times per week when he wasn’t working. He began to experience numbness in his feet, which made his work difficult. He had no health insurance, so he went to the emergency department, where he was noted to have alcohol on his breath and was diagnosed as having peripheral neuropathy due to alcohol use. He realized that his alcohol use was out of control and was now threatening his livelihood, but he had no means to pay for treatment, and he didn’t know what to do. When he saw a newspaper ad regarding our prazosin study, and he came in for an evaluation. The screening evaluation indicated that he met DSM-IV criteria for alcohol dependence (the study was conducted before DSM-5 was published) and had no other psychiatric disorders except nicotine dependence. In addition to the neuropathy diagnosis, Mr. A was also noted to have a sitting blood pressure of 150/95 and elevations in liver enzymes (AST and ALT) on laboratory testing. Mr. A was eligible for the study and consented to participate with an initial goal of achieving alcohol abstinence. He tolerated the 2-week medication titration well, with initial complaints of slight dizziness that soon resolved; he experienced no orthostatic hypotension. By the end of the titration period, Mr. A’s sitting blood pressure was noted to be 140/90. He attended all of his medical management sessions and consistently answered the daily interactive voice response questions. Pill counts and urine riboflavin indicated full medication adherence. Mr. A gradually decreased his alcohol consumption during the 12 weeks of the study. By the end of the study, he had not achieved his goal of total abstinence, but he was typically consuming 3 to 4 beers and no whiskey on the days he used alcohol and was abstaining 1 or 2 days per week. He had attended two meetings of Alcoholics Anonymous, and he found them helpful and planned to continue attending. His neuropathy had not worsened. He was referred to a federally qualified health center for ongoing follow-up of his alcohol use, his tobacco use, his neuropathy, and his blood pressure. He wanted to continue the study medication but because of the blinded nature of the study, he was informed that he would have to retitrate on prazosin received clinically. He signed a release of information for study staff to communicate the titration schedule to his new primary care provider.
1 : Brain stress systems in the amygdala and addiction. Brain Res 2009; 1293:61–75Crossref, Medline, Google Scholar
2 : The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res 2009; 33:264–272Crossref, Medline, Google Scholar
3 : Prazosin selectively antagonizes neuronal responses mediated by alpha1-adrenoceptors in brain. Naunyn Schmiedebergs Arch Pharmacol 1981; 317:273–275Crossref, Medline, Google Scholar
4 : alpha1-Noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol 2008; 42:91–97Crossref, Medline, Google Scholar
5 : Effects of prazosin and doxazosin on yohimbine-induced reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2016; 233:2197–2207Crossref, Medline, Google Scholar
6 : Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011; 218:89–99Crossref, Medline, Google Scholar
7 : Prazosin effects on stress- and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res 2012; 36:351–360Crossref, Medline, Google Scholar
8 : A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res 2009; 33:255–263Crossref, Medline, Google Scholar
9 : A randomized, placebo-controlled, clinical trial of prazosin for the treatment of alcohol use disorder. J Addict Med (Epub ahead of print, April 16, 2018)Google Scholar
10 : A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res 2015; 39:808–817Crossref, Medline, Google Scholar
11 : Prazosin for veterans with posttraumatic stress disorder and comorbid alcohol dependence: a clinical trial. Alcohol Clin Exp Res 2016; 40:178–186Crossref, Medline, Google Scholar
12 : Role of the α1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol 2016; 21:904–914Crossref, Medline, Google Scholar
13 : Higher pretreatment blood pressure is associated with greater alcohol drinking reduction in alcohol-dependent individuals treated with doxazosin. Drug Alcohol Depend 2017; 177:23–28Crossref, Medline, Google Scholar
14 : A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry 2013; 170:1003–1010Link, Google Scholar
15 : Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006; 295:2003–2017Crossref, Medline, Google Scholar
16 : Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Text Revision. Washington, DC, American Psychiatric Association, 2000Google Scholar
17 : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-I/P). New York, Biometrics Research Department, New York State Psychiatric Institute, 1995Google Scholar
18 : The development of a Clinician-Administered PTSD Scale. J Trauma Stress 1995; 8:75–90Crossref, Medline, Google Scholar
19 : Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl 1994; 12:112–118Crossref, Medline, Google Scholar
20 : R: A Language and Environment for Statistical Computing. Vienna, R Foundation for Statistical Computing, 2017Google Scholar
21 : Fitting linear mixed-effects models using lme4. J Stat Softw 2015; 67:1–48Crossref, Google Scholar
22 Magnusson A, Skaug H, Nielsen A, et al: glmmTMB: generalized linear mixed models using template model builder: R package, version 0.1.3, 2017. https://cran.r-project.org/web/packages/glmmTMB/index.htmlGoogle Scholar
23 : EnvStats: An R Package for Environmental Statistics. New York, Springer, 2013Crossref, Google Scholar
24 : Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry 2014; 171:445–452Link, Google Scholar
25 : A comparison of bootstrap approaches for estimating uncertainty of parameters in linear mixed-effects models. Pharm Stat 2013; 12:129–140Crossref, Medline, Google Scholar
26 : Random effects structure for confirmatory hypothesis testing: keep it maximal. J Mem Lang 2013; 68(3)Crossref, Medline, Google Scholar
27 : Conclusions beyond support: overconfident estimates in mixed models. Behav Ecol 2009; 20:416–420Crossref, Medline, Google Scholar
28 : Higher pretreatment blood pressure is associated with greater posttraumatic stress disorder symptom reduction in soldiers treated with prazosin. Biol Psychiatry 2016; 80:736–742Crossref, Medline, Google Scholar
29 : Reductions in healthcare costs following alcohol treatment: moving toward low-risk drinking end points in alcohol clinical trials. Alcohol Clin Exp Res 2016; 40:1415–1417Crossref, Medline, Google Scholar
30 : Effect of cocaine on the disposition of noradrenaline labelled with tritium. Nature 1960; 187:604–605Crossref, Medline, Google Scholar
31 : Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci 2002; 22:2873–2884Crossref, Medline, Google Scholar
32 : Norepinephrine in the prefrontal cortex is critical for amphetamine-induced reward and mesoaccumbens dopamine release. J Neurosci 2003; 23:1879–1885Crossref, Medline, Google Scholar
33 : Effects of prazosin, an α1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res 2012; 36:881–886Crossref, Medline, Google Scholar
34 : Previous exposure to cocaine enhances cocaine self-administration in an alpha 1-adrenergic receptor dependent manner. Neuropsychopharmacology 2007; 32:638–645Crossref, Medline, Google Scholar
35 : The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav 2009; 91:295–302Crossref, Medline, Google Scholar
36 : Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 2010; 35:1751–1760Crossref, Medline, Google Scholar
37 : The alpha-1 adrenergic antagonist doxazosin for treatment of cocaine dependence: a pilot study. Drug Alcohol Depend 2013; 131:66–70Crossref, Medline, Google Scholar
38 : Posttreatment effects of topiramate treatment for heavy drinking. Alcohol Clin Exp Res 2014; 38:3017–3023Crossref, Medline, Google Scholar
39 : Naltrexone vs placebo for the treatment of alcohol dependence: a randomized clinical trial. JAMA Psychiatry 2015; 72:430–437Crossref, Medline, Google Scholar
40 : Combining naltrexone and prazosin in a single oral medication decreases alcohol drinking more effectively than does either drug alone. Alcohol Clin Exp Res 2013; 37:1763–1770Medline, Google Scholar
41 : Prazosin + naltrexone decreases alcohol drinking more effectively than does either drug alone in P rats with a protracted history of extensive voluntary alcohol drinking, dependence, and multiple withdrawals. Alcohol Clin Exp Res 2015; 39:1832–1841Crossref, Medline, Google Scholar



