Common Dimensional Reward Deficits Across Mood and Psychotic Disorders: A Connectome-Wide Association Study
Abstract
Objective:
Anhedonia is central to multiple psychiatric disorders and causes substantial disability. A dimensional conceptualization posits that anhedonia severity is related to a transdiagnostic continuum of reward deficits in specific neural networks. Previous functional connectivity studies related to anhedonia have focused on case-control comparisons in specific disorders, using region-specific seed-based analyses. Here, the authors explore the entire functional connectome in relation to reward responsivity across a population of adults with heterogeneous psychopathology.
Method:
In a sample of 225 adults from five diagnostic groups (major depressive disorder, N=32; bipolar disorder, N=50; schizophrenia, N=51; psychosis risk, N=39; and healthy control subjects, N=53), the authors conducted a connectome-wide analysis examining the relationship between a dimensional measure of reward responsivity (the reward sensitivity subscale of the Behavioral Activation Scale) and resting-state functional connectivity using multivariate distance-based matrix regression.
Results:
The authors identified foci of dysconnectivity associated with reward responsivity in the nucleus accumbens, the default mode network, and the cingulo-opercular network. Follow-up analyses revealed dysconnectivity among specific large-scale functional networks and their connectivity with the nucleus accumbens. Reward deficits were associated with decreased connectivity between the nucleus accumbens and the default mode network and increased connectivity between the nucleus accumbens and the cingulo-opercular network. In addition, impaired reward responsivity was associated with default mode network hyperconnectivity and diminished connectivity between the default mode network and the cingulo-opercular network.
Conclusions:
These results emphasize the centrality of the nucleus accumbens in the pathophysiology of reward deficits and suggest that dissociable patterns of connectivity among large-scale networks are critical to the neurobiology of reward dysfunction across clinical diagnostic categories.
Anhedonia, defined as diminished reward responsivity, is central to a wide range of psychiatric disorders. In mood disorders such as major depressive disorder and bipolar disorder, depression is frequently associated with anhedonia, significantly affecting psychosocial function (1). Similarly, in psychotic disorders such as schizophrenia, anhedonia is one of the negative symptoms that lack effective treatments and cause substantial disability (2). The presence of anhedonia across multiple psychiatric disorders suggests common underlying deficits in reward system function. Such a conceptualization accords with the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) effort to map transdiagnostic dimensions of psychopathology, such as anhedonia, to abnormalities in specific brain circuitry (3).
Both animal and human studies consistently implicate the mesolimbic reward system, particularly the ventral striatum and nucleus accumbens, in the neurobiology of anhedonia (4). Neuroimaging studies in unipolar depression report ventral striatum hyporesponsivity during reward-related tasks (5). A similar blunting of striatal activation is seen in bipolar depression (6), schizophrenia (7), and psychosis risk populations (8). Given that this system has been implicated in multiple disorders, dimensional paradigms have recently been employed to identify common reward valuation abnormalities across disorders (9). We recently demonstrated, using task-based functional MRI (fMRI), that depression severity is related to blunted ventral striatum responses to monetary rewards across unipolar and bipolar depression (6). Others have reported similar results across a diverse group of patients with major depression, schizophrenia, alcohol dependence, and ADHD, as well as healthy control subjects (10). These findings highlight the value of dimensional approaches in identifying common neurobehavioral brain abnormalities (11).
One approach that is increasingly utilized to investigate circuit-level abnormalities in psychiatric disorders is resting-state (intrinsic) functional connectivity, which examines correlations in activity across different regions and can be used to delineate large-scale functional networks. Functional connectivity abnormalities are found in diverse psychiatric conditions (12, 13), suggesting that psychiatric disorders can be studied as syndromes of dysconnectivity. Using resting-state fMRI, several studies in distinct psychiatric disorders have employed seed-based analyses, preselecting specific brain regions (seeds) and examining how their activity correlates with activity in the rest of the brain. These studies implicate corticostriatal abnormalities in reward-related symptomatology (14, 15).
Studies investigating the relationship between reward-related deficits and functional network abnormalities have been limited, in part, by two factors. First, only a few studies have evaluated these deficits across multiple psychiatric disorders. This diminishes the ability to identify common brain phenotypes underlying reward system deficits associated with anhedonia. Second, most studies have examined functional connectivity on a regional basis using traditional seed-based analyses restricted to a few brain regions. By definition, this approach cannot reveal potentially important effects in brain regions not included in the analysis. To address these limitations, we evaluated a large, heterogeneous sample of adults with psychiatric conditions associated with reward abnormalities—major depression, bipolar disorder, schizophrenia, and genetic and clinical psychosis risk—as well as healthy individuals. We conducted a connectome-wide association study (CWAS), in which alterations in functional connectivity are examined across all the interregional connections in the brain (16). For this analysis, we used multivariate distance-based matrix regression (MDMR), a statistical procedure suited to analyzing complex neuroimaging data when there are many biological variables (e.g., image voxels) per subject. MDMR examines the overall pattern of connectivity for each individual voxel with all other brain voxels in relation to a clinical phenotype of interest, such as dimensional reward responsiveness. As suggested by the NIMH RDoC initiative (17), we measured anhedonia as a reduction in reward responsivity, using the reward sensitivity subscale of the Behavioral Activation Scale (18).
We hypothesized that across the dimension of reward responsivity, this data-driven analysis would reveal common patterns of dysconnectivity involving key elements of the reward system, such as the nucleus accumbens. Our analytic approach was not biased by a priori network selection but rather explored the entire complexity of the functional connectome using MDMR. This strategy was facilitated by a large sample of adults evaluated using a common imaging and phenotyping protocol. As described below, we offer novel evidence of functional network abnormalities associated with deficits in reward responsiveness across clinical diagnostic categories.
Method
Participants
For this study, 244 participants were assessed at two half-day visits using a common imaging and phenotyping protocol. On the first visit, the Structured Clinical Interview for DSM-IV was administered. On the second visit, assessment of reward responsiveness and neuroimaging was conducted. Individuals were enrolled if they met criteria for major depressive disorder, bipolar disorder, or schizophrenia; were at genetic or clinical risk of psychosis (e.g., had a first-degree relative with a psychotic disorder or met clinical high risk criteria for psychosis); or had no axis I diagnoses (healthy control subjects). After quality assurance procedures, the final sample for analysis included 225 individuals (Table 1). (For a list of medications by class, see Table S1 in the data supplement that accompanies the online edition of this article.) The University of Pennsylvania Institutional Review Board approved all study procedures, and all participants gave written informed consent. Additional details on participants are provided in the Supplementary Methods section of the data supplement.
| Characteristic | Control Group (N=53) | Bipolar Disorder Group (N=50) | Major Depression Group (N=32) | Schizophrenia Group (N=51) | Psychosis Risk Group (N=39) | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 32.52 | 13.0 | 30.58 | 10.6 | 40.13 | 13.0 | 37.54 | 12.0 | 32.84 | 16.2 | <0.01 |
| Education (years) | 14.86 | 2.3 | 14.47 | 2.2 | 14.10 | 2.8 | 13.65 | 2.2 | 14.34 | 2.4 | 0.12 |
| Behavioral Activation Scale, reward sensitivity subscale score | 17.72 | 1.8 | 17.06 | 2.5 | 16.09 | 2.8 | 16.74 | 2.6 | 17.44 | 1.9 | <0.05 |
| In-scanner motion (mm) | 0.08 | 0.03 | 0.07 | 0.04 | 0.09 | 0.05 | 0.09 | 0.06 | 0.09 | 0.06 | 0.18 |
| N | % | N | % | N | % | N | % | N | % | ||
| Female | 28 | 53 | 31 | 62 | 16 | 50 | 24 | 47 | 16 | 41 | 0.36 |
| Taking psychotropic medication | 46 | 92 | 21 | 66 | 49 | 96 | 6 | 15 | <0.001 | ||
TABLE 1. Basic Demographic and Clinical Characteristics of Participants in a Study of the Functional Connectome in Relation to Reward Responsivity (N=225)
Dimensional Assessment of Reward Responsiveness
To assess reward-related functioning, we administered the Behavioral Activation Scale (BAS) (18). The BAS reward sensitivity subscale has been identified as a transdiagnostic measure of reward responsivity (17) and has been used to index anhedonia (19). The scale captures a broad range of reward functioning, and it is useful for dimensional analyses across both clinical and nonclinical samples. Because the initial descriptions of the BAS factor structure were based on healthy young adults, we conducted a factor analysis on item-level data to confirm previously identified subfactors, including the reward sensitivity subscale, in our clinical sample (see Table S2 in the data supplement). Additionally, we measured the BAS reward sensitivity subscale in each subject to confirm a broad distribution of reward functioning across disorders (Figure 1).
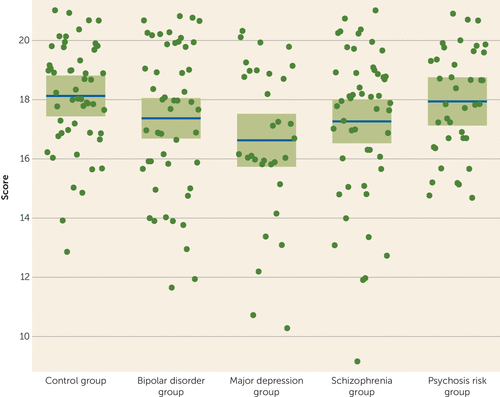
FIGURE 1. Score on the Reward Sensitivity Subscale of the Behavioral Activation Scale, by Diagnostic Groupa
a Each dot represents a study participant. The horizontal lines indicate mean values, and the shaded regions indicate 95% confidence bands. Values adjusted for age and sex.
Image Acquisition and Processing
All MRI data were acquired on the same Siemens 3-T scanner using the same imaging sequences, as detailed in the Supplementary Methods section of the data supplement. Resting-state fMRI bold-oxygen-level-dependent (BOLD) time-series data were processed to correct image distortion and to minimize the influence of in-scanner motion (20), transformed into a standard anatomical brain space (Montreal Neurological Institute), and downsampled to a lower spatial resolution prior to CWAS (16).
CWAS Using MDMR
MDMR relating reward sensitivity to whole-brain connectivity patterns was conducted in three steps (Figure 2). In the first step, the processed voxel-wise subject resting-state fMRI data were used to conduct a seed-based connectivity analysis at each gray matter voxel. In this step, the Pearson’s correlation between each voxel’s BOLD time series and that of every other voxel within gray matter was calculated. In the second step, the overall pattern of connectivity for each voxel was compared between subjects using a distance metric. The distance metric quantifies the similarity in the pattern of connectivity between each pair of subjects (16). Finally, in the third step, MDMR was used to test how well the dimensional variable of interest (BAS reward sensitivity subscore) explained the distances between each subject’s pattern of connectivity at that seed voxel, while controlling for the effects of nuisance covariates such as clinical group status, age, sex, and in-scanner motion (21). This MDMR procedure identified voxels where BAS reward sensitivity subscore affected the overall pattern of connectivity. As in Shehzad et al. (16), the false positive error rate (type I error) of MDMR was controlled using cluster correction with a voxel height of z>1.64 and utilized a cluster-extent probability threshold p<0.01 (22). Cortical projections of MDMR statistical maps were displayed using the Caret software package (23). See the Supplementary Methods section of the data supplement for additional details.
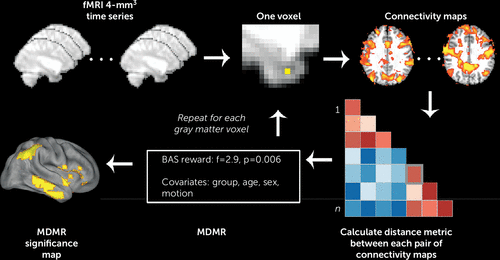
FIGURE 2. Connectome-Wide Analysis Using Multivariate Distance-Based Matrix Regression (MDMR)a
a Template-space functional time series were resampled at 4 mm3 for computational feasibility. For each gray matter voxel, a connectivity map was created for each subject, and the maps were compared in a pairwise manner to create a distance matrix. MDMR used these distance matrices to evaluate the complex multivariate pattern of connectivity in association with reward responsivity (Behavioral Activation Scale [BAS] reward sensitivity subscale score) across subjects while controlling for clinical group, age, sex, and in-scanner motion. This yielded a pseudo-F statistic and a corresponding p value through permutation testing. This procedure was repeated for each gray matter voxel, yielding a voxel-wise significance map. Adapted from Shehzad et al. (16) with permission from Elsevier.
Follow-Up Seed-Based Analyses
Although MDMR identifies clusters where the overall multivariate pattern of connectivity is dimensionally related to BAS reward sensitivity subscore, it does not describe the specific pattern of connectivity that drives the significant result. As in previous studies (24, 25), we conducted post hoc seed-based analyses from each cluster returned by MDMR, followed by network analyses of these regions (described below). Group-level seed analyses included the same covariates as those listed above. These follow-up analyses subsequent to MDMR are applied descriptively, as the seeds were selected on the basis of the significance of the MDMR result. Further details are provided in the Supplementary Methods section of the data supplement.
Network Construction and Analysis
In order to summarize the observed pairwise interactions among the implicated brain regions, we evaluated the data within a network framework. We constructed a graph of cortical nodes consisting of clusters identified by MDMR. As in previous work, subcortical nodes such as the nucleus accumbens were not included in detecting cortical modules (26). The graph was separated into distinct network modules using community detection techniques (described in the Supplementary Methods section of the data supplement). Differences in connectivity among the cortical modules and with the nucleus accumbens were investigated using measures of within-network and between-network connectivity (27). Within-network connectivity was defined as the mean correlation strength of all edges within a network module. In contrast, between-network connectivity was defined on a pairwise basis as the mean strength of edges between nodes within a network module and nodes outside the module (27). The relationship of BAS reward sensitivity subscore to these connectivity measures was examined using linear regression, with the same covariates as listed above.
Supplementary Analyses
To evaluate within-group dimensional effects, we conducted separate analyses examining network-level associations for each diagnostic group and specific subgroups, as well as for a psychopathology-only sample that excluded healthy control subjects. Additionally, we explored differences in network-level measures among categorical diagnostic groups. Furthermore, to assess specificity for our clinical phenotype, we compared network associations among the BAS subscales and with diagnosis-specific illness severity measures (see the Supplementary Methods section of the data supplement). Although the above analyses accounted for variables including clinical group, age, sex, and in-scanner motion, we also conducted additional analyses including smoking status as a covariate. Finally, we also included composite medication load as a confounding variable in network analyses, based on a previously described method (28).
Results
MDMR Identifies Multiple Foci of Connectivity Related to Reward Responsivity
MDMR revealed multiple regions where the multivariate pattern of connectivity was dimensionally related to reward sensitivity across clinical diagnostic categories (Figure 3). These regions included the left and right nucleus accumbens and a set of widely distributed cortical regions (left and right temporoparietal junction, right insular cortex, right inferior and left superior lateral temporal cortex, left lateral orbitofrontal cortex, and left dorsomedial frontal cortex) (see Table S3 in the online data supplement). Next, because these results do not describe which specific connections form the basis for the observed multivariate results, each significant MDMR cluster was evaluated using a standard seed-based connectivity analysis.
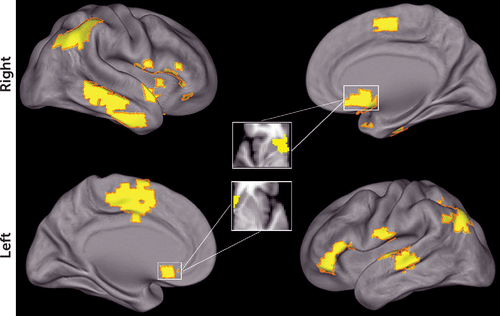
FIGURE 3. Multivariate Distance-Based Matrix Regression (MDMR) Identified Multiple Foci of Connectivity Related to Reward Responsivitya
a Cortical projection displaying clusters identified by MDMR where score on the reward sensitivity subscale of the Behavioral Activation Scale affected the overall multivariate pattern of functional connectivity. All clusters corrected for multiple comparisons at z>1.64, p<0.01.
Seed-Based Connectivity Analyses Explain Patterns of Connectivity That Drive MDMR Results
Follow-up analyses used the regions identified by MDMR as the basis for seed-based connectivity analyses, which examined the connectivity from a given region with the rest of the brain on a voxel-wise basis. These analyses demonstrated that the multivariate results from MDMR were driven by altered patterns of connectivity affecting elements of the default mode network and the cingulo-opercular network (Figure 4). With increasing BAS reward sensitivity subscore, nucleus accumbens connectivity increased with elements of the default mode network, including the temporoparietal junction, lateral temporal cortex, anterior medial prefrontal cortex, and posterior cingulate cortex (Figure 4A). Conversely, nucleus accumbens connectivity decreased with elements of the cingulo-opercular network, including the insular cortex and dorsomedial frontal cortex (including supplementary motor regions). Within clusters in the default mode network, higher BAS reward sensitivity subscore was associated with a dissociable pattern of diminished connectivity with other elements of the default mode network, as well as increased connectivity with cingulo-opercular network regions (Figure 4B). Similarly, for clusters in the cingulo-opercular network, higher BAS reward sensitivity subscore was associated with decreased connectivity with other parts of the cingulo-opercular network and increased connectivity with default mode network regions (Figure 4C).
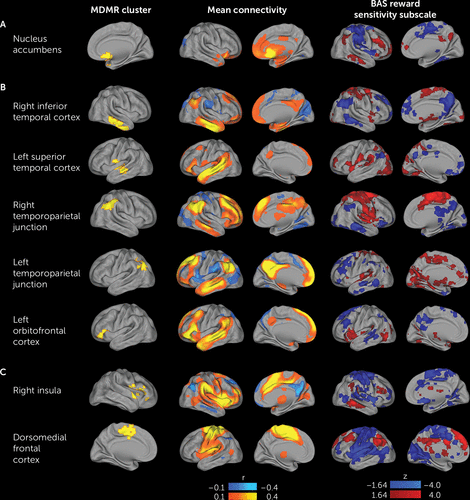
FIGURE 4. Follow-Up Seed-Based Connectivity Analyses Explain Patterns of Connectivity That Drive Multivariate Distance-Based Matrix Regression (MDMR) Resultsa
a The multivariate results of the connectome-wide association study (CWAS) identified the nucleus accumbens (section A), default mode network regions (section B), and cingulo-opercular network regions (section C) where the overall pattern of connectivity is related to reward responsivity, but it did not delineate the nature of those patterns. Accordingly, each cluster identified by the CWAS (left column) was used as a seed to identify what changes in connectivity led to the significant finding. The middle column displays the mean connectivity across all subjects from each seed. The right column displays the association with score on the reward sensitivity subscale of the Behavioral Activation Scale (BAS) for each seed.
Reward Deficits Are Associated With a Dissociable Pattern of Within- and Between-Network Connectivity
The results of the seed-based analyses suggested common patterns of dysconnectivity involving the nucleus accumbens, the default mode network, and the cingulo-opercular network. In order to concisely summarize these effects, we conducted network analyses in which nodes were centered on the clusters identified by MDMR (Figure 5). Application of community detection procedures to the cortical regions identified by MDMR revealed two network modules (Figure 5A): a default mode network module and a cingulo-opercular network module. The integrity of these modules was confirmed through permutation testing (default mode network: p=1.95×10−4; cingulo-opercular network: p=2.39×10−4).
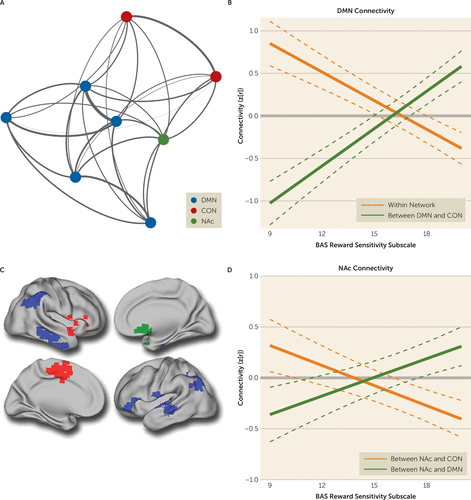
FIGURE 5. Dissociable Patterns of Within- and Between-Network Connectivity Are Dimensionally Related to Reward Deficits Across Psychiatric Disordersa
a Panel A presents the layout of mean connectivity within a network of nodes defined by multivariate distance-based matrix regression (MDMR). DMN=default mode network; CON=cingulo-opercular network; NAc=nucleus accumbens. In panel B, default mode network hyperconnectivity and decreased connectivity between default mode network and cingulo-opercular network regions is associated with reward deficits. Panel C shows a cortical projection displaying the nucleus accumbens (green) along with default mode network (blue) and cingulo-opercular network regions (red). In panel D, reward deficits are related to dissociable patterns of nucleus accumbens dysconnectivity, including diminished connectivity with the default mode network and increased connectivity with the cingulo-opercular network.
These network modules were used to derive summary measures of within-network and between-network connectivity for each cortical network as well as the nucleus accumbens. This approach demonstrated that deficits in reward responsivity were associated with default mode network hyperconnectivity (t=3.75, p=2.3×10−4) and decreased integration between the default mode network and the cingulo-opercular network (t=−5.17, p=5.3×10−7). In addition, reward deficits were associated with decreased connectivity between the nucleus accumbens and the default mode network (t=−2.45, p=1.5×10−2) and increased connectivity between the nucleus accumbens and the cingulo-opercular network (t=3.35, p=9.4×10−4).
An MDMR analysis evaluating for effects of clinical diagnosis did not identify hubs of the reward system such as the nucleus accumbens (see Table S4 in the online data supplement). Notably, observed dimensional effects with BAS reward sensitivity subscore were present within each clinical diagnostic category (see Table S5 in the data supplement). In control subjects, most effects were in the same direction but were attenuated in magnitude; exclusion of control subjects from the sample strengthened all associations. Although differences in BAS subfactor scores were present between groups (see Table S6 in the data supplement), there were no group differences in network-level summary measures. Inclusion of smoking status or composite medication load did not affect the results (see Table S7 in the data supplement). Network-level associations were directionally similar for other BAS subscales but were reduced in magnitude (see Table S8 in the data supplement). The network associations were not related to other disorder-specific illness severity measures (see Table S9 in the data supplement).
Discussion
We used a fully data-driven survey of the functional connectome to identify regions where the multivariate pattern of brain connectivity was dimensionally related to reward responsivity across a large, heterogeneous population of adults with psychiatric disorders. This approach identified multivariate patterns of connectivity centered on regions within the default mode network, the cingulo-opercular network, and the nucleus accumbens. Network-based analyses revealed that reward deficits were associated with hyperconnectivity within the default mode network and with diminished connectivity between default mode network and cingulo-opercular network regions. Furthermore, nucleus accumbens connectivity was decoupled from default mode network regions and showed increased connectivity with cingulo-opercular network regions. Taken together, these findings delineate a common pattern of large-scale network dysconnectivity associated with reward deficits across clinical diagnostic categories.
MDMR Allows Full Exploration of the Connectome in Relation to Reward Responsivity
To date, the majority of studies examining reward-related functional connectivity abnormalities in psychiatric disorders have employed a priori defined seed-based approaches (14). In contrast, we used MDMR to conduct a data-driven analysis of the entire functional connectome in relation to reward responsiveness. MDMR has the advantage of simultaneously assessing associations between reward responsiveness and each connection within the functional connectome while controlling for the effects of potential confounders. MDMR thus eliminates the need for researchers to select specific brain regions of interest, minimizing bias in the experimental design. Remarkably, this exploratory multivariate analysis identified abnormalities in the nucleus accumbens, a region critical for reward processing (5). Single-disorder case-control studies have consistently implicated the nucleus accumbens in reward-related pathophysiology in mood and psychotic disorders (7). Furthermore, abnormalities involving the nucleus accumbens have specifically been linked with symptoms of anhedonia (15). However, whether circuit-level abnormalities involving the nucleus accumbens can be identified across these disorders has not previously been evaluated. As discussed below, dimensional deficits of reward responsivity are associated with connectivity abnormalities between the nucleus accumbens and major functional networks.
Hyperconnectivity Within a Default Mode Network Subsystem Is Associated With Reward Impairments
The default mode network comprises brain regions that are important for internally directed modes of cognition, including memory, prospection, theory of mind, and reward valuation. Abnormally enhanced connectivity within the default mode network has been reported in multiple psychiatric conditions in which anhedonia is prominent (29, 30). In our diagnostically heterogeneous sample, MDMR identified default mode network regions, including the temporoparietal junction and the lateral temporal cortex. Follow-up seed-based connectivity analyses identified additional default mode network regions, including the posterior cingulate cortex and the anterior medial prefrontal cortex. These regions map to a functionally specific default mode network subsystem (the dorsomedial prefrontal subsystem) linked to present-state, self-referential, and affective cognition (31). Notably, regions belonging to another default mode network subsystem—the medial temporal lobe system, which is preferentially active during memory rather than affective processing—were not identified by MDMR (31). Hyperconnectivity within the dorsomedial prefrontal default mode network subsystem was associated with impairments in reward responsivity. Additionally, there was diminished connectivity between this default mode network subsystem and the cingulo-opercular network, a cognitive-control network involved in detecting salient external stimuli (32). These findings were present in each clinical group, highlighting the relevance of these abnormalities across diagnostic categories. Speculatively, default mode network hyperconnectivity and diminished default mode network connectivity with the cingulo-opercular network may represent a deficit in network integration necessary for reward responsiveness. Symptoms of anhedonia may also be associated with increased rumination and ineffective transitioning from internal to external modes of cognition (29).
Reduced Connectivity Between the Nucleus Accumbens and a Default Mode Network Subsystem Is Associated With Reward Deficits
Corticostriatal abnormalities involving the nucleus accumbens have been reported in diverse psychiatric disorders. In our study, reward deficits were related to dissociable patterns of nucleus accumbens dysconnectivity, including decreased connectivity between the nucleus accumbens and the default mode network and increased connectivity between the nucleus accumbens and the cingulo-opercular network. Together, these findings implicate a pattern of dysconnectivity between the nucleus accumbens and major functional networks for which dysconnectivity has commonly been reported in both mood and psychotic disorders (13–15). Notably, these effects were consistent across the groups with major depression, bipolar disorder, schizophrenia, and psychosis risk. This raises the interesting possibility that nucleus accumbens dysconnectivity is associated with reward responsiveness deficits, regardless of clinical diagnostic category. Diminished integration between the nucleus accumbens and the default mode network may reflect a brain phenotype corresponding to impairments in reward-oriented internal cognition (33). Increased integration seen between the nucleus accumbens and the cingulo-opercular network may be related to elevated cognitive control over reward system activity (34).
Strengths and Limitations of Examining Dimensions in Heterogeneous Populations
Establishing common patterns of brain dysconnectivity across clinical diagnostic categories is a central aim of the NIMH RDoC (3). In the present study, inclusion of a heterogeneous population of adults with diverse psychopathology allowed identification of common dimensional patterns of dysconnectivity related to reward functioning. However, certain limitations of our approach should be noted. First, the cross-sectional analyses we used here do not allow determination of causation or the temporal pattern of changes. Second, evaluating whole-brain connectivity using MDMR may have decreased sensitivity to localized patterns of dysconnectivity related to reward functioning. While comparisons of different MDMR distance measures have generally yielded similar findings, it remains unclear whether there is an optimal metric depending on the question of interest (16). Third, while this study characterizes anhedonia in terms of diminished self-reported reward responsivity, this may be distinct from other elements of anhedonia, including reward anticipation, effort, and satiety (2, 35). Fourth, while our findings suggest no significant impact of composite medication load on neural activity and were similar for psychosis risk groups with few medicated subjects, future studies should confirm these findings in unmedicated populations. Finally, the dimensional analysis of a heterogeneous sample may not identify important disorder-specific brain phenotypes related to reward dysfunction, and the results may not extend to other psychiatric disorders with reward dysfunction, such as addiction and ADHD (36).
Consideration of Findings Within the Framework of Traditional Diagnostic Categories
Neuroimaging studies in different psychiatric disorders have previously reported corticostriatal abnormalities involving the reward system (13, 14). In our study, dissociable patterns of dysconnectivity among large-scale cortical networks and the nucleus accumbens were identified across the dimension of reward responsiveness. These neurobiological patterns were found in subjects with both mood and psychotic disorders. This suggests that common pathophysiological mechanisms may underlie the development of anhedonia in different psychiatric disorders. However, distinct pathological mechanisms have also been shown to underlie aspects of anhedonia seen in these disorders (37). Consequently, a focus on the etiological and neurodevelopmental aspects of reward-related dysfunction is needed to clarify common and dissociable mechanisms. This will be important for developing interventions that target shared and unique processes underlying anhedonia in these disorders.
Conclusions
Our results corroborate previous research using case-control designs and emphasize that corticostriatal dysconnectivity is implicated in reward-related abnormalities across clinical diagnostic categories and in individuals at risk for these disorders. Specifically, common abnormalities among large-scale cortical networks and the nucleus accumbens may underlie reward deficits. These results suggest that development of interventions to treat anhedonia in different psychiatric disorders may effectively target shared neural abnormalities in critical functional networks. Research employing longitudinal designs may allow for evaluation of early interventions that promote resilience against shared reward-related psychopathology before disabling symptoms develop.
1 : Long-term morbidity in bipolar-I, bipolar-II, and unipolar major depressive disorders. J Affect Disord 2015; 178:71–78Crossref, Medline, Google Scholar
2 : Anhedonia in schizophrenia. Curr Psychiatry Rep 2006; 8:322–328Crossref, Medline, Google Scholar
3 : Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167:748–751Link, Google Scholar
4 : The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 2013; 76:412–427Crossref, Medline, Google Scholar
5 : Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009; 166:702–710Link, Google Scholar
6 : Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology 2015; 40:2258–2268Crossref, Medline, Google Scholar
7 : Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull 2014; 40:1328–1337Crossref, Medline, Google Scholar
8 : Striatal response to reward anticipation: evidence for a systems-level intermediate phenotype for schizophrenia. JAMA Psychiatry 2014; 71:531–539Crossref, Medline, Google Scholar
9 : Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: a replicated cross-diagnostic finding. Front Psychol 2015; 6:1280Crossref, Medline, Google Scholar
10 : Dimensional psychiatry: reward dysfunction, and depressive mood across psychiatric disorders. Psychopharmacology (Berl) 2015; 232:331–341Crossref, Medline, Google Scholar
11 : Diminished effort on a progressive ratio task in both unipolar and bipolar depression. J Affect Disord 2016; 196:97–100Crossref, Medline, Google Scholar
12 : A systematic literature review of resting state network: functional MRI in bipolar disorder. J Affect Disord 2013; 150:727–735Crossref, Medline, Google Scholar
13 : Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 2009; 106:1279–1284Crossref, Medline, Google Scholar
14 : A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 2014; 171:829–843Link, Google Scholar
15 : Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychol Med 2016; 46:125–135Crossref, Medline, Google Scholar
16 : A multivariate distance-based analytic framework for connectome-wide association studies. Neuroimage 2014; 93:74–94Crossref, Medline, Google Scholar
17 Positive Valence Systems: Workshop Proceedings (National Institute of Mental Health, Rockville, Md, June 19–21, 2011). http://www.nimh.nih.gov/research-priorities/rdoc/positive-valence-systems-workshop-proceedings.shtmlGoogle Scholar
18 : Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol 1994; 67:319–333Crossref, Google Scholar
19 : Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res 2007; 93:253–260Crossref, Medline, Google Scholar
20 : An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 2013; 64:240–256Crossref, Medline, Google Scholar
21 : Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage 2012; 60:623–632Crossref, Medline, Google Scholar
22 : AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
23 : An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc 2001; 8:443–459Crossref, Medline, Google Scholar
24 : Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivty. Mol Psychiatry 2016; 21:894–902Crossref, Medline, Google Scholar
25 : Connectome-wide network analysis of youth with psychosis-spectrum symptoms. Mol Psychiatry 2015; 20:1508–1515Crossref, Medline, Google Scholar
26 : Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex 2016; 26:288–303Crossref, Medline, Google Scholar
27 : Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci USA 2015; 112:13681–13686Crossref, Medline, Google Scholar
28 : Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord 2008; 10:916–927Crossref, Medline, Google Scholar
29 : Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry 2011; 70:327–333Crossref, Medline, Google Scholar
30 : Aberrant default mode functional connectivity in early onset schizophrenia. PLoS One 2013; 8:e71061Crossref, Medline, Google Scholar
31 : Functional-anatomic fractionation of the brain’s default network. Neuron 2010; 65:550–562Crossref, Medline, Google Scholar
32 : Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res 2013; 148:74–80Crossref, Medline, Google Scholar
33 : Frontal cortex and reward-guided learning and decision-making. Neuron 2011; 70:1054–1069Crossref, Medline, Google Scholar
34 : Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol 2015; 66:83–113Crossref, Medline, Google Scholar
35 : Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev 2016; 65:21–35Crossref, Medline, Google Scholar
36 : A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA 2010; 107:13509–13514Crossref, Medline, Google Scholar
37 : Reward processing dysfunction in major depression, bipolar disorder, and schizophrenia. Curr Opin Psychiatry 2015; 28:7–12Crossref, Medline, Google Scholar



