Thalamo-Cortical Activation and Connectivity During Response Preparation in Adults With Persistent and Remitted ADHD
Abstract
Objective
The neural correlates of stimulus-driven processes, such as response preparation, have been posited to be associated with the onset of attention deficit hyperactivity disorder (ADHD) while being distinct from the neural mechanisms associated with recovery. The authors tested this hypothesis in adults with remitted and persistent ADHD.
Method
Thirty-eight young adults who were diagnosed with combined-type ADHD in childhood (probands) and 32 carefully matched comparison subjects were followed longitudinally and scanned with functional MRI while performing an event-related cued reaction time task. Probands were characterized as individuals with persistent or remitted ADHD. Differences in thalamo-cortical activation and functional connectivity during response preparation between comparison subjects and probands and between individuals with persistent ADHD and those with remitted ADHD were assessed by contrasting neural activation and functional connectivity during cue or noncue events.
Results
Probands exhibited less cue-related activation than comparison subjects in the thalamus, anterior cingulate cortex, supplementary motor area, inferior parietal lobe, and dorsolateral prefrontal cortex despite similar overall patterns of activation. There were no differences in activation between individuals in the remitted ADHD group and those in the persistent ADHD group in any hypothesized regions. However, cue-related functional connectivity between the right thalamus and brainstem was greater in comparison subjects relative to probands, and cue-related connectivity was greater between the right thalamus and prefrontal regions in individuals with remitted ADHD relative to those with persistent ADHD.
Conclusions
Decreased thalamo-cortical activation during response preparation was present in adults diagnosed with ADHD in childhood regardless of symptom remission in adulthood, and may be partly driven by less functional coordination between the brainstem and thalamus. Greater functional integration of the thalamo-cortical network might parallel symptom recovery.
Attention deficit hyperactivity disorder (ADHD) is a prevalent neurodevelopmental disorder characterized by age-inappropriate inattention and hyperactivity/impulsivity. Up to 65% of children who are diagnosed with ADHD will continue to show varying levels of ADHD symptoms in adulthood (1), with significant functional (2) and cognitive impairment (3). However, little is known about the neural mechanisms that determine the persistence or remission of ADHD into adulthood and the neural correlates of cognitive deficits that persist despite symptom remission (4). A neurodevelopmental theory of ADHD posits that early-developing bottom-up processes, such as stimulus-driven response preparation, play a primary role in the onset of ADHD. These potentially causal deficits are hypothesized to be distinct from mechanisms of recovery, and as such, should remain relatively static over time (4).
Stimulus-driven response preparation encompasses multiple neurocognitive processes that allow for swift task-appropriate behavioral responses, including attention allocation, maintenance of task set, and motor planning. These processes are mediated by a thalamo-cortical network that includes the thalamus, striatum, anterior cingulate cortex, supplementary motor area, and dorsolateral prefrontal cortex (5–7). Insufficient response preparation manifests behaviorally as slow and variable reaction time on a variety of tasks in both children (8) and adults (9) with ADHD and has been associated with increased activation of the presupplementary motor area and reduced prefrontal activation in children with ADHD (10). Children with ADHD have also demonstrated diminished activation in the anterior cingulate cortex, putamen, and supplementary motor cortex in response to cues signaling impending targets (11, 12) and reduced activation of the thalamus, basal ganglia, and parietal cortex during attention allocation (13, 14). Prospectively followed adults with persistent ADHD have demonstrated reduced dorsolateral prefrontal cortex activation during attention allocation and lower thalamus activity during simple motor tasks (15, 16). Volumetric anomalies in thalamo-cortical regions have also been reported in both children (17, 18) and adults with ADHD (19, 20), irrespective of the current diagnostic status of adults diagnosed with ADHD in childhood (21). Similar comparisons of brain function between prospectively followed adults with and without current ADHD diagnoses are necessary to fully elucidate the role of response preparation deficits in the persistence or remission of ADHD.
If deficits in response preparation are associated with the onset of ADHD and are distinct from the neural processes that underlie symptom recovery, such deficits should be present in adulthood regardless of symptom remission (4). However, it is possible that the protracted development of the prefrontal cortex contributes to both ADHD remission and increased top-down control of response preparation (4). We utilized functional MRI (fMRI) with a cued reaction time task (5) to test these hypotheses in a prospectively followed sample of adults diagnosed with combined-type ADHD in childhood and well-matched comparison subjects with no history of ADHD. The prospective sample allowed for the comparison of individuals with childhood ADHD who had substantial remission of symptoms and those with persistent ADHD. We predicted that adults with a childhood diagnosis of ADHD would demonstrate less activation than comparison subjects throughout the thalamo-cortical system, including the thalamus, striatum, parietal lobe, anterior cingulate cortex, and supplementary motor area. We also predicted that increased top-down control of response preparation would be associated with ADHD remission, as evidenced by greater activation of the dorsolateral prefrontal cortex in individuals with remitted ADHD than in those with persistent ADHD. Finally, exploratory analyses utilizing psychophysiological interactions were conducted to test functional connectivity within the thalamo-cortical network during cues or noncues in comparison subjects and probands and in individuals with remitted or persistent ADHD.
Method
Participants
The probands were from a sample of 169 children diagnosed with combined-type ADHD between 1990 and 1997 when they were 7–11 years old (mean age, 9.08 years [SD=1.38]). All of the children had elevated ADHD symptoms in school based on the IOWA Conners’ Teachers Rating Scale (22). Psychiatric status was confirmed with the Diagnostic Interview Schedule for Children (23). The exclusion criteria were chronic medical illness; neurological disorder; diagnosis of schizophrenia, autism spectrum disorder, or chronic tic disorder; full scale IQ <70; and not speaking English. Sixty probands were reevaluated in adulthood, and 38 (63%; 32 men) provided usable scan data (mean age, 24.38 years [SD=2.40]). Those not providing usable scan data were excluded as a result of missing the scanning session (N=2), claustrophobia (N=6), poor performance (N=3), metal inside the body (N=3), structural brain abnormality (N=3), positive toxicology screen (N=1), or not fitting inside the scanner (N=4).
No significant differences were found in childhood full-scale IQ, age, race, or rates of oppositional-defiant, conduct, or mood disorders between the original sample and those evaluated in adulthood; a greater proportion of individuals not examined in adulthood had childhood anxiety disorders. Of those with usable scan data, 18 (47%) met criteria for oppositional-defiant disorder in childhood, and four (10%) met criteria for conduct disorder in childhood.
The comparison group was recruited during an adolescent follow-up study. Comparison subjects had no history of childhood ADHD and no more than three inattentive or hyperactive/impulsive symptoms reported by parents on the Diagnostic Interview Schedule for Children. Other psychiatric disorders that were allowed in the original ADHD sample were not exclusionary. Of the original 85 adolescent comparison subjects, 46 were clinically re-evaluated in adulthood, and 32 (68%; 27 men) provided usable scan data (mean age, 24.38 years [SD=2.40]). The 14 individuals who did not provide usable scan data were excluded as a result of being diagnosed in adulthood with ADHD not otherwise specified (N=3), psychosis (N=1), claustrophobia (N=4), poor performance (N=2), structural brain abnormality (N=2), positive toxicology screen (N=1), or not fitting inside the scanner (N=1).
The adult assessment took place an average of 15.58 years (SD=2.17) after the probands’ initial evaluation. The study was approved by the institutional review boards of the participating institutions. Participants provided signed informed consent and were compensated for their time and travel expenses. Psychiatric status was assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (24), supplemented by a semistructured interview comprised of the 18 DSM-IV ADHD symptoms. Prompts for the interview were adapted from the Schedule for Affective Disorders and Schizophrenia for School-Age Children (25) and the Conners’ Adult ADHD Diagnostic Interview for DSM-IV (26). The adapted interview demonstrated strong internal consistency in our sample (α=0.92). Probands endorsed a range of 0 to 17 current ADHD symptoms (mean=6.18, SD=4.63).
Based on group status in childhood and symptom endorsement in adulthood, we classified participants into three groups: comparison subjects with no ADHD, individuals with persistent ADHD, and individuals with remitted ADHD. Comparison subjects had no history of childhood ADHD, endorsed no more than three inattentive or hyperactive/impulsive symptoms in adulthood, and had no more than five symptoms. Individuals with persistent ADHD met DSM-IV criteria for combined-type ADHD in childhood, met DSM-IV criteria for ADHD (any subtype including not otherwise specified) during adulthood, and endorsed at least three inattentive and three hyperactive/impulsive symptoms during adulthood. Individuals with remitted ADHD met criteria for combined-type ADHD in childhood, endorsed no more than three inattentive or three hyperactive/impulsive symptoms in adulthood, and had no more than five symptoms. Nineteen (50%) probands were classified with remitted ADHD and 16 (42%) with persistent ADHD. Three (8%) probands did not meet criteria for either the remitted or persistent ADHD groups and were excluded from the analyses. Of the 16 with persistent ADHD, five (31%) had combined-type ADHD, six (38%) had inattentive-type ADHD, one had hyperactive/impulsive-type ADHD (6%), and four (25%) had ADHD not otherwise specified. The groups did not differ in prevalence of other disorders except anxiety disorders, which was accounted for by more simple phobias in the group with persistent ADHD. The comparison subjects had a significantly higher mean socioeconomic status (27) than the probands, while individuals with remitted ADHD and those with persistent ADHD did not differ in socioeconomic status. Characteristics of the final sample are summarized in Table 1.
| Characteristic | Comparison Subjects (N=32) | ADHD (N=35) | Remitted ADHD (N=19) | Persistent ADHD (N=16) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | p | |
| Age | 24.38 | 2.40 | 24.60 | 2.04 | 0.68 | 24.74 | 2.10 | 24.44 | 2.02 | 0.66 |
| Full-scale IQ | 102.81 | 15.78 | 96.94 | 16.15 | 0.13 | 98.53 | 16.68 | 95.06 | 15.83 | 0.51 |
| Socioeconomic status | 50.28 | 14.13 | 42.46 | 16.94 | 0.05 | 43.53 | 15.22 | 41.19 | 19.21 | 0.69 |
| Conners' Adult ADHD Diagnostic Interview for DSM-IV (number of symptoms) | ||||||||||
| Inattentive | 45.09 | 8.37 | 55.42 | 12.65 | <0.001 | 49.29 | 11.54 | 61.94 | 10.55 | <0.01 |
| Hyperactive/impulsive | 42.78 | 6.23 | 52.61 | 12.50 | <0.001 | 45.65 | 9.43 | 60.00 | 11.18 | <0.001 |
| ADHD symptom total | 43.41 | 7.86 | 55.36 | 14.38 | <0.001 | 47.76 | 11.91 | 63.44 | 12.43 | <0.001 |
| ADHD semistructured interview (number of symptoms) | 0.81 | 1.26 | 6.26 | 4.82 | <0.001 | 2.52 | 1.84 | 10.69 | 3.16 | <0.01 |
| N | % | N | % | p | N | % | N | % | p | |
| Male | 27 | 84.4 | 29 | 82.9 | 0.83 | 17 | 89.5 | 12 | 75.0 | 0.51 |
| Right-handed | 30 | 93.8 | 31 | 88.6 | 0.67 | 16 | 84.2 | 15 | 93.8 | 0.60 |
| Race | 0.26 | 0.50 | ||||||||
| Caucasian | 13 | 40.6 | 20 | 57.1 | 9 | 47.4 | 11 | 68.8 | ||
| African American | 11 | 34.4 | 7 | 20.0 | 4 | 21.1 | 3 | 18.8 | ||
| More than one race | 6 | 18.8 | 7 | 20.0 | 5 | 26.3 | 2 | 12.5 | ||
| Asian | 2 | 6.3 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Ethnicity | 0.24 | 0.88 | ||||||||
| Hispanic/Latino | 11 | 34.4 | 17 | 48.6 | 9 | 47.4 | 8 | 50.0 | ||
| Current mood disorder | 4 | 12.5 | 8 | 22.9 | 0.27 | 3 | 15.8 | 5 | 31.3 | 0.42 |
| Current anxiety disorder | 8 | 25.0 | 8 | 22.9 | 0.83 | 1 | 5.3 | 7 | 43.8 | 0.01 |
| Current substance disorder | 8 | 25.0 | 15 | 42.9 | 0.12 | 9 | 47.4 | 6 | 37.5 | 0.56 |
Twenty-five probands had a history of treatment with psychostimulants. The duration of treatment did not differ significantly between the remitted ADHD (mean=2.61 years, SD=2.78) and persistent ADHD (mean=4.78 years, SD=4.19) groups; however, fewer individuals with remitted ADHD (N=9; 47.0%) relative to persistent ADHD (N=13; 81.3%) received treatment (χ2=4.27, p=0.04). Two individuals with persistent ADHD were taking psychostimulants at the time of this study, but they refrained from treatment for at least 48 hours before the scan.
Positive urine toxicology results for amphetamines, opiates, and cocaine were exclusionary. Individuals refrained from cannabis use for at least 24 hours before the scan. Twenty-nine participants (12 comparison subjects and 17 probands) tested positive for cannabis. Among these, 12 reported their last cannabis use was 24 hours before the scan, 12 reported their last cannabis use was 2−4 days before the scan, and five reported their last cannabis use was 10 or more days before the scan. Patterns of cannabis use did not differ between groups (χ2=7.62, p=0.47).
Procedures
Cued reaction time paradigm.
The cued reaction time task (5) was an event-related paradigm consisting of four 300-second runs that began and ended with a 30-second fixation cross, which served as a baseline measure. Each block contained a series of 120 letters, including 24 targets (“X”), half of which were preceded by a cue (“A”). The other half of the targets were preceded by a noncue letter (“B” through “H”), yielding a total of 48 cued and 48 noncued targets across all runs. Cues were always followed by a target. The task did not include blank or partial trials, which have been shown to change the cue-target relationship and attenuate the effect of cues on neural activity (6). The task temporally segregated the neural effect of warning cues and targets. The stimuli were presented individually at fixation for 200 ms. The interstimulus interval was pseudo-randomized from 1,550 to 2,050 ms (mean=1,800 ms/block). Participants were instructed to respond with their right index finger as rapidly as possible to every target and were told that some targets would be preceded by the cue, which would always be followed by the target. Participants practiced one block on a desktop computer prior to the scan.
Image acquisition.
All participants were scanned on the same 3.0-T Siemens Allegra (Siemens, Erlangen, Germany) head-dedicated MRI scanner. A high resolution T2-weighted anatomical volume of the brain was acquired in the axial plane with a turbo spin-echo pulse sequence (TR=4,050 ms, TE=99 ms, flip angle=170°, field of view=240 mm, matrix=512×336, 40 slices, slice thickness=4 mm, in-plane resolution=0.41 mm2). Functional T2*-weighted images depicting the blood-oxygen-level-dependent (BOLD) signal were acquired at the same 40 slice locations as the T2 image using gradient-echo echo-planar images (TR=2,500 ms, TE=27 ms, flip angle=82°, matrix=64×64, slice thickness=4 mm, gap=4 mm, in-plane resolution=3.75 mm2). Images were acquired with slices positioned parallel to the anterior commissure-posterior commissure line. Stimuli were projected onto a rear projection screen mounted at the head of the magnet bore that was viewed through a mirror on the head coil.
Statistical Analysis
Performance.
Separate mixed-design analyses of variance with target type (cued or noncued) as a within-subjects factor and group as a between-subjects factor were used to compare reaction time and reaction time standard deviation measures between 1) comparison subjects and probands and 2) individuals with remitted and persistent ADHD. Group differences in overall error rate and rates of anticipation, commission, and omission errors were assessed with t tests. Alpha was set at 0.05 for all analyses.
Neuroimaging.
Imaging data were preprocessed and analyzed with SPM8 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm/) implemented on a MATLAB platform (version 13, Mathworks, Natick, Mass.). Each functional time series was slice time-corrected, realigned and unwarped, coregistered to the T2 structural image, normalized to the Montreal Neurological Institute (MNI) template, and spatially smoothed with an 8-mm Gaussian kernel.
First-level analyses were conducted for each participant with a general linear model to determine the relationship between the observed event-related BOLD signals and regressors that represented expected neural responses to events. Regressors were created by convolving a train of delta functions that represented the individual trial events with the default basis function. Regressors representing cues, noncues (letters other than “A” and “X”), and targets (“X”) were included in the model; if necessary, anticipation errors (response to the cue), omission errors (no response to “X”), and commission errors were also included. Six motion parameters created during realignment were included as covariates of no interest. A contrast map for cue minus noncue was created for each participant and entered into the group-level analysis.
Activation during response preparation, defined by the cue minus noncue contrast, was assessed separately for the comparison subjects and probands with one-sample t tests. The a priori hypotheses were tested with independent-samples t tests to compare 1) comparison subjects and probands and 2) individuals with remitted and persistent ADHD. The resultant voxel-wise statistical maps were thresholded for significance at a height intensity of p<0.01 and an extent of 100 voxels based on a Monte Carlo simulation (28), which took into account the image resolution parameters and the 8-mm full width at half maximum smoothing parameter and established that a cluster extent of 100 contiguous resampled voxels (2×2×2 mm3) was necessary to correct for multiple voxel comparisons at p<0.01. MNI coordinates were assigned anatomic labels by converting them to the Talairach and Tournoux (29) system.
Psychophysiological interaction.
Psychophysiological interaction is a regression-based method that tests for variations in physiological connectivity between brain regions as a function of changes in the psychological context (30, 31). This analysis was conducted for each participant to determine the whole-brain connectivity of the right thalamus for correct cue and noncue events. The thalamus was selected as a seed region based on the assumption that stimulus-driven response preparation is a bottom-up process, with the thalamus serving as a critical entry point for information en route to the cortex. Furthermore, we selected the right thalamus as the seed after our analysis of comparison subjects and probands, which indicated a right-lateralized difference in thalamus activation.
The seed volume of interest was defined as 8-mm radius spheres centered on subject-specific maxima that were within 4 mm of the group maxima in the right thalamus (coordinates: x=8, y=−12, z=0). The volume of interest was restricted to the thalamus using a mask derived from the Anatomical Automated Labeling Atlas (aal.002) (32) and generated with the PickAtlas toolbox (33). The time series of the first eigenvariate of the BOLD signal, adjusted for the effects of interest, was extracted from the right thalamus (average volume=161 mm3).
Time-series data of the first eigenvariate of the seed volume of interest were temporally filtered and mean corrected as in conventional SPM analysis. Bayesian estimation was used to deconvolve the time series of the BOLD signals to generate separate time series of the neuronal signal for the volume of interest for correct cue and noncue events, generating regressors that represented interactions between the psychological and physiological factors, as well as separate regressors representing the main effects of interest and the baseline time courses for the right thalamus. These regressors were forward-convolved with the hemodynamic response function and then entered into a regression model for correct cue and noncue events for the right thalamus, along with the six motion correction parameters entered as events of no interest. The resultant contrast maps were entered into second-level one-sample t tests to assess right thalamus connectivity in comparison subjects and probands, and independent-samples t tests were used to test for differences in connectivity between comparison subjects and probands and between individuals with remitted or those with persistent ADHD.
Results
Performance
Behavioral data are presented in Table 2. The reaction time analysis for comparison subjects and probands revealed a significant main effect of target type (F=857.72, df=1, 65, p<0.001, η2=0.90) but no main effect of group (p=0.12, η2= 0.03) and no interaction (p=0.15, η2=0.002). The reaction time analysis for individuals with remitted ADHD and those with persistent ADHD revealed a significant main effect of target type (F=307.60, df=1, 33, p<0.001, η2=0.90) but no main effect of group (p=0.993, η2=0.000002) and no group-by-target type interaction (p=0.25, η2=0.004). The reaction time standard deviation analysis for comparison subjects and probands revealed a significant main effect of group (F=5.69, df=1, 65, p<0.02, η2=0.01) but no main effect of target type (p=0.08, η2=0.46) and no interaction (p=0.08, η2=0.05). The reaction time standard deviation comparison for individuals with remitted ADHD and those with persistent ADHD revealed no main effect of target type (p=0.98, η2=0.00002), no main effect of group (p=0.07, η2=0.002), and no interaction (p=0.73, η2=0.004). No significant group differences were found in commission or omission errors.
| Variablea | Comparison Subjects (N=32) | ADHD (N=35) | Remitted ADHD (N=19) | Persistent ADHD (N=16) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Reaction time cued target | 311.67 | 52.51 | 346.87 | 82.37 | 341.82 | 60.38 | 352.86 | 104.59 |
| Reaction time noncued target | 491.19 | 61.26 | 509.24 | 89.18 | 514.07 | 86.33 | 503.50 | 94.97 |
| Reaction time standard deviation cued target | 79.54 | 26.25 | 100.23 | 36.51 | 98.33 | 27.29 | 102.50 | 45.58 |
| Reaction time standard deviation noncued target | 93.19 | 23.94 | 100.26 | 24.45 | 100.17 | 23.29 | 100.37 | 26.53 |
| Commission errors | 0.11 | 0.28 | 0.27 | 0.49 | 0.24 | 0.51 | 0.30 | 0.49 |
| Omission errors | 4.30 | 5.64 | 6.39 | 6.93 | 4.82 | 4.93 | 8.66 | 8.92 |
Neuroimaging
Comparison subjects and probands demonstrated similar patterns of thalamo-cortical activation during response preparation (Figure 1; see also Table S1 in the data supplement that accompanies the online edition of this article). For comparison subjects, the largest cluster of activation had a peak in the anterior cingulate cortex (Brodmann’s area [BA] 24) and extended to the supplementary motor area (BA 6), putamen, caudate, and thalamus. Probands had a large cluster of activation with a peak in the supplementary motor area (BA 6) that extended to the anterior cingulate cortex (BA 24) and a cluster of activation with a peak in the putamen extending to thalamus. Comparison subjects and probands also demonstrated overlapping clusters of cue-related activation bilaterally in the inferior parietal lobule (BA 40), regions of the temporal lobe and the cerebellum, and both showed activation in the dorsolateral prefrontal cortex.
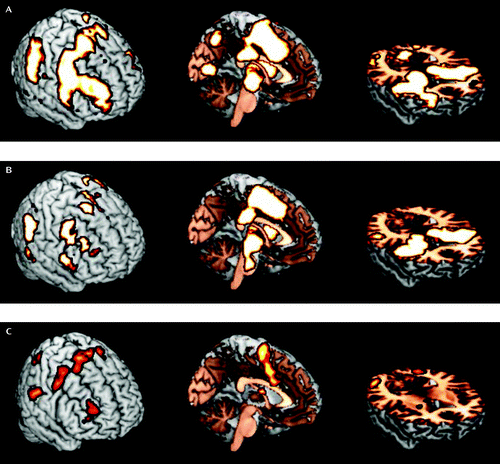
a Images are from comparison subjects (panel A), probands (panel B), and comparison subjects minus probands (panel C). The significance threshold was set at p<0.01, with an extent threshold at 100 voxels.
The comparison of probands and comparison subjects revealed significant differences in cue-related activation in several mainly right-lateralized regions (Table 3, Figure 1). Comparison subjects had greater cue-evoked activation than probands in the dorsolateral prefrontal cortex (BA 9), supplementary motor area (BA 6), anterior cingulate cortex (BA 24), inferior parietal lobule (BA 40), fusiform gyrus (BA 37), thalamus, striatum, and cerebellum. There were no regions of significantly greater activation for probands than comparison subjects. The comparison of individuals with persistent ADHD and those with remitted ADHD revealed no significant differences in the thalamo-cortical regions that were hypothesized to be associated with response preparation. However, there was a small cluster of greater activation in the posterior insula cortex in the remitted ADHD group (Table 3).
| MNI Coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast and Region | BA | Cluster Size | x | y | z | t |
| Comparison subjects > probands | ||||||
| Supplementary motor area | 6 | 1,279 | 8 | –2 | 68 | 4.56 |
| Anterior cingulate gyrus | 24 | 6 | 6 | 42 | 4.02 | |
| Middle frontal gyrus | 6 | 163 | 46 | 2 | 50 | 3.45 |
| Middle frontal gyrus | 10 | 217 | 34 | 48 | 18 | 3.90 |
| Dorsolateral prefrontal cortex | 9 | 32 | 42 | 30 | 2.73 | |
| Inferior parietal lobule | 40 | 356 | 62 | –30 | 24 | 3.92 |
| Inferior parietal lobule | 40 | 392 | –64 | –30 | 24 | 3.73 |
| Fusiform gyrus | 19 | 112 | 26 | –56 | 12 | 3.24 |
| Thalamus | 208 | 4 | –4 | 14 | 3.15 | |
| Putamen | 24 | 4 | –2 | 2.97 | ||
| Caudate | 14 | –2 | 14 | 3.07 | ||
| Precuneus | 7 | 318 | –6 | –74 | 38 | 2.99 |
| Middle occipital gyrus | 19 | 353 | 50 | –64 | –10 | 3.80 |
| Cerebellum | 408 | –34 | –56 | –24 | 3.79 | |
| Remitted ADHD > persistent ADHD | ||||||
| Posterior insula | 13 | 125 | 44 | –12 | 2 | 3.34 |
Comparison subjects and probands demonstrated distinct patterns of increased thalamo-cortical connectivity during cues relative to noncues (see Table S2 in the online data supplement). However, the only significant difference between comparison subjects and probands was greater functional connectivity among comparison subjects for cues relative to noncues between the right thalamus and the brainstem at the level of the pons (Figure 2). In contrast, compared with individuals with persistent ADHD, those with remitted ADHD demonstrated greater connectivity for cues relative to noncues between the right thalamus and several areas of the prefrontal cortex, including the frontopolar cortex bilaterally (BA 10) and the left dorsolateral prefrontal cortex (BA 9/46) (Figure 2).
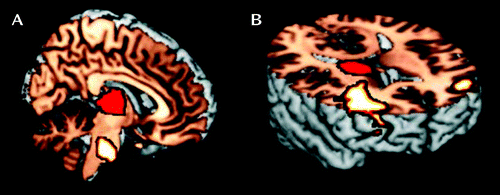
a Panel A depicts significantly greater functional connectivity during cues relative to noncues for comparison subjects than probands between the right thalamus (mask shown in red) and the brainstem at the level of the pons (x=2, y=−26, z=−34). Panel B depicts significantly greater functional connectivity during cues relative to noncues for individuals with remitted ADHD than those with persistent ADHD between the right thalamus (mask shown in red) and the prefrontal cortex, including the left (x=−38, y=44, z=14) and right (x=38, y=36, z=22) frontopolar cortex (Brodmann’s area [BA] 10) and the left (x=−46, y=46, z=10) dorsolateral prefrontal cortex (BA 46). The significance threshold was set at p<0.01, with an extent threshold at 100 voxels.
The pattern of results for all contrasts remained the same after controlling for medication history, substance use disorders, anxiety disorders, and mood disorders. Furthermore, there were no significant differences between individuals who reported cannabis use in the days before the scan and those who did not.
Discussion
Probands and comparison subjects demonstrated similar patterns of neural activation during response preparation, including greater activation for cues relative to noncues in the thalamus, striatum, anterior cingulate cortex, supplementary motor area, and inferior parietal lobule, indicating that probands did not utilize distinct brain regions to perform the task. However, relative to comparison subjects, probands demonstrated increased reaction time variability for cued targets and less activation in regions associated with neurocognitive processes linked to response preparation. Decreased activation among probands relative to comparison subjects in the thalamus and inferior parietal lobule suggest deficits in sustained attention and target detection (13). Furthermore, deficits in motor preparation among probands relative to comparison subjects were evidenced by decreased activation in the anterior cingulate cortex, supplementary motor area, and the culmen of the cerebellum, which has connections to premotor regions (34).
Deficient activation of the noted brain regions might be driven, in part, by reduced functional connectivity between the right thalamus and brainstem. The brainstem region identified in our connectivity analysis potentially encompassed cortico-pontine and ponto-cerebellar motor nuclei. Information used in motor planning and initiation is conveyed to the anterior pons by cortico-pontine fibers that originate from motor regions of the cortex and course through the thalamus (35). This information is conveyed to the cerebellum by the pontocerebellar tract. Probands demonstrated less functional connectivity between key nodes in this motor preparatory system. It is conceivable that the brainstem finding is related to movement-related artifacts. However, we are fairly confident this is not case because we added regressors to control for movement in our connectivity analyses, all participants demonstrated less than 2 mm of translational movement, and there were no differences in movement between groups.
Deficient activation of the dorsolateral prefrontal cortex among probands also indicates insufficient stimulus-driven top-down control. Persistent firing in the dorsolateral prefrontal cortex microcircuits during delay periods strengthens network connectivity and is responsible for integration and maintenance of information (36) and provides control of response preparation through fronto-striatal and fronto-parietal loops (37). Although activation differences in the prefrontal cortex were not found between individuals with remitted ADHD and those with persistent ADHD, individuals with remitted ADHD demonstrated greater functional connectivity for cues relative to noncues between the thalamus and the frontopolar and dorsolateral prefrontal cortices. While deficits in neural activation might be more closely linked to childhood status than adult status, the degree of functional integration between the right thalamus and prefrontal regions appears to parallel symptom recovery in adulthood.
Our results are partially consistent with those from other studies using different tasks. Decreased activation and functional connectivity of the thalamo-cortico and cortico-striatal loops during sustained attention (16), inhibitory control (15, 16), and cognitive switching (15) have been reported in prospectively followed adults with a current ADHD diagnosis relative to comparison subjects. However, the lack of activation differences between individuals with persistent ADHD and those with remitted ADHD may be task specific and a result of the stimulus-driven nature our task. Two fMRI studies directly compared activation during response inhibition in small groups of individuals with persistent ADHD and those with remitted ADHD in adolescence (38) and adulthood (39). In adolescence, linear trends existed for activation in the inferior frontal gyrus bilaterally (BA 47) and the left inferior parietal lobule (BA 40), such that individuals with persistent ADHD demonstrated greater activation than those with remitted ADHD, and individuals with remitted ADHD had greater activation than comparison subjects (38). In adulthood, higher neural activation during response inhibition was observed in individuals with remitted ADHD in the premotor and prefrontal cortex, while individuals with persistent ADHD demonstrated higher activation in the temporal lobes, cerebellum, and thalamus (39). Thus, it appears that changes in neural activation during goal-directed behavior might parallel symptom recovery, while activation during stimulus-driven processes remains deficient despite increased functional connectivity and symptom recovery. Further studies are needed to confirm this dissociation.
Our results must be interpreted within the context of several methodological considerations. Twenty-five probands had a history of stimulant treatment, and significantly more individuals were treated in the persistent ADHD group than the remitted ADHD group. However, the imaging results remained unchanged after controlling for treatment history. We also did not exclude individuals with psychiatric disorders other than ADHD from either the ADHD or comparison groups, but rates of these other disorders were well balanced across groups. We view this sampling method as advantageous. Most individuals with ADHD present with at least one comorbid disorder (40), and by avoiding a “supernormal” group of comparison subjects, we have greater confidence that our group differences are attributable to ADHD rather than more generalized psychopathology. Furthermore, our imaging results were unchanged by controlling for treatment or other diagnoses. A large portion of our sample not only reported chronic cannabis use but also use of cannabis during the days prior to the scan. No significant differences in brain activation were found between those who reported using cannabis before the scan and those who did not. Therefore, we have confidence that our results can be attributed to ADHD and not substance use.
In conclusion, this study provides evidence that ADHD is a neurodevelopmental disorder associated with lasting aberrations in thalamo-cortical activation during response preparation, despite significant remittance of symptoms, as well as adaptive changes in the functional integration of this neural circuit that parallel symptom recovery. Prospective studies with fMRI data collected at multiple time points are needed to fully understand the developmental trajectory of the neural correlates of response preparation and other neurocognitive functions in individuals with ADHD.
1 : The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol 2002; 111:279–289Crossref, Medline, Google Scholar
2 : Functional impairment and occupational outcome in adults with ADHD. J Atten Disord 2012; 16:544–552Crossref, Medline, Google Scholar
3 : Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters, and controls. J Child Psychol Psychiatry 2008; 49:958–966Crossref, Medline, Google Scholar
4 : Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull 2006; 132:560–581Crossref, Medline, Google Scholar
5 : Guanfacine potentiates the activation of prefrontal cortex evoked by warning signals. Biol Psychiatry 2009; 66:307–312Crossref, Medline, Google Scholar
6 : Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study. J Neurosci 2007; 27:2272–2282Crossref, Medline, Google Scholar
7 : Preparatory activity and connectivity in dorsal anterior cingulate cortex for cognitive control. Neuroimage 2011; 57:242–250Crossref, Medline, Google Scholar
8 : Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia 2009; 47:2389–2396Crossref, Medline, Google Scholar
9 : Perceptual and motor inhibition in adolescents/young adults with childhood-diagnosed ADHD. Neuropsychology 2010; 24:424–434Crossref, Medline, Google Scholar
10 : fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J Am Acad Child Adolesc Psychiatry 2008; 47:1141–1150Crossref, Medline, Google Scholar
11 : Alerting deficits in children with attention deficit/hyperactivity disorder: event-related fMRI evidence. Brain Res 2008; 1219:159–168Crossref, Medline, Google Scholar
12 : Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biol Psychiatry 2006; 59:643–651Crossref, Medline, Google Scholar
13 : Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am J Psychiatry 2006; 163:1033–1043Link, Google Scholar
14 : Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry 2007; 62:999–1006Crossref, Medline, Google Scholar
15 : Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res 2010; 44:629–639Crossref, Medline, Google Scholar
16 : Fronto-striatal underactivation during interference inhibition and attention allocation in grown-up children with attention deficit/hyperactivity disorder and persistent symptoms. Psychiatry Res 2011; 193:17–27Crossref, Medline, Google Scholar
17 : Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry 2010; 167:397–408Link, Google Scholar
18 : Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry 2009; 166:74–82Link, Google Scholar
19 : Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry 2006; 60:1071–1080Crossref, Medline, Google Scholar
20 : Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol Psychiatry 2011; 69:857–866Crossref, Medline, Google Scholar
21 : Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry 2011; 68:1122–1134Crossref, Medline, Google Scholar
22 : Hyperactivity, inattention, and aggression in clinical practice, in Advances in Developmental and Behavioral Pediatrics. Edited by Wolraich MRouth D. Greenwich, Conn, JAI Press, 1982, pp 113–147Google Scholar
23 : Diagnostic Interview Schedule for Children-Parent Version (DISC-2.1P). New York, New York State Psychiatric Institute, 1989Google Scholar
24 : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I-NP). New York, Biometrics Institute, New York State Psychiatric Institute, 2002Google Scholar
25 : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
26 : Conners’ Adult ADHD Diagnostic Interview for DSM-IV. North Tonawanda, NY, Multi-Health Systems, 2006Google Scholar
27 : Updating occupational prestige and socioeconomic scores: how the new measures measure up. Sociol Methodol 1994; 24:1–72Crossref, Google Scholar
28 : A sensory signature that distinguishes true from false memories. Nat Neurosci 2004; 7:664–672Crossref, Medline, Google Scholar
29 : Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical Publishers, 1988Google Scholar
30 : Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997; 6:218–229Crossref, Medline, Google Scholar
31 : Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage 2003; 19:200–207Crossref, Medline, Google Scholar
32 : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289Crossref, Medline, Google Scholar
33 : An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239Crossref, Medline, Google Scholar
34 : Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 2003; 23:8432–8444Crossref, Medline, Google Scholar
35 : The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from macaque monkeys and humans. Cereb Cortex 2006; 16:811–818Crossref, Medline, Google Scholar
36 : Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci 1995; 769:71–83Crossref, Medline, Google Scholar
37 : Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 2007; 104:11073–11078Crossref, Medline, Google Scholar
38 : Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. J Am Acad Child Adolesc Psychiatry 2005; 44:47–54Crossref, Medline, Google Scholar
39 : Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults: a functional magnetic resonance imaging (fMRI) study. Psychiatry Res 2010; 183:75–84Crossref, Medline, Google Scholar
40 : Psychiatric comorbidity and functional impairment in a clinically referred sample of adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci 2007; 257:371–377Crossref, Medline, Google Scholar



