A Critical Appraisal of Neuroimaging Studies of Bipolar Disorder: Toward a New Conceptualization of Underlying Neural Circuitry and a Road Map for Future Research
Abstract
Objective
In this critical review, the authors appraise neuroimaging findings in bipolar disorder in emotion-processing, emotion-regulation, and reward-processing neural circuitry in order to synthesize the current knowledge of the neural underpinnings of bipolar disorder and provide a neuroimaging research road map for future studies.
Method
The authors examined findings from all major studies in bipolar disorder that used functional MRI, volumetric analysis, diffusion imaging, and resting-state techniques, integrating findings to provide a better understanding of larger-scale neural circuitry abnormalities in bipolar disorder.
Results
Bipolar disorder can be conceptualized, in neural circuitry terms, as parallel dysfunction in prefrontal cortical (especially ventrolateral prefrontal cortical)-hippocampal-amygdala emotion-processing and emotion-regulation circuits bilaterally, together with an “overactive” left-sided ventral striatal-ventrolateral and orbitofrontal cortical reward-processing circuitry, resulting in characteristic behavioral abnormalities associated with bipolar disorder: emotional lability, emotional dysregulation, and heightened reward sensitivity. A potential structural basis for these functional abnormalities is gray matter volume decreases in the prefrontal and temporal cortices, the amygdala, and the hippocampus and fractional anisotropy decreases in white matter tracts connecting prefrontal and subcortical regions.
Conclusions
Neuroimaging studies of bipolar disorder clearly demonstrate abnormalities in neural circuits supporting emotion processing, emotion regulation, and reward processing, although there are several limitations to these studies. Future neuroimaging research in bipolar disorder should include studies adopting dimensional approaches; larger studies examining neurodevelopmental trajectories in youths with bipolar disorder or at risk for bipolar disorder; multimodal neuroimaging studies using integrated systems approaches; and studies using pattern recognition approaches to provide clinically useful individual-level data. Such studies will help identify clinically relevant biomarkers to guide diagnosis and treatment decision making for individuals with bipolar disorder.
Identifying biomarkers is a key goal of neuroimaging research in bipolar disorder, to provide objective neurobiological markers to increase diagnostic precision, identify markers of risk for future bipolar disorder, and pave the way for personalized treatments based on an enhanced understanding of underlying neuropathophysiological processes. Our purpose in this critical review is fourfold. We first provide a new conceptualization of neural circuitry abnormalities in bipolar disorder based on the most consistent themes emerging from neuroimaging research. We then identify areas of neuroimaging research that are needed to confirm this model of neural circuitry in bipolar disorder and elucidate as yet unknown neuropathophysiological processes in the disorder. Next, we describe other areas of bipolar disorder neuroimaging research that are currently understudied but will be critical in fully informing our understanding of the neuropathophysiology of bipolar disorder. We end the review with a suggested road map for future neuroimaging studies of bipolar disorder to provide a research framework that will permit elucidation of the neuropathophysiology of bipolar disorder and eventually identify biomarkers for diagnosis, risk identification, and targets to guide personalized treatment.
The Scope of Neuroimaging Studies in the Review
We integrate findings from all major studies of bipolar disorder that have used neuroimaging modalities, to provide a better understanding of larger-scale neural circuitry abnormalities in bipolar disorder. We first review functional neuroimaging studies examining the functional integrity of neural circuits relevant to neuropathophysiological processes in bipolar disorder by measuring regional activity (using blood-oxygen-level-dependent signal change) and functional connectivity, using techniques that examine the extent of coupling of time series of activity between neural regions of interest. We also review studies examining gray matter regional volumes and diffusion imaging studies examining the structure of white matter in key tracts in these circuits, to help inform interpretation of functional abnormalities in these circuits in bipolar disorder. We include findings from studies examining intrinsic (resting state) connectivity in neural circuits of relevance to bipolar disorder. We have not included findings from studies using other methods (e.g., magnetic resonance spectroscopy or positron emission tomography) that examine regional neurotransmitter concentration and neuroreceptor density, given our focus here on larger-scale circuits relevant to bipolar disorder.
Major Themes That Emerge From Neuroimaging Studies
Theme 1: Abnormalities in Emotion-Processing and Emotion-Regulation Neural Circuitry
Emotional overreactivity and emotion dysregulation are characteristic symptoms of bipolar disorder (1). A large number of functional neuroimaging studies have thus examined emotion-processing and emotion-regulation neural circuitry function in individuals with bipolar disorder during performance of emotion-processing and emotion-regulation tasks (Table 1; see also Table S1 in the data supplement that accompanies the online edition of this article). This circuitry includes the amygdala, a region with a key role in emotion processing (2), threat, and salience perception (3), and prefrontal cortical regions implicated in emotion regulation. Emotion regulation has been categorized into voluntary and automatic (implicit) subprocesses, each implicating different prefrontal cortical regions, including the orbitofrontal cortex, ventrolateral prefrontal cortex, dorsolateral prefrontal cortex, and medial prefrontal cortex (encompassing the anterior cingulate cortex and mediodorsal prefrontal cortex) (4). Here, a predominantly lateral prefrontal cortical system (centered on the dorsolateral prefrontal cortex and ventrolateral prefrontal cortex) mediates voluntary emotion-regulation subprocesses, while a medial prefrontal cortical system (including the orbitofrontal cortex, anterior cingulate cortex, mediodorsal prefrontal cortex, and hippocampus) mediates automatic emotion-regulation subprocesses. The dense connections between temporal limbic regions and the ventrolateral prefrontal cortex (5) and the role of this region in set shifting and reversal learning (6, 7) (inhibitory control processes common to voluntary emotion-regulation subprocesses) have motivated a focus on the ventrolateral prefrontal cortex in particular in research on voluntary emotion-regulation circuitry (for example, see reference 8).
| Theme 1 | Abnormally decreased ventrolateral prefrontal cortex activity during emotion processing, emotion regulation, and response inhibition (9–19) |
| Theme 2 | Abnormally increased amygdala, striatal, and medial prefrontal cortical activity and decreased functional connectivity between amygdala and prefrontal cortex, to positive emotional stimuli (9, 10, 20–22) |
| Theme 3 | Abnormally increased amygdala, orbitofrontal cortex, and temporal cortical activity during nonemotional, cognitive task performance (23–28) |
| Theme 4 | Abnormally increased left ventrolateral prefrontal cortex and orbitofrontal cortex, and ventral striatum activity during reward processing (40–45) |
Early neuroimaging studies of individuals with bipolar disorder indicated predominant patterns of abnormally elevated amygdala activity in response to emotional stimuli (9, 10) and abnormally reduced activity in lateral and medial prefrontal cortical regions supporting emotion regulation (4, 11). More recent studies have used more sophisticated functional connectivity techniques and identified fronto-subcortical functional abnormalities in adults with bipolar disorder during emotion regulation and inhibitory control during different mood states (12). Specifically, these studies report abnormally decreased inferior frontal cortical activity, especially in the ventrolateral prefrontal cortex, and abnormally decreased ventrolateral prefrontal cortex-amygdala functional connectivity during different positive and negative emotion-processing and emotion-regulation tasks in adults with bipolar disorder across different mood states (13–18). These findings parallel earlier findings of abnormally decreased ventrolateral prefrontal cortex activity and ventrolateral prefrontal cortex-amygdala functional connectivity during emotion processing in manic adults with bipolar disorder (19).
Theme 2: Abnormal Activity in Emotion-Processing Circuitry to Positive Emotional Stimuli
A second theme, building on the first, is a pattern of abnormally elevated amygdala and striatal and medial prefrontal cortical activity in response to positive emotional stimuli in individuals with bipolar disorder (9, 10) (Table 1; see also Table S1 in the online data supplement). More recent studies in adults with bipolar disorder have demonstrated abnormally increased amygdala and medial prefrontal cortex activity (20, 21) and abnormally decreased positive orbitofrontal cortex-amygdala effective connectivity bilaterally (22) in response to emotional—especially happy—faces, suggesting a dysregulated amygdala response to these stimuli. These findings may reflect an underlying attentional bias to positive emotional stimuli in bipolar disorder, predisposing to mania.
Theme 3: Abnormal Activity in Emotion-Processing Neural Circuitry During Performance of Nonemotional Tasks
A third theme is abnormal activity in emotion processing circuitry including the amygdala, orbitofrontal cortex, and temporal cortex during nonemotional cognitive task performance in bipolar disorder (23, 24) (Table 1; see also Table S1 in the online data supplement). For example, studies have reported abnormally elevated amygdala activity in adults with bipolar disorder across different mood states during performance of a variety of cognitive tasks (25–27) and increased amygdala activity during motor response inhibition in manic compared with remitted adults with bipolar disorder (28). These findings suggest a heightened perception of emotional salience in nonemotional contexts in bipolar disorder.
Theme 4: Abnormalities in Reward-Processing Neural Circuitry
In addition to emotional overreactivity and emotion dysregulation, another feature of bipolar disorder is heightened reward sensitivity, indicated by behavioral and event-related-potential studies (29–31). The key role of the ventral striatum (nucleus accumbens) in response to reward cues and reward receipt is well established (32, 33), although specific prefrontal cortical regions also have roles. The ventrolateral prefrontal cortex, in addition to its role in emotion regulation described above, is activated during arousal in the context of emotional stimuli (34, 35), while the orbitofrontal cortex plays a role in encoding reward value (36). In humans, both of these regions may have excitatory afferent connections with the ventral striatum, as suggested by studies reporting excitatory afferent connections between the homologues of these prefrontal cortical regions and the ventral striatum in rodents (37). The medial prefrontal cortex regulates the ventral striatum and appetitive behaviors in potentially rewarding contexts (38, 39).
Functional neuroimaging studies of reward-processing neural circuitry in individuals with bipolar disorder indicate abnormally elevated activity in the ventral striatum and left prefrontal cortex, in particular the left orbitofrontal cortex and left ventrolateral prefrontal cortex, during reward processing. Studies have reported abnormally increased left ventrolateral prefrontal cortex and ventral striatal activity in response to reward anticipation in adults with bipolar disorder in different mood states (40–42); abnormally elevated left orbitofrontal cortex and amygdala activity in response to reward reversal and elevated left orbitofrontal cortex activity in response to reward in euthymic adults with bipolar disorder (43); and elevated ventral striatal activity in response to reward cues and outcomes in individuals with subthreshold hypomania (44). One study, however, reported no differential activity in the ventral striatum in response to reward receipt versus omission in manic adults with bipolar disorder (45) (Table 1; see also Table S1 in the online data supplement).
Structural Neuroimaging Studies Providing Support for the Main Themes
Early studies focused on structural neuroanatomical changes in bipolar disorder. Key findings were an increased number of white matter hyperintensities (46), as well as enlarged amygdala gray matter volumes (47, 48), highlighting the potential role of abnormalities in emotion processing neural circuitry in the disorder. More recent studies have examined regional gray matter volumes in cortical and subcortical regions in adults with bipolar disorder, and emerging findings coalesce into two main themes largely relating to emotion-processing and emotion-regulation neural circuits (Table 2; see also Table S2 in the online data supplement). First, many studies have examined cortical regions implicated in emotion processing and cognitive processes that are important for emotion regulation—the prefrontal and anterior temporal cortices—and cortical regions that underlie salience perception—the insula and the dorsal anterior cingulate cortex (4, 12). Findings indicate a predominant pattern of abnormally decreased gray matter volume, decreased white matter volume, and decreased cortical thickness in these regions in individuals with bipolar disorder or at risk for bipolar disorder (49–57, although see also references 58, 59). Another study reported a negative association between right ventrolateral prefrontal cortex gray matter volume and illness duration and smaller right ventrolateral prefrontal cortex gray matter volume in adults with bipolar disorder with long-term illness and minimal lifetime exposure to lithium compared with healthy adults, but abnormally increased right ventrolateral prefrontal cortex gray matter volume in relatives of individuals with bipolar disorder and in younger adults in early stages of bipolar disorder (60). Orbitofrontal cortical volume reductions in adults with bipolar disorder may be more evident during depressive episodes (61). Prefrontal cortical gray matter volumes in general may decrease with illness progression (62) but normalize (or even increase) with lithium treatment (60, 63). Studies have also reported widespread decreases in frontal cortical thickness bilaterally, especially in the right hemisphere, and abnormally decreased temporal and parietal cortical thickness bilaterally in adults with bipolar disorder (64, 65).
| Main findings from structural neuroimaging studies supporting the main themes from functional neuroimaging studies |
| Cortical findings |
| Decreased gray matter volume, decreased white matter volume, and decreased cortical thickness in prefrontal, anterior temporal and insula cortices. Decreased gray matter volume in particular in right ventrolateral prefrontal cortex and orbitofrontal cortex (49–65) |
| Subcortical findings |
| Decreased volume of amygdala and hippocampus. Altered striatal volumes (55, 56, 60, 63, 65–74) |
| Main findings from diffusion imaging studies supporting the main themes from functional neuroimaging studies |
| White matter tract findings |
| Altered fractional anisotropy, and increased radial diffusivity, in frontally situated white matter (51, 56, 75–103) |
A second key finding relating to emotion processing and regulation neural circuits is decreased subcortical regional volumes, especially in the amygdala and hippocampus. Studies have reported decreased amygdala volume in adults with bipolar disorder (66), particularly during depressive episodes (67), that may normalize with lithium treatment (68). One meta-analysis reported amygdala volume decreases in youths but not adults with bipolar disorder (69), suggesting a normalization of this structure over the course of development in bipolar disorder, potentially due to medication (60, 63). Abnormally decreased hippocampal and parahippocampal volumes have also been reported in adults with bipolar disorder (55, 65, 66), although such abnormalities may be masked by lithium treatment (68, 70, 71). Furthermore, one study demonstrated that larger hippocampal volumes in adult bipolar disorder may decrease with illness duration and number of illness episodes (72).
In addition, a small number of studies have reported altered volumes of striatal nuclei in adults with bipolar disorder compared with healthy adults, paralleling functional neuroimaging findings of altered functioning in these regions, especially during reward processing. These findings include decreased volume and resulting change in shape of the ventromedial surface of the caudate nucleus (73); decreased left putamen volume (55); decreased right caudate, putamen, and ventral striatum volumes (56); and increased right putamen volume (71).
Overall, the predominant pattern of reduced gray matter in the ventral prefrontal cortex in adults with bipolar disorder, especially in the right ventrolateral prefrontal cortex, suggests a structural basis for functional MRI (fMRI) findings of decreased activity in this region during emotion processing and emotion regulation tasks. The predominant pattern of reduced subcortical gray matter volumes in adults with bipolar disorder may result from a neurotoxic effect of elevated activity in these structures, indicated by fMRI studies, and may become more apparent with increasing illness duration but may be normalized, or increased, by lithium (74).
Diffusion Imaging Studies Providing Support for the Main Themes
Diffusion imaging techniques identify changes in white matter by measuring the extent of diffusion of water molecules along longitudinal and perpendicular axes of white matter tracts. Owing to the hydrophobic nature of axonal membranes and myelin sheaths, in white matter tracts that have densely packed, collinear axons, water molecules will diffuse predominantly along the longitudinal direction. In white matter containing noncollinear axons (e.g., in white matter containing crossing tracts), however, water molecules will diffuse in two or more directions. Diffusion imaging measures include longitudinal/axial diffusivity—the diffusivity along the principal axis; radial diffusivity—the diffusivity along transverse directions perpendicular to the longitudinal axis; and fractional anisotropy—the ratio of longitudinal to transverse diffusivity in white matter tracts. Fractional anisotropy will thus be high in white matter tracts with densely packed collinear axons, but low in white matter with noncollinear axons. Radial diffusivity will be high in white matter with noncollinear axons, but also high in white matter with damaged axonal membranes and/or myelin sheaths. The combination of fractional anisotropy and radial diffusivity measures in between-group studies can thus help determine group differences in the collinearity of axons in specific white matter regions; it can also help identify specific white matter tracts that may show pathological changes in the non-control group (e.g., individuals with bipolar disorder). These studies can thus provide important information about the structure of key white matter tracts in neural circuits showing functional and gray matter abnormalities in individuals with bipolar disorder.
Initial diffusion imaging studies reported white matter abnormalities in frontally situated tracts in adults with bipolar disorder (75–79). The more specific finding from recent diffusion imaging studies of adults with bipolar disorder is abnormally reduced fractional anisotropy, paralleled in many cases by abnormally increased radial diffusivity, in frontally situated white matter (80–85), including white matter tracts connecting prefrontal cortical and anterior limbic structures (86, 87) supporting emotion regulation, and in temporal white matter (88, 89) (Table 2; see also Table S3 in the online data supplement). White matter tracts that most consistently show these abnormalities are the anterior regions of the corpus callosum (56, 82, 85, 87, 90), the anterior cingulum (82, 84, 87), the uncinate fasciculus (81, 82, 86, 91), and the superior longitudinal fasciculus (51, 81, 87, 90, 92). These abnormalities may be more apparent in depressive episodes than in remission (93). Studies have also reported decreased fronto-temporal white matter fractional anisotropy in at-risk relatives of individuals with bipolar disorder (51, 88, 90, 94). A recent diffusion imaging study reported abnormal nodal networks in the left ventrolateral prefrontal cortex, the left hippocampus, and the left and right mid-anterior cingulate cortex in adults with bipolar disorder compared with healthy adults (95), which may suggest a specific white matter structural basis for the observed pattern of abnormal activity in the left ventrolateral prefrontal cortex and orbitofrontal cortex during reward processing in bipolar disorder. There are some discrepant findings of abnormally increased fractional anisotropy in frontal white matter in adults with bipolar disorder (81, 96, 97), while other studies have reported more widespread reductions in fractional anisotropy and increases in radial diffusivity (98–101).
Findings from diffusion imaging studies in adults with bipolar disorder thus suggest either abnormal myelination or abnormal orientation of axons in predominantly frontal and temporal white matter regions that include tracts connecting prefrontal cortical and subcortical regions in neural circuits important for emotion regulation and reward processing. While abnormal white matter in these tracts may be associated with the functional and gray matter abnormalities in emotion-processing, emotion-regulation, and reward-processing neural circuitry (102, 103), the causal nature of these structure-function relationships remains to be clarified.
Conceptualizing Bipolar Disorder in Terms of Abnormalities in Large-Scale Neural Circuits
Findings from functional neuroimaging studies indicate abnormalities in adults with bipolar disorder in prefrontal cortical-amygdala-centered emotion-regulation circuitry and prefrontal cortical-striatal reward circuitry. Altered functioning within, and functional coupling between, the ventrolateral prefrontal cortex and the amygdala may represent a neural mechanism for the emotion dysregulation that characterizes bipolar disorder, given the key roles of these regions in emotion regulation (2–4, 6, 8). Abnormally elevated activity in the left ventrolateral prefrontal cortex and orbitofrontal cortex during reward anticipation and processing in adults with bipolar disorder may represent a neural mechanism for heightened reward sensitivity, given the association of these regions with arousal in potentially rewarding contexts and reward value encoding (32, 35–37). These findings also suggest a left hemisphere focus for abnormally increased activity in reward-processing neural circuitry in bipolar disorder, consistent with EEG studies showing increased left frontal activity in response to challenging and potentially rewarding events (104) and an association between increased left frontal activity and conversion to bipolar I disorder in individuals with cyclothymia or bipolar II disorder (105). Given the hypothesized role of the left hemisphere in approach-related emotions (106), the left-lateralized nature of reward-circuitry findings in bipolar disorder provides further support for heightened processing of reward- and approach-related stimuli, which may predispose to mania or hypomania. Parallel gray matter decreases in the prefrontal and temporal cortices, the amygdala, and the hippocampus and fractional anisotropy decreases in white matter tracts connecting prefrontal and subcortical regions suggest a structural basis for the functional abnormalities in emotion-processing, emotion-regulation, and reward-processing circuitry in bipolar disorder.
Bipolar disorder can thus be conceptualized, in neural circuitry terms, as parallel dysfunction in prefrontal cortical (especially ventrolateral prefrontal cortex and orbitofrontal cortex)-hippocampal-amygdala emotion processing and emotion regulation circuits bilaterally, together with an “overactive” left-sided ventral striatal-ventrolateral prefrontal cortex reward-processing circuitry, that may, together, result in the characteristic behavioral abnormalities associated with the disorder—emotional lability, emotional dysregulation, and reward sensitivity (Figures 1 and 2).
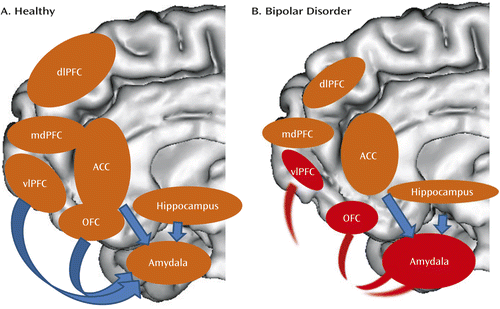
a Panel A is a schematic diagram highlighting key nodes in emotion-processing and emotion-regulation neural circuitry in healthy individuals; arrows represent key regulatory connections between prefrontal cortical regions and the amygdala. In panel B, key functional abnormalities in individuals with bipolar disorder are highlighted in red (in regions and connections between regions); these include abnormally increased amygdala activity during emotion processing, emotion regulation, and performance of nonemotional tasks; abnormally decreased activity in the ventrolateral prefrontal cortex and orbitofrontal cortex during emotion regulation; and decreased functional connectivity between these prefrontal cortical regions and the amygdala during emotion regulation. In parallel, there are widespread abnormal decreases in gray matter volume and cortical thickness in prefrontal cortical regions, decreased gray matter volume in the amygdala and hippocampus, and abnormally decreased fractional anisotropy in white matter tracts connecting the ventral prefrontal cortex and anterior temporal regions. These changes are indicated by smaller sizes of ovals representing these regions. ACC=anterior cingulate cortex; dlPFC=dorsolateral prefrontal cortex; mdPFC=mediodorsal prefrontal cortex; OFC=orbitofrontal cortex; vlPFC=ventrolateral prefrontal cortex.
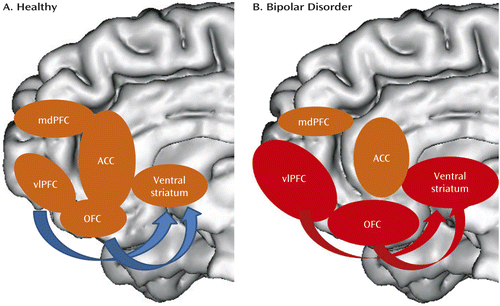
a Panel A is a schematic diagram highlighting key nodes in reward-processing circuitry in healthy individuals. In panel B, key functional abnormalities in individuals with bipolar disorder are highlighted in red; these include abnormally increased activity in the ventral striatum, ventrolateral prefrontal cortex, and orbitofrontal cortex during reward processing, especially during reward anticipation. While not yet reported in the literature, it is likely that patterns of aberrant functional connectivity among these regions are exhibited by individuals with bipolar disorder during reward processing. In parallel, there are widespread decreases in gray matter volume and cortical thickness in prefrontal cortical regions and striatal regions in individuals with bipolar disorder. ACC=anterior cingulate cortex; mdPFC=mediodorsal prefrontal cortex; OFC=orbitofrontal cortex; vlPFC=ventrolateral prefrontal cortex.
What remains to be determined is the extent to which dysfunction in these circuits produces switching between mood states and the extent to which intrinsic functional abnormalities in these circuits play a role in the neuropathophysiology of bipolar disorder. We next examine findings from the small number of studies comparing neural circuitry in individuals with bipolar disorder in different mood states and findings from resting-state studies in bipolar disorder as areas of research that need further study before our conceptualization of bipolar disorder can be confirmed.
Neuroimaging Research Needed to Support This Conceptualization of Bipolar Disorder Neural Circuitry
Mood State-Specific Functional Abnormalities in Circuits Supporting Emotion Processing, Emotion Regulation, and Reward Processing
A small number of cross-sectional studies have examined individuals with bipolar disorder in different mood states during emotion processing and emotion regulation. One finding is abnormally decreased orbitofrontal cortex activity during emotion processing across different mood states (107, 108). Mood state-specific patterns of decreased right orbitofrontal cortex activity in response to fearful and neutral faces in adults with bipolar disorder in hypomanic, manic, or mixed mood states compared with healthy adults were also reported (107). Other findings indicate differing roles of the insula and the ventrolateral prefrontal cortex in adults with bipolar disorder across different mood states during emotion regulation (109); different mood state-specific increases in amygdala activity in response to negative emotional faces (110); abnormally decreased right dorsolateral prefrontal cortex activity during nonemotional working memory across different mood states (111); and increased ventrolateral prefrontal cortical-thalamic activity during response inhibition in adults with bipolar disorder in mixed mood episodes compared with depressive episodes (27). Longitudinal studies have reported differential patterns of amygdala functional connectivity in the same individuals with bipolar disorder during mania and depression (112) and normalized activity in the amygdala and prefrontal cortical regions in remitted compared with manic adults with bipolar disorder during reward and cognitive tasks (28, 42). While there are no clear patterns of mood state-specific functional neural abnormalities in bipolar disorder, findings suggest amygdala-prefrontal cortical functional abnormalities across different mood states and normalization with remission, which supports our conceptualization of bipolar disorder neural circuitry. No studies have yet examined mood state differences in reward-processing circuitry in bipolar disorder, however. Clearly, more longitudinal within-subject studies are required to identify functional abnormalities in neural circuits that predispose to switches between different mood states in bipolar disorder.
Intrinsic (Resting-State) Connectivity Studies
These studies provide measures of tonic functional connectivity, rather than of stimulus-related, phasic functional connectivity, in neural circuits of interest and can thereby identify context-independent functional abnormalities, which potentially represent core functional abnormalities in such circuits in a given disorder. The majority of these studies in bipolar disorder have employed a region-of-interest approach to examine functional connectivity among a priori regions of interest at rest, measuring, for example, correlations between time series of low-frequency fluctuations in activity among these regions. The main finding is abnormally decreased positive or negative (inverse) resting connectivity among frontal, temporal, and subcortical regions in adults with bipolar disorder (113–115), which suggests a decoupling of resting connectivity among these regions, although one study reported abnormally increased resting connectivity between the right amygdala and the right ventrolateral prefrontal cortex (116). Studies focusing on larger-scale networks have reported, in adults with bipolar disorder in different mood states, diverse patterns of abnormally increased resting connectivity in paralimbic and fronto-temporal/paralimbic networks (117), abnormally decreased connectivity in the medial prefrontal cortex (118), and abnormal positive resting connectivity between the medial prefrontal cortex and the ventrolateral prefrontal cortex and between the medial prefrontal cortex and the insula, together with abnormal decoupling between the mediodorsal prefrontal cortex and the dorsolateral prefrontal cortex (119). Other studies have reported reduced global connectivity with the mediodorsal prefrontal cortex and thalamo-cortical disconnectivity in euthymic adults with bipolar disorder with a history of psychosis compared with healthy adults (115, 120). Recent studies have employed different techniques to examine the amplitude of low-frequency fluctuations and the homogeneity of time series within specific neural regions and have reported increased amplitude of low-frequency fluctuations in fronto-temporal-striatal regions, decreased amplitude of low-frequency fluctuations in left postcentral-parahippocampal regions (121), and greater regional homogeneity in left fronto-parietal cortices (122) in depressed adults with bipolar disorder (subtype unspecified) compared with healthy adults.
These studies indicate intrinsic, context-independent abnormalities in adults with bipolar disorder, both in functional connectivity between regions and in the amplitude and homogeneity of low-frequency fluctuations within neural regions, predominantly within fronto-temporal-striatal circuitry. The findings thereby provide some support for our conceptualization of bipolar disorder neural circuitry, but they are highly variable across studies. Furthermore, given the paucity of studies combining resting state with other neuroimaging modalities, it is difficult to determine how these findings relate to the functional and structural abnormalities in neural circuits relevant to bipolar disorder.
Future Neuroimaging Research in Bipolar Disorder
Limitations of Extant Studies
The above inferences aside, there are many limitations of existing neuroimaging studies in bipolar disorder. First, the majority of studies, especially those employing fMRI or resting state, recruited relatively modest numbers of participants per group (e.g., <30), thereby allowing only limited conclusions to be drawn about the generalizability of the findings to the wider population of individuals with bipolar disorder. Similarly, few studies have compared individuals with bipolar disorder across different mood states, and there have been few replication findings, especially for fMRI and resting-state studies. For resting-state studies, this is likely due to the different resting-state methodologies used, in addition to modest sample sizes. Clearly, there is a need in bipolar disorder research for more neuroimaging studies with larger samples and for more resting-state studies using similar techniques. Many studies have focused solely on a priori prefrontal cortical-subcortical regions of interest, with little reporting of findings in other regions, thereby limiting inferences about the potential roles of other neural circuits in bipolar disorder. Furthermore, there is a dearth of neuroimaging studies directly comparing different bipolar disorder subtypes (e.g., bipolar I and II disorders) or bipolar disorder and other major psychiatric disorders (e.g., schizophrenia). It is therefore difficult to determine the extent to which bipolar disorder subtypes, or different psychiatric disorders, share, or are distinguished by, underlying neural mechanisms. Such studies have the potential to identify neural biomarkers reflecting these neural mechanisms and thus aid diagnosis and treatment choice, particularly for disorders that are often difficult to distinguish on the basis of clinical assessment alone—for example, bipolar I disorder versus bipolar II disorder, bipolar I and II disorders versus major depressive disorder, and bipolar disorder versus schizophrenia. There have also been few multimodal neuroimaging studies examining relationships between structure and function in neural circuits of interest in bipolar disorder or between resting and task-related functional connectivity in these circuits in bipolar disorder. Such studies will facilitate a more in-depth understanding of neural mechanisms underlying bipolar disorder.
Another major criticism of neuroimaging studies in bipolar disorder is the potentially confounding effects of psychotropic medication on neuroimaging measures. An increasing number of studies in bipolar disorder suggest that psychotropic medications either have normalizing effects on neuroimaging measures or do not have a significant impact on these measures (123), although, as is apparent from the description of studies above, lithium in particular may have neurotrophic effects in some neural regions in bipolar disorder, while antipsychotic medications are associated with gray matter decreases (124). Further studies are thus needed to determine the nature of effects specific medications have on the neural circuits of interest in bipolar disorder. Longitudinal neuroimaging studies examining individuals before and after medication can address this important point, as can large cross-sectional studies comparing medication-free individuals with those taking different medication types.
Newer Research Areas
Neuroimaging studies of different bipolar disorder subtypes.
Few MRI studies have focused on adults with bipolar II disorder. One fMRI study that focused exclusively on bipolar II disorder reported decreased activity in the right amygdala and the left and right ventrolateral prefrontal cortex and reduced functional connectivity between the amygdala and both the orbitofrontal cortex and the dorsolateral prefrontal cortex during emotional face processing in depressed adults with bipolar II disorder compared with healthy adults (125). The finding of decreased ventrolateral prefrontal cortex activity parallels that reported for similar tasks in euthymic and depressed adults with bipolar I disorder (13–16, 19) and suggests that reduced ventrolateral prefrontal cortex activity during emotion processing and emotion regulation may be a trait marker of bipolar I and II disorders. An fMRI study that directly compared euthymic adults with bipolar I and II disorders and healthy adults reported significantly increased activity in the ventral striatum and the left ventrolateral prefrontal cortex in adults with bipolar II disorder compared to those with bipolar I disorder and healthy adults during reward anticipation (126), again paralleling previous studies that highlighted abnormally increased activity in the left ventrolateral prefrontal cortex and ventral striatum during reward anticipation in euthymic and depressed adults with bipolar I disorder (40, 41). Interestingly, findings from that study are the first to suggest that bipolar II disorder may be characterized by a greater magnitude of functional abnormalities in reward neural circuitry than bipolar I disorder, supporting findings associating bipolar II disorder with more disabling functional impairments in daily living than bipolar I disorder (127, 128). One structural study reported more widespread gray matter reduction in prefrontal, temporal, and parietal cortices in euthymic or moderately depressed adults with bipolar I disorder compared with depressed adults with bipolar II disorder (129). The two diffusion imaging studies that compared individuals with bipolar I and II disorders reported abnormalities in fronto-thalamic-temporal white matter in both disorders, although findings across the two studies were inconsistent (130, 131). There is clearly a need for more neuroimaging studies comparing individuals with bipolar I disorder with those with bipolar II disorder and those with other bipolar disorder subtypes.
Neuroimaging studies comparing bipolar disorder with other major psychiatric disorders.
An increasing number of studies have focused on identifying measures of neural circuitry structure and function that distinguish bipolar disorder from other disorders. Studies have compared depressed adults with bipolar disorder and those with major depressive disorder (132) and adults with bipolar disorder and those with schizophrenia (117, 133). These studies suggest that functional abnormalities in prefrontal cortical-subcortical circuitry may distinguish different disorders; they are paving the way to identification of clinically useful biomarkers guiding diagnosis and treatment choices.
Multimodal neuroimaging studies.
Another area for future neuroimaging research in bipolar disorder is the use of multimodal techniques to identify structure-function relationships in neural circuitry. A small number of studies have examined structure-function relationships in prefrontal cortical-amygdala circuitry in adults with bipolar I and II disorders (102, 103, 126), but there is a need for more such studies in individuals across the mood disorders spectrum. In parallel, studies are beginning to identify relationships between genetic variants and functioning within neural circuitry in adults and youths with bipolar disorder (134). Ultimately, an integrated systems approach will help identify biomarkers that reflect neuropathophysiological processes in individuals with mood, psychotic, and other psychiatric disorders that span genetic, molecular, neural circuitry, and behavioral levels of investigation (135–137).
In addition, neuroimaging studies in more novel areas of bipolar disorder research can further elucidate neural mechanisms of the disorder and provide clinically useful biomarkers. These include 1) studies in youths with bipolar disorder and those at risk for bipolar disorder to help identify biomarkers conferring risk for future development of the disorder without confounding by potential scarring effects due to present illness and illness history; 2) studies identifying dimensions of pathology that may cut across conventionally defined diagnostic categories; and 3) studies using pattern recognition approaches to help provide clinically useful individual-level neuroimaging biomarkers.
Neuroimaging studies in youths with bipolar disorder and those at risk for bipolar disorder.
These studies have examined youths with bipolar disorder and those at risk for bipolar disorder to determine the extent to which abnormalities in neural circuitry identified in adult bipolar disorder originated in youth. Studies have reported patterns in youths with bipolar disorder similar to those in adult bipolar disorder, with abnormally increased amygdala activity and decreased prefrontal cortical-amygdala functional connectivity during emotion-processing and emotion-regulation paradigms (138–142). Abnormally increased amygdala activity may be more evident in youths than in adults with bipolar disorder (139). Studies in youths with bipolar disorder have also demonstrated abnormally decreased amygdala volumes (69, 142, 143); decreased orbitofrontal cortex and anterior cingulate cortex gray matter volumes (142); abnormally reduced fractional anisotropy in white matter tracts connecting prefrontal and subcortical regions (144–147) (and similar findings in at-risk youths [148]); and altered resting state in prefrontal cortical circuitry (149) and large-scale networks (150). Longitudinal studies are clearly needed to examine developmental trajectories of structural and functional changes in prefrontal cortical-subcortical circuitry in individuals with bipolar disorder and in youths at risk for future mood and psychotic disorders. Such studies will help identify abnormal developmental trajectories in this circuitry that are associated with bipolar disorder or other mood disorders in youth and biomarkers that can help identify which at-risk youths are most likely to develop these disorders.
Neuroimaging studies adopting dimensional approaches.
Guided by the National Institute of Mental Health’s Research Domain Criteria initiative, neuroimaging studies are beginning to adopt a dimensional approach to bipolar disorder. One recent study reported a positive correlation between reward sensitivity (fun-seeking) and ventral striatal activity across adults with bipolar I disorder, adults with bipolar II disorder, and healthy adults (126). The study thus associated patterns of function in reward circuitry with information-processing domains that cut across diagnostic boundaries. Conceptualizing bipolar I disorder, bipolar II disorder, other bipolar disorder subtypes, and even major depressive disorder in terms of a mood disorders spectrum may lead to a better understanding of neuropathophysiological processes in these illnesses (136).
Neuroimaging studies using pattern recognition approaches.
A key criticism of neuroimaging studies is their reliance on group-level statistics, rather than providing data that are useful at the individual level. If neuroimaging techniques are to provide clinically relevant information, then useful individual-level measures of brain structure and function must be obtained from these techniques. One recent advance has been to combine neuroimaging with pattern recognition approaches that develop algorithms to automatically learn and recognize complex patterns to inform decision making based on large data sets. Studies combining these approaches have been able to help classify individuals, case by case, into different diagnostic categories, including bipolar disorder versus major depression, based on their patterns of neural function (151, 152). They have also accurately discriminated between individual healthy youths at high genetic risk for bipolar disorder and those at low risk (153). Combining neuroimaging with pattern recognition techniques thus holds much promise for the elucidation of clinically useful individual-level biomarkers to inform diagnosis, risk identification, and personalized treatment choice.
A Neuroimaging Research Road Map For Bipolar Disorder
The field of neuroimaging in bipolar disorder is progressing considerably, with research findings making significant contributions to our understanding of the neuropathophysiology of bipolar disorder. In order to move the field forward, the next wave of bipolar disorder neuroimaging studies should aim to adopt the following strategies.
1. Studies should examine functional, structural, white matter, and intrinsic connectivity abnormalities in emotion-processing, emotion-regulation, and reward-processing neural circuitry associated with dimensions of pathological behaviors that cut across conventionally defined bipolar disorder and other mood disorder diagnostic categories. These studies should also include longitudinal designs to identify the extent to which alterations in these neural circuitry abnormalities are associated with changes in affective state. This approach has the potential to identify neural circuitry markers that better reflect neuropathophysiological processes in bipolar disorder and other mood disorders and the nature of neural mechanisms underlying abnormal mood switches.
2. Studies should use longitudinal follow-up designs to examine the developmental trajectories of these neural circuits in individuals with bipolar disorder across the lifespan and in at-risk youths. This approach will identify neural circuitry markers that can help identify those individuals at highest risk of developing affective pathology, and thereby pave the way for studies that utilize these markers to guide early intervention and prevention strategies.
3. Studies should incorporate multimodal neuroimaging techniques and biological system-level approaches to examine the impact of genetic variation and molecular-level processes on neural circuitry development in at-risk individuals and individuals with bipolar disorder and other mood disorders.
4. Studies should take advantage of advances in the application of pattern recognition techniques to neuroimaging to identify individual-level neural circuitry markers that help classify individuals into diagnostic groups and also help predict clinical course at the individual level.
Collectively, these four approaches will help elucidate more complex neuropathophysiological processes underlying dimensions of abnormal behaviors associated with affective pathology and yield individual-level biological markers reflecting these processes. Such markers will have clinical utility for diagnosis, prediction of future illness development, and the guiding of personalized treatment choice in individuals with mood disorders or those at risk of developing mood disorders (Table 3).
| Strategy 1: Dimensional approaches | Dimensional approaches to identify emotion-processing, emotion-regulation, and reward-processing neural circuitry abnormalities associated with dimensions of pathological behaviors that cut across conventionally defined bipolar disorder and other mood disorder diagnostic categories. The inclusion of longitudinal designs will help identify the extent to which alterations in these circuitry abnormalities are associated with changes in affective state. |
| Strategy 2: Developmental studies | Longitudinal follow-up studies examining developmental trajectories of these neural circuits in individuals with bipolar disorder across the lifespan and in youths at risk for mood disorders |
| Strategy 3: Multimodal neuroimaging and systems-level approaches | Multimodal neuroimaging studies and studies adopting biological system-level approaches to examine the impact of genetic variation and molecular-level processes on neural circuitry development in at-risk individuals and individuals with bipolar disorder and other mood disorders |
| Strategy 4: Techniques to identify individualized patterns of neuroimaging measures | Studies using neuroimaging in combination with pattern recognition techniques to identify individual-level neural circuitry markers that help classify individuals into diagnostic groups and help predict individual-level future clinical course |
| Strategy 5: Combinations of the above strategies | These studies will a) help elucidate more complex neuropathophysiological processes underlying dimensions of abnormal behaviors associated with affective pathology across different diagnostic categories; and b) yield individual-level biological markers reflecting these processes with clinical utility for diagnosis, predicting future illness development, and guiding personalized treatment choice. |
1 : Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression, 2nd ed. New York, Oxford University Press, 2007Google Scholar
2 : The amygdala and its place in the cerebral hemisphere. Ann N Y Acad Sci 2003; 985:174–184Crossref, Medline, Google Scholar
3 : The amygdala: vigilance and emotion. Mol Psychiatry 2001; 6:13–34Crossref, Medline, Google Scholar
4 : A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008; 13:829, 833–857Crossref, Medline, Google Scholar
5 : Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 2002; 16:291–310Crossref, Medline, Google Scholar
6 : Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 2000; 11:43–48Crossref, Medline, Google Scholar
7 : Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci 2010; 30:14552–14559Crossref, Medline, Google Scholar
8 : The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 2008; 63:577–586Crossref, Medline, Google Scholar
9 : Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 2004; 55:578–587Crossref, Medline, Google Scholar
10 : Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005; 183:308–313Crossref, Medline, Google Scholar
11 : Neurobiology of emotion perception, II: implications for major psychiatric disorders. Biol Psychiatry 2003; 54:515–528Crossref, Medline, Google Scholar
12 : The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord 2012; 14:313–325Crossref, Medline, Google Scholar
13 : Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage 2012; 59:738–744Crossref, Medline, Google Scholar
14 : Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry 2013; 73:127–135Crossref, Medline, Google Scholar
15 : Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord 2008; 10:708–717Crossref, Medline, Google Scholar
16 : Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry 2011; 69:381–388Crossref, Medline, Google Scholar
17 : Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord 2012; 14:442–450Crossref, Medline, Google Scholar
18 : Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol 2012; 22:100–113Crossref, Medline, Google Scholar
19 : Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res 2008; 162:27–37Crossref, Medline, Google Scholar
20 : Dissociable patterns of medial prefrontal and amygdala activity to face identity versus emotion in bipolar disorder. Psychol Med 2012; 42:1913–1924Crossref, Medline, Google Scholar
21 : Exaggerated neural response to emotional faces in patients with bipolar disorder and their first-degree relatives. Neuroimage 2010; 53:58–64Crossref, Medline, Google Scholar
22 : Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry 2009; 66:451–459Crossref, Medline, Google Scholar
23 : A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology 2004; 29:1734–1740Crossref, Medline, Google Scholar
24 : Assessing working memory via N-back task in euthymic bipolar I disorder patients: a review of functional magnetic resonance imaging studies. Neuropsychobiology 2013; 68:63–70Crossref, Medline, Google Scholar
25 : Pathological amygdala activation during working memory performance: evidence for a pathophysiological trait marker in bipolar affective disorder. Hum Brain Mapp 2010; 31:115–125Medline, Google Scholar
26 : Functional MRI of sustained attention in bipolar mania. Mol Psychiatry 2012; 17:325–336Crossref, Medline, Google Scholar
27 : Preliminary evidence for increased frontosubcortical activation on a motor impulsivity task in mixed episode bipolar disorder. J Affect Disord 2011; 133:333–339Crossref, Medline, Google Scholar
28 : Remission from mania is associated with a decrease in amygdala activation during motor response inhibition. Bipolar Disord 2009; 11:530–538Crossref, Medline, Google Scholar
29 : High behavioral approach system (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: a prospective behavioral high-risk design. J Abnorm Psychol 2012; 121:339–351Crossref, Medline, Google Scholar
30 : I want it now! Neural correlates of hypersensitivity to immediate reward in hypomania. Biol Psychiatry 2012; 71:530–537Crossref, Medline, Google Scholar
31 : The neural basis of decision-making and reward processing in adults with euthymic bipolar disorder or attention-deficit/hyperactivity disorder (ADHD). PLoS ONE 2012; 7:e37306Crossref, Medline, Google Scholar
32 : Splitting the difference: how does the brain code reward episodes? Ann N Y Acad Sci 2007; 1104:54–69Crossref, Medline, Google Scholar
33 : Dopamine, learning, and motivation. Nat Rev Neurosci 2004; 5:483–494Crossref, Medline, Google Scholar
34 : Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage 2004; 23:64–74Crossref, Medline, Google Scholar
35 : Get aroused and be stronger: emotional facilitation of physical effort in the human brain. J Neurosci 2009; 29:9450–9457Crossref, Medline, Google Scholar
36 : Value, pleasure, and choice in the ventral prefrontal cortex. Trends Cogn Sci 2011; 15:56–67Crossref, Medline, Google Scholar
37 : Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 2010; 35:27–47Crossref, Medline, Google Scholar
38 : Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry 2004; 55:594–602Crossref, Medline, Google Scholar
39 : Differential magnitude coding of gains and omitted rewards in the ventral striatum. Brain Res 2011; 1411:76–86Crossref, Medline, Google Scholar
40 : Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord 2012; 14:249–260Crossref, Medline, Google Scholar
41 : Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord 2013; 15:839–854 Crossref, Medline, Google Scholar
42 : Altered representation of expected value in the orbitofrontal cortex in mania. Hum Brain Mapp 2010; 31:958–969Crossref, Medline, Google Scholar
43 : Increased medial orbitofrontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder. Am J Psychiatry 2012; 169:316–325Link, Google Scholar
44 : fMRI evidence of a relationship between hypomania and both increased goal-sensitivity and positive outcome-expectancy bias. Neuropsychologia 2011; 49:2825–2835Crossref, Medline, Google Scholar
45 : Abnormal reward system activation in mania. Neuropsychopharmacology 2008; 33:2217–2227Crossref, Medline, Google Scholar
46 : The anatomy of mood disorders: review of structural neuroimaging studies. Biol Psychiatry 1997; 41:86–106Crossref, Medline, Google Scholar
47 : Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry 1998; 55:663–664Medline, Google Scholar
48 : Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry 1999; 56:254–260Crossref, Medline, Google Scholar
49 : Structural abnormalities of ventrolateral and orbitofrontal cortex in patients with familial bipolar disorder. Bipolar Disord 2009; 11:135–144Crossref, Medline, Google Scholar
50 : New structural brain imaging endophenotype in bipolar disorder. Mol Psychiatry 2012; 17:412–420Crossref, Medline, Google Scholar
51 : Genetic and environmental influences on focal brain density in bipolar disorder. Brain 2010; 133:3080–3092Crossref, Medline, Google Scholar
52 : Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry 2009; 66:142–151Crossref, Medline, Google Scholar
53 : Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord 2012; 14:135–145Crossref, Medline, Google Scholar
54 : Prefrontal-temporal gray matter deficits in bipolar disorder patients with persecutory delusions. J Affect Disord 2010; 120:54–61Crossref, Medline, Google Scholar
55 : Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res 2009; 171:54–68Crossref, Medline, Google Scholar
56 : Combined analysis of grey matter voxel-based morphometry and white matter tract-based spatial statistics in late-life bipolar disorder. J Psychiatry Neurosci 2011; 36:391–401Crossref, Medline, Google Scholar
57 : Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry 2013; 170:1285–1296Link, Google Scholar
58 : Brain gray matter phenotypes across the psychosis dimension. Psychiatry Res 2012; 204:13–24Crossref, Medline, Google Scholar
59 : No change to grey and white matter volumes in bipolar I disorder patients. Eur Arch Psychiatry Clin Neurosci 2008; 258:345–349Crossref, Medline, Google Scholar
60 : Brain structural signature of familial predisposition for bipolar disorder: replicable evidence for involvement of the right inferior frontal gyrus. Biol Psychiatry 2013; 73:144–152Crossref, Medline, Google Scholar
61 : Orbitofrontal cortex gray matter volumes in bipolar disorder patients: a region-of-interest MRI study. Bipolar Disord 2009; 11:145–153Crossref, Medline, Google Scholar
62 : Preliminary evidence for progressive prefrontal abnormalities in adolescents and young adults with bipolar disorder. J Int Neuropsychol Soc 2009; 15:476–481Crossref, Medline, Google Scholar
63 : A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry 2009; 70:699–705Crossref, Medline, Google Scholar
64 : Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. Am J Psychiatry 2011; 168:530–539Link, Google Scholar
65 : Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry 2010; 68:41–50Crossref, Medline, Google Scholar
66 : Hippocampal and amygdala volumes in an older bipolar disorder sample. Int Psychogeriatr 2013; 25:54–60Crossref, Medline, Google Scholar
67 : Mood-state effects on amygdala volume in bipolar disorder. J Affect Disord 2012; 139:298–301Crossref, Medline, Google Scholar
68 : Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport 2008; 19:221–224Crossref, Medline, Google Scholar
69 : Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2008; 47:1289–1298Crossref, Medline, Google Scholar
70 : Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: a meta-analysis. J Psychiatry Neurosci 2012; 37:333–343Crossref, Medline, Google Scholar
71 : Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry 2011; 69:326–335Crossref, Medline, Google Scholar
72 : Hippocampal volumes in adults with bipolar disorder. J Neuropsychiatry Clin Neurosci 2010; 22:55–62Crossref, Medline, Google Scholar
73 : Size and shape of the caudate nucleus in individuals with bipolar affective disorder. Aust N Z J Psychiatry 2012; 46:340–351Crossref, Medline, Google Scholar
74 : Progressive neurostructural changes in adolescent and adult patients with bipolar disorder. Bipolar Disord 2011; 13:396–405Crossref, Medline, Google Scholar
75 : Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry 2007; 12:1001–1010Crossref, Medline, Google Scholar
76 : White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord 2007; 9:504–512Crossref, Medline, Google Scholar
77 : Diffusion-weighted magnetic resonance imaging of white matter in bipolar disorder: a pilot study. Bipolar Disord 2006; 8:188–195Crossref, Medline, Google Scholar
78 : Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology 2005; 30:2225–2229Crossref, Medline, Google Scholar
79 : Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord 2004; 6:197–203Crossref, Medline, Google Scholar
80 : Limbic and callosal white matter changes in euthymic bipolar I disorder: an advanced diffusion magnetic resonance imaging tractography study. Biol Psychiatry 2013; 73:194–201Crossref, Medline, Google Scholar
81 : Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry 2008; 65:1041–1052Crossref, Medline, Google Scholar
82 : Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry 2014; 19:200-208 Crossref, Medline, Google Scholar
83 : A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev 2010; 34:533–554Crossref, Medline, Google Scholar
84 : Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. Br J Psychiatry 2008; 193:126–129Crossref, Medline, Google Scholar
85 : Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry 2008; 64:730–733Crossref, Medline, Google Scholar
86 : Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar Disord 2011; 13:414–424Crossref, Medline, Google Scholar
87 : Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol Psychiatry 2011; 69:309–317Crossref, Medline, Google Scholar
88 : Abnormal temporal lobe white matter as a biomarker for genetic risk of bipolar disorder. Biol Psychiatry 2013; 73:177–182Crossref, Medline, Google Scholar
89 : White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar Disord 2008; 10:460–468Crossref, Medline, Google Scholar
90 : White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry 2009; 194:527–534Crossref, Medline, Google Scholar
91 : Impaired anatomical connectivity and related executive functions: differentiating vulnerability and disease marker in bipolar disorder. Biol Psychiatry 2013; 74:908–916Crossref, Medline, Google Scholar
92 : Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol Psychiatry 2010; 68:560–567Crossref, Medline, Google Scholar
93 : State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci 2009; 259:316–328Crossref, Medline, Google Scholar
94 : White matter integrity in individuals at high genetic risk of bipolar disorder. Biol Psychiatry 2011; 70:350–356Crossref, Medline, Google Scholar
95 : Impaired inter-hemispheric integration in bipolar disorder revealed with brain network analyses. Biol Psychiatry 2013; 73:183–193Crossref, Medline, Google Scholar
96 : Microstructural white matter changes in euthymic bipolar patients: a whole-brain diffusion tensor imaging study. Bipolar Disord 2009; 11:504–514Crossref, Medline, Google Scholar
97 : A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology 2009; 34:1590–1600Crossref, Medline, Google Scholar
98 : Cortical and subcortical white matter abnormalities in adults with remitted first-episode mania revealed by tract-based spatial statistics. Bipolar Disord 2010; 12:383–389Crossref, Medline, Google Scholar
99 : Assessment of white matter abnormalities in paranoid schizophrenia and bipolar mania patients. Psychiatry Res 2011; 194:347–353Crossref, Medline, Google Scholar
100 : White matter microstructure in untreated first episode bipolar disorder with psychosis: comparison with schizophrenia. Bipolar Disord 2011; 13:604–613Crossref, Medline, Google Scholar
101 : White matter microstructural abnormalities in euthymic bipolar disorder. Br J Psychiatry 2010; 196:52–58Crossref, Medline, Google Scholar
102 : Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry 2010; 67:422–431Crossref, Medline, Google Scholar
103 : Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 2009; 66:516–521Crossref, Medline, Google Scholar
104 : Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biol Psychiatry 2008; 63:693–698Crossref, Medline, Google Scholar
105 : Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. J Abnorm Psychol 2012; 121:592–601Crossref, Medline, Google Scholar
106 : The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 2003; 160:64–75Link, Google Scholar
107 : Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disord 2012; 14:432–441Crossref, Medline, Google Scholar
108 : Trait and state dependent functional impairments in bipolar disorder. Psychiatry Res 2010; 184:135–142Crossref, Medline, Google Scholar
109 : Emotional response inhibition in bipolar disorder: a functional magnetic resonance imaging study of trait- and state-related abnormalities. Biol Psychiatry 2013; 73:136–143Crossref, Medline, Google Scholar
110 : Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biol Psychiatry 2012; 71:603–610Crossref, Medline, Google Scholar
111 : fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic, and depressed bipolar subjects. Psychiatry Res 2010; 182:22–29Crossref, Medline, Google Scholar
112 : A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disord 2012; 14:175–184Crossref, Medline, Google Scholar
113 : Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res 2010; 182:207–210Crossref, Medline, Google Scholar
114 : Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res 2009; 171:189–198Crossref, Medline, Google Scholar
115 : Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry 2013; 73:565–573Crossref, Medline, Google Scholar
116 : Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar Disord 2013; 15:156–166Crossref, Medline, Google Scholar
117 : Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol Psychiatry 2012; 71:881–889Crossref, Medline, Google Scholar
118 : Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res 2010; 183:59–68Crossref, Medline, Google Scholar
119 : Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 2011; 36:2009–2017Crossref, Medline, Google Scholar
120 : Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex (Epub ahead of print, July 3, 2013)Google Scholar
121 : Abnormal baseline brain activity in bipolar depression: a resting state functional magnetic resonance imaging study. Psychiatry Res 2012; 203:175–179Crossref, Medline, Google Scholar
122 : Regional homogeneity within the default mode network in bipolar depression: a resting-state functional magnetic resonance imaging study. PLoS ONE 2012; 7:e48181Crossref, Medline, Google Scholar
123 : Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord 2012; 14:375–410Crossref, Medline, Google Scholar
124 : A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med 2010; 40:1409–1422Crossref, Medline, Google Scholar
125 : Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. Am J Psychiatry 2012; 169:831–840Link, Google Scholar
126 : Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry 2013; 170:533–541Link, Google Scholar
127 : Health-related quality of life in euthymic bipolar disorder patients: differences between bipolar I and II subtypes. J Clin Psychiatry 2007; 68:207–212Crossref, Medline, Google Scholar
128 : The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord 2003; 73:19–32Crossref, Medline, Google Scholar
129 : Regional brain gray matter abnormalities in patients with bipolar II disorder: a comparison study with bipolar I patients and healthy controls. Neurosci Lett 2009; 456:44–48Crossref, Medline, Google Scholar
130 : Differences in white matter abnormalities between bipolar I and II disorders. J Affect Disord 2010; 127:309–315Crossref, Medline, Google Scholar
131 : Similarities and differences of white matter connectivity and water diffusivity in bipolar I and II disorder. Neurosci Lett 2011; 505:150–154Crossref, Medline, Google Scholar
132 : Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biol Psychiatry 2013; 73:111–118Crossref, Medline, Google Scholar
133 : Review of functional magnetic resonance imaging studies comparing bipolar disorder and schizophrenia. Bipolar Disord 2012; 14:411–431Crossref, Medline, Google Scholar
134 : A genome-wide association study of amygdala activation in youths with and without bipolar disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:33–41Crossref, Medline, Google Scholar
135 : Toward a systems biology of mood disorder. Biol Psychiatry 2013; 73:107–108Crossref, Medline, Google Scholar
136 : Bipolar disorder diagnosis: challenges and future directions. Lancet 2013; 381:1663–1671Crossref, Medline, Google Scholar
137 : Brain-behavior biomarkers of illness and illness risk in bipolar disorder: present findings and next steps. Biol Psychiatry 2013; 74:870–871Crossref, Medline, Google Scholar
138 : Reduced functional connectivity of prefrontal regions and amygdala within affect and working memory networks in pediatric bipolar disorder. Brain Connect 2012; 2:320–334Crossref, Medline, Google Scholar
139 : Differing amygdala responses to facial expressions in children and adults with bipolar disorder. Am J Psychiatry 2012; 169:642–649Link, Google Scholar
140 : Abnormal amygdala and prefrontal cortex activation to facial expressions in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2012; 51:821–831Crossref, Medline, Google Scholar
141 : Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. J Am Acad Child Adolesc Psychiatry 2011; 50:1275–1289.e2Crossref, Medline, Google Scholar
142 : Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2009; 48:636–642Crossref, Medline, Google Scholar
143 : Progression of amygdala volumetric abnormalities in adolescents after their first manic episode. J Am Acad Child Adolesc Psychiatry 2011; 50:1017–1026Crossref, Medline, Google Scholar
144 : Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry 2009; 66:238–244Crossref, Medline, Google Scholar
145 : Combined diffusion tensor imaging and transverse relaxometry in early-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:1260–1268Medline, Google Scholar
146 : Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. J Am Acad Child Adolesc Psychiatry 2009; 48:79–86Crossref, Medline, Google Scholar
147 : Microstructural abnormalities of white matter differentiate pediatric and adult-onset bipolar disorder. Bipolar Disord 2012; 14:597–606Crossref, Medline, Google Scholar
148 : Altered development of white matter in youth at high familial risk for bipolar disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry 2010; 49:1249–1259.e1Medline, Google Scholar
149 : Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol Psychiatry 2010; 68:839–846Crossref, Medline, Google Scholar
150 : Altered affective, executive, and sensorimotor resting state networks in patients with pediatric mania. J Psychiatry Neurosci 2013; 38:232–240Crossref, Medline, Google Scholar
151 : Pattern recognition analyses of brain activation elicited by happy and neutral faces in unipolar and bipolar depression. Bipolar Disord 2012; 14:451–460Crossref, Medline, Google Scholar
152 : Pattern recognition analysis of anterior cingulate cortex blood flow to classify depression polarity. Br J Psychiatry 2013; 203:310–311Crossref, Medline, Google Scholar
153 : Pattern recognition and functional neuroimaging help to discriminate healthy adolescents at risk for mood disorders from low risk adolescents. PLoS ONE 2012; 7:e29482Crossref, Medline, Google Scholar



