Parietal Attentional System Aberrations During Target Detection in Adolescents With Attention Deficit Hyperactivity Disorder: Event-Related fMRI Evidence
Abstract
Objective: Directed attention, the ability to allocate and direct attention toward a salient stimulus, is impaired in attention deficit hyperactivity disorder (ADHD). This construct is often assessed with target detection or oddball tasks, and individuals with ADHD perform poorly on such tasks. However, to date, the specific brain structures or neural mechanisms underlying target detection dysfunction in individuals with ADHD have not been identified. The authors’ goal was to investigate neural correlates of target detection dysfunction in ADHD using event-related fMRI. Method: Behavioral and brain activation data were collected while subjects performed a visual oddball task. Participants included 14 right-handed male adolescents with ADHD (combined type) and 12 typically developing age- and handedness-matched male comparison subjects. Results: Individuals with ADHD made significantly more errors of commission than comparison subjects. Further, relative to comparison subjects, individuals with ADHD showed significantly less activation in the bilateral parietal lobes (including the superior parietal gyrus and supramarginal and angular gyri of the inferior parietal lobe), right precuneus, and thalamus. Conclusions: Adolescents with ADHD demonstrated significant impairments in their ability to direct and allocate attentional resources. These difficulties were associated with significant aberrations in the parietal attentional system, which is known to play a significant role in attention shifting and detecting specific or salient targets. Thus, dysfunction in the parietal attentional system may play a significant role in the behavioral phenotype of ADHD.
Attention deficit hyperactivity disorder (ADHD) is a behavioral disorder characterized by marked problems with inattention, impulsivity, and hyperactivity that causes significant functional impairment in multiple settings (e.g., school and home). Although behavioral and cognitive deficits in ADHD are well described, there is little consensus regarding the specific brain structures or neural mechanisms underlying dysfunction in individuals with this disorder (1) . To further elucidate this issue, a variety of laboratory paradigms have been used, including tasks involving target detection and response inhibition.
One such paradigm is the “oddball task,” which involves detection and response to infrequent (oddball) target events embedded in a series of repetitive events. Following initial sensory processing, target detection involves the process of bringing a salient stimulus into conscious awareness for further evaluation, categorization, and response. This task is thought to capture the construct of directed attention and reflects an individual’s ability to monitor the environment for change and decide on a course of action, a critical survival skill (2 , 3) .
Studies that have investigated target detection or oddball task performance have reported that individuals with ADHD commit more errors of commission (failure to withhold a response to frequent or standard stimuli) and often have slower or more variable reaction times than comparison subjects (4 , 5) . Further, individuals with ADHD show aberrant event-related potentials (i.e., decreased P300 latency and amplitude) in response to oddball or rare target stimuli in conjunction with poorer behavioral performance relative to comparison subjects (4 – 8) . Thus, it appears that impairments in directed attention are characteristic of ADHD. A clearer understanding of the underlying neural correlates of these deficits in ADHD will be important in diagnosis and treatment planning.
Although event-related potential studies index the timing and sequence of neuronal activity underlying cognitive processes, this technique is limited because of poor spatial resolution (i.e., a number of current distributions in the brain can give rise to identical field distributions on the scalp [2] ). Thus, complementary methods are needed to determine the neural correlates of target detection. In particular, the advent of noninvasive functional MRI (fMRI) has allowed investigation of the neural correlates of such functions as target detection, thus contributing to a better understanding of executive dysfunction observed in neuropsychiatric disorders such as ADHD.
To date, there have been no fMRI studies investigating target detection or oddball task performance in an ADHD population. However, five fMRI studies have investigated response inhibition in ADHD populations using go/no go type tasks and block (9 – 11) and event-related designs (12 , 13) . The go/no go and oddball tasks are somewhat like mirror images of one another, with go/no go type tasks involving response inhibition in the context of frequent response generation, and oddball tasks involving response generation in the context of frequent response inhibition (14) . It is unclear whether response inhibition (the act of withholding or suppressing a response) differs from that of selecting an alternative response.
To examine these processes and associated dysfunction in ADHD, we designed two event-related fMRI tasks, a go/no go task controlling for novelty (13) and an oddball task. In this study, we used a standard visual-based oddball task similar to that used in other fMRI studies (15) , with a modification that controls for motor responding. This type of task also has been referred to as a “change task” (16) or a response selection task (14) . Individuals with ADHD are known to perform poorly on these tasks (16) .
We predicted that individuals with ADHD would make more errors of commission than typically developing healthy comparison subjects on the oddball task. Although no previous fMRI studies have investigated oddball task performance in ADHD subjects, previous fMRI studies of normal subject performance on oddball paradigms have reported activation to target stimuli in the inferior and middle frontal gyri (15 , 17–19) as well as temporal regions (superior temporal gyrus [Brodmann’s area 22] and middle temporal gyrus) and parietal regions (inferior parietal lobules [including supramarginal gyrus] and superior parietal lobe) (2 , 15 , 17–20) . On the basis of morphological studies (21) and functional imaging studies of response inhibition (9 – 13) implicating these regions in ADHD, we predicted that activation might be aberrant in the frontal and striatal regions in the ADHD group. Further, since the parietal regions and frontal lobes play a critical role in sustained attention or vigilance (22 – 24) (also known to be impaired in ADHD [24] ), we also predicted aberrant activation in these regions.
Method
Participants
Male participants diagnosed with ADHD (N=14) and 12 typically developing male comparison subjects participated in the study after giving written informed consent and assent in accordance with Stanford Human Subjects Institutional Review Board requirements. All participants were paid $100 for participating in the study. Participants were right-handed and ranged in age from 14 to 18 years (group-matched; mean age=15.64 years, SD=1.15). Participants were predominantly of Caucasian ethnicity (80%), with the remaining participants identifying themselves as mixed-Asian (12%) and Hispanic (8%). Five participants in the ADHD group reported current use of stimulant medication; all five were subjected to an 18-hour washout of their stimulant medication prior to the scan. In addition, all participants did not have caffeine at least 2 hours before the scan, and of those reporting drug use (two in the ADHD group, one in the comparison group) and alcohol use (three in the ADHD group, six in the comparison group), a 3-week washout period for drugs (typically marijuana) and a 1-week washout period for alcohol were observed.
Typically developing comparison subjects were screened (via telephone interview with their primary caretaker and a follow-up written questionnaire) for absence of neurological, developmental, and psychiatric disorders and no family history of specific psychiatric disorders (i.e., ADHD, bipolar disorder, substance abuse/dependence, and conduct disorder/antisocial personality disorder). All individuals in the comparison group scored in the normative range (<65) on the Child Behavior Checklist (25) .
ADHD diagnosis was determined through a structured clinical interview conducted with the primary caregiver of each participant during the initial telephone screening and was verified during the initial visit (Diagnostic Interview for Children and Adolescents–Version IV [DICA-IV] completed by primary caregiver). In addition, each primary caregiver completed the Conner ADHD/DSM-IV scale–Parent Version about their adolescent (ADHD Index Score: mean=75.5, SD=10.0). Individuals with a family history of bipolar disorder were excluded from participation. One participant in the ADHD group met diagnostic criteria for current major depression and was excluded from analyses to avoid confounds related to psychomotor retardation.
To familiarize subjects with the MRI environment and to minimize artifacts related to movement, a protocol using an MRI simulator was administered to subjects in the ADHD group. Specifically, a movie viewed by the subject during the simulated scan would cutoff for 3 seconds whenever the subject moved beyond a set criterion. Increasingly stringent movement criteria (decreasing from 3 to 1 mm) and increasing time periods (2 minutes, 5 minutes, 10 minutes) were selected until all subjects met a criterion of less than three movements exceeding 1 mm within a 10-minute period. Exclusion criteria of head movement greater than 3 mm and rotation less than 3° during fMRI scanning were established; no participants exceeded this movement threshold.
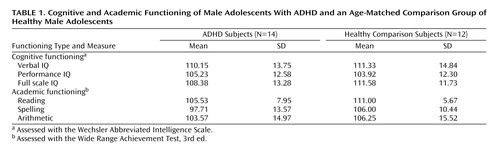
Cognitive functioning was assessed with the Wechsler Abbreviated Intelligence Scale (WAIS), and academic functioning (i.e., reading, spelling, and arithmetic) was assessed with the Wide Range Achievement Test, Third Edition (WRAT-III).
Experimental Task
In the event-related oddball task, stimuli were presented using a fast event-related design (2 , 26–28) . A jittered stimulus presentation was used with mean inter-oddball interval of 10.9 sec (SD=9.8) (28 , 29) . Such a jittered stimulus presentation has been shown to be efficient for separating brain activation to individual events (28) . To ensure that events could be statistically separated, we further verified that the predicted responses for each pair of stimuli were mutually orthogonal. Expected waveforms for each of the two conditions were computed after convolution with a hemodynamic response function (30) . The correlation between expected responses to each pair of conditions was less than 0.01, allowing us to independently assess brain activation to each condition (31) .
The event-related oddball experiment consisted of a 20-second rest epoch at the beginning and end of the task, during which subjects passively viewed a screen showing the word “rest.” Following the initial rest period, green circles (standard stimulus, 80% of trials) or green triangles (target or oddball stimulus, 20% of trials) were presented sequentially for 250 msec with an interstimulus interval of 1750 msec. Total number of trials was 225, which were presented in a single run. Participants were instructed to respond to the standard stimuli (circles) by pressing one button on the response box and to target stimuli (triangles) by pressing a second button on the response box. Specifically, the instructions read, “For this task, the computer will show you circles and triangles. Your job is to press 1 for triangles or press 2 for circles, as quickly as you can. Remember you want to be quick but also accurate, so do not go too fast.” Participants responded using the forefinger and middle finger of the right hand. Errors of commission (pressing 1 instead of 2/pressing 2 instead of 1) and reaction times to standard and target stimuli were recorded.
Image Acquisition
Images were acquired on a 1.5-T GE Signa scanner with echo speed gradients using a custom-built whole head coil that provides a 50% advantage in signal-to-noise ratio over that of the standard GE coil (32) . A custom-built headholder was used to prevent head movement. Eighteen axial slices (6 mm thick, 1 mm skip) parallel to the anterior and posterior commissures covering the whole brain were imaged with a temporal resolution of 2 seconds using a T2* weighted gradient echo spiral pulse sequence (TR=2000 msec, TE=30 msec, flip angle=89° and 1 interleave) (33) . The field of view was 240 mm, and the effective in-plane spatial resolution was 3.75 mm. To aid in localization of functional data, high-resolution T1-weighted inversion recovery spoiled grass gradient recalled (SPGR) three-dimensional MRI sequence with the following parameters was used: TR=9, TE=1.9, flip angle=15°, 124 slices in coronal plane, 256×192 matrix, acquired resolution=1.5×0.9×1.2 mm. The images were reconstructed as a 124×256×256 matrix with a 1.5×0.9×0.9 mm spatial resolution.
The task was programmed using PsyScope (http://psyscope.psy.cmu.edu) on a Macintosh notebook computer. Initiation of scan and task was synchronized using a TTL pulse delivered to the scanner timing microprocessor board from a “CMU Button Box” microprocessor connected to the Macintosh. Stimuli were presented visually at the center of a screen using a custom-built magnet compatible projection system (Resonance Technology, Calif.).
Image Preprocessing
Images were reconstructed, by inverse Fourier transform, for each of the 120 time points into 64×64×18 image matrices (voxel size: 3.75×3.75×7 mm). fMRI data were preprocessed using SPM99. Images were corrected for movement using least square minimization without higher-order corrections for spin history, and normalized to Montreal Neurological Institute (MNI) template provided with SPM. Images were then resampled every 2 mm using sinc interpolation.
Statistical Analysis
Statistical analysis was performed on group data by using a random effects model (34) along with the theory of Gaussian random fields as implemented in SPM99. This method takes advantage of multivariate regression analysis and corrects for temporal and spatial autocorrelations in the fMRI data (35) .
Confounding effects of fluctuations in global mean were removed by proportional scaling where, for each time point, each voxel was scaled by the global mean at that time point. Low-frequency noise was removed with a high-pass filter (0.5 cycles/minute) applied to the fMRI time series at each voxel. A temporal smoothing function (4 mm Gaussian kernel corresponding to dispersion of 8 seconds) was applied to the fMRI time series to enhance the temporal signal-to-noise ratio. Voxel-wise t statistics were computed using the random effects model and normalized to z scores to provide a statistical measure of activation independent of sample size. Finally, to determine the presence of significant clusters of activation, a joint expected probability distribution of height (z>1.67, p<0.05) and extent (p<0.05) threshold (36) was used to correct for spatial correlations in the data.
For group analysis, a random effects model was used to determine voxel-wise t statistics contrasting specific events of interest (i.e., oddball versus standard stimuli). This model estimates the error variance for each condition of interest across subjects rather than across scans (34) . The random effects model provides better generalization to the subject population, albeit with some loss in power due to averaging in the time domain and small N. This analysis proceeded in two steps. In the first step, adjusted images corresponding to the conditions/events of interest were determined. For each condition, a weighted average of the images was computed, taking into account the hemodynamic response. In the second step, these condition-specific images were contrasted in a general linear model to determine appropriate t statistics. The t statistics were normalized to z scores to determine significant clusters of activation. We examined activation to oddball versus standard events.
Neuroanatomical locations of activation were first determined using the standard Talairach atlas and then refined using the more detailed and thorough Duvernoy atlas (37) .
Functional ROI Analysis
Following these analyses, exploratory analyses were conducted to assess the relationship between brain activation and performance. Specifically, regions in which the comparison subjects exhibited significantly greater activation than the ADHD group, i.e., functional regions of interest, were identified. Pearson correlations between percentage of voxels activated (height threshold: z>1.67) between the functional regions of interest and errors of commission were then computed for each group.
Results
Movement Analysis
Although no participant exceeded minimum movement thresholds of 3 mm in any direction, we further ensured that there were no significant between-group movement differences by comparing the groups’ average displacement from the mean head position in six dimensions (translation in x, y, and z, and rotation around the x, y, and z axes). No significant group differences emerged.
IQ and Achievement
The two groups did not significantly differ with regard to verbal, performance, or full scale IQ scores or on reading, spelling, or arithmetic scores. Table 1 includes descriptive data for these variables. Two subjects in the ADHD group met DSM-IV criteria for a learning disability in spelling.
Behavioral Data
An initial investigation of the dependent variables (both errors of commission types, reaction time to triangles [oddballs], reaction time to circles [standards], and total number correct) using stem-and-leaf plots in SPSS revealed the presence of outliers in both groups for the errors of commission variables. To prevent any confounds in the data resulting from non-normality of the data, nonparametric Mann-Whitney tests were conducted to compare the groups on the errors of commission variables. Independent sample t tests were conducted for the reaction time variables.
The results of these analyses revealed significant group differences for both errors of commission variables, with the ADHD group making significantly more errors of commission than comparison subjects, pressing 1 instead of 2 (Mann-Whitney U=38.0, p<0.05) and pressing 2 instead of 1 (Mann-Whitney U=24.5, p<0.01). The two groups did not differ in reaction time to either novel triangles or standard circles. Means and standard deviations for these variables can be found in Table 2 .
Observation of the data reported in Table 2 suggests a higher number of errors in which 2 was pressed instead of 1 (pressing standard button when novel stimulus appears) than errors in which 1 was pressed instead of 2 (pressing novel button when standard stimulus appears); however, a paired samples t test comparing the two types of errors was not significant.
Brain Activation
Within-group activations to oddball versus standard events were examined for each group. A between-group analysis was then conducted to examine group differences in activation. To control for the potentially confounding influences of deactivation in the ADHD group, which has been shown to significantly influence between-group analyses, a mask of deactivation (activation to standard versus oddball events) was created for the between-group analyses. For a detailed discussion about deactivation influences on between-group activation comparisons, see Tamm et al. (38) . Only data that survived masking with deactivation are reported for the between-group analyses.
ADHD group
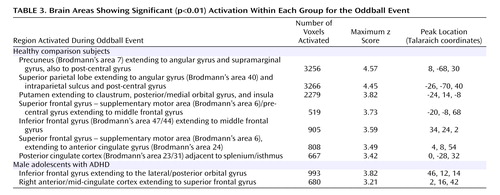
Male subjects with ADHD showed significant activation for the oddball versus standard events in the right inferior frontal gyrus extending to the orbital gyrus, and in the right anterior to mid-cingulate cortex extending to the superior frontal gyrus ( Table 3 , Figure 1 ).

Comparison group
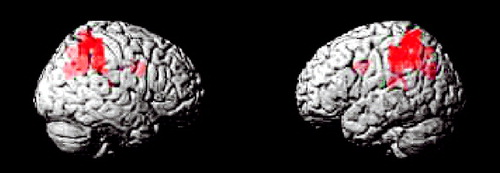
Comparison subjects showed several peaks of activation associated with the oddball versus standard events ( Table 3 , Figure 1 ). Significant activation was observed in the bilateral parietal lobe, including the angular gyrus, supramarginal gyrus, extending to the postcentral gyrus. Significant activation also was observed in the left putamen extending to the claustrum, insula, and posterior orbital gyrus. Bilateral activation in the superior frontal gyrus/supplementary motor area also emerged, which extended ventrally to the middle frontal gyrus. Finally, activation was also observed in the right inferior frontal gyrus and right posterior cingulate cortex.
Group differences
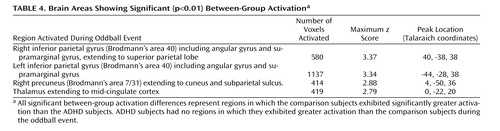
The ADHD group did not show greater activation to oddball versus standard events relative to healthy subjects on the between-group comparisons. In contrast, for the oddball versus standard events, the comparison group showed significantly more activation than the ADHD group in the bilateral parietal association cortex (Brodmann’s area 40/5/7), including the angular and supramarginal gyri, as well as the right precuneus/cuneus and thalamus/mid-cingulate ( Table 4 , Figure 2 , Figure 3 ).
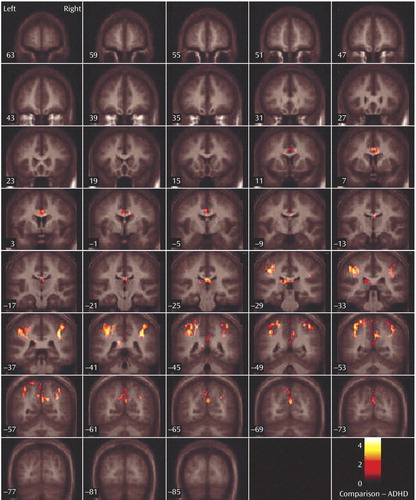
a Regions mapped onto an average T1-weighted image of healthy and ADHD brains.
Exploratory Functional Region of Interest Analysis
Bivariate correlations were conducted between the two errors of commission variables, number correct, and percent voxels activated in functional regions of interest (i.e., regions in which comparison subjects exhibited greater activation than the ADHD group). A significant negative correlation was observed in comparison subjects between percent voxels activated in the left parietal cortex and the commission error of pressing the standard button when the novel stimulus appeared ( Table 5 ). Activation in the right parietal cortex, precuneus, and thalamus was not significantly correlated with errors of commission. No significant correlations were observed in the ADHD group.
Discussion
This study is the first to examine the performance and brain activation of individuals with ADHD relative to comparison subjects on an oddball task using fMRI. Results indicated significant behavioral differences between the two groups accompanied by significant differences in brain activation. Adolescents in the ADHD group made significantly more errors of commission than comparison subjects on the oddball task, but the two groups did not differ significantly on the reaction time variables. Individuals with ADHD exhibited significantly less activation than comparison subjects in the bilateral parietal association cortex, in the right precuneus, and in the thalamus. These findings suggest a critical role for these brain regions in accurate target detection and task performance, which may underlie known deficits in directed attention in individuals with ADHD.
Within-group analyses revealed that the comparison group activated several regions during task performance, including peaks of activation in the bilateral parietal lobe, left putamen, bilateral superior frontal gyrus/supplementary motor area, left middle frontal gyrus, right posterior cingulate gyrus, and right inferior frontal gyrus. These regions of activation are consistent with those reported by other fMRI studies of oddball tasks in normal comparison subjects (2 , 12 , 13 , 15 , 17 – 20 , 39–41) . It is probable that activation in the supplementary motor area reflects higher-order motor planning (14 , 42 , 43) and potentially attentional shifting (43) . Activation in the parietal lobes, cingulate, and frontal lobes is likely associated with the process of target detection/directed attention (including activation related to response inhibition, vigilance, and working memory), since these regions are consistently activated in visual oddball tasks (19 , 44) .
More specifically, anterior cingulate activation to oddball stimuli observed in the comparison group likely reflects response conflict (i.e., simultaneous activation of incompatible response tendencies [14] ), and inferior frontal gyrus activation is thought to be related to the need to “stop” prepotent motor responses (26 , 45 , 46) . Putamen activation during target detection in normal subjects has been reported previously (47) and may be associated with response selection during controlled processing (48) and response inhibition (9) . Overall, those regions in which comparison subjects showed significant activation to oddball stimuli in our study are similar to those reported by target detection studies, suggesting that the task used in this study captured the construct of directed attention.
In contrast to the comparison group, individuals with ADHD showed fewer peaks of activation. Within-group analyses revealed activation in the right inferior frontal gyrus and in the right cingulate gyrus extending to the superior frontal gyrus. Again, activation in the right inferior frontal gyrus likely reflects response inhibition (26 , 45 , 46) , and activation in the cingulate gyrus likely reflects response conflict (14) . Notably, activation in the cingulate region was more anterior in the ADHD group than that observed in the comparison group. It has been suggested that the cingulate can be subdivided in terms of function into the caudal cingulate zone, the rostral cingulate zone, and the anterior rostral cingulate zone (49) , with the anterior rostral cingulate zone thought to be an “error-sensitive” region (14) . Because individuals in the ADHD group made significantly more errors of commission than comparison subjects, activation in this region may reflect response to errors or increased conflict resulting in errors of commission (14) . However, to assess the validity of this hypothesis, continued investigation is warranted. It was not possible to investigate brain activation specifically related to errors of commission because of insufficient power.
Adolescents with ADHD did not show more activation than comparison subjects in any region on the between-group analyses. However, the ADHD group did make significantly more errors of commission and showed significantly less activation in several posterior brain regions. Exploratory functional regions of interest analyses indicated that activation in the left parietal lobe was significantly negatively correlated with errors for the comparison group, supporting the hypothesis that for comparison subjects, the left parietal cortex may be directly involved in improving behavioral performance. The fact that no significant correlations were observed between errors of commission and other regions with group differences suggests that the right parietal cortex and right cingulate are either related to performance indirectly or via a mechanism not measured in this study. Regardless, it does appear that activation in these regions plays a role in task performance (i.e., target detection) and may be associated with improving performance in the comparison group.
Male adolescents with ADHD activated the bilateral superior parietal lobe, angular and supramarginal gyri, the right precuneus, and the thalamus significantly less than comparison subjects. There is strong evidence to suggest that activation in the superior and inferior parietal lobes is related to sustained attention/vigilance and attentional or set shifting (50 , 51) , or more generally, effortful attention (52 , 53) . Similarly, the precuneus has been shown to activate in response to relevancy and context (54 , 55) , as well as attentional shifting (43 , 51 , 56 , 57) . The majority of fMRI studies investigating a number of oddball paradigm variants report activation in the bilateral supramarginal gyrus (most typically in Brodmann’s area 40), which suggests a critical role for this region in target detection (2 , 15 , 19 – 20 , 58) . Other studies suggest a role for the left parietal cortex, and in particular the supramarginal gyrus, in motor attention (59 , 60) , and the right supramarginal gyrus in attentional shifting (61) . Thus, the confluence of evidence suggests that the bilateral supramarginal gyrus plays a role in postsensory processing of salient stimuli for evaluation, categorization, response, and decision making (2) . It is interesting that the pattern of results for the ADHD group is consistent with impairments in the regions thought to be associated with goal-directed attention (i.e., a top-down process for attention to features of a stimulus) including the fronto-parietal network and in particular the posterior parietal region, which activates in response to attention switched between objects presented in the same location [e.g., the oddball stimuli (53) ].
Group differences in angular gyrus activation were somewhat unexpected, since this region is most frequently reported as involved in language (62) and number processing (63 , 64) . However, angular gyrus activation also has been observed in response to visuospatial functioning (65) , visual pattern recognition (66) , and perception of spatial features of objects (67) , which are clearly relevant for the visual oddball task. Activation in the thalamus has been reported in previous oddball studies (2 , 17 , 18 , 41) and is thought to reflect engagement of the hippocampal-hypothalamic network in mediating the initial orienting response (2 , 3) .
Failure of the ADHD group to activate to the same degree as comparison subjects in parietal/precuneus regions suggests that they had difficulties in shifting attention and alternating motor responses to target stimuli, possibly one reason why this group made more errors than comparison subjects. A hypothesis that individuals with ADHD have impaired attention shifting is supported by our finding that this group made more of both errors of commission types. That is, they appeared to have trouble inhibiting a prepotent response and shifting to make an alternative response to target stimuli (i.e., pressing 1 instead of 2), but they also had difficulty shifting back to making the prepotent response (i.e., pressing 2 instead of 1). Thus, the difficulties expressed by individuals with ADHD appear to reflect more than impaired response inhibition; they also reflect fundamental impairments in the ability to shift attention and make alternative responses. In addition, errors of commission signal impulsivity, which also is indicative of impaired directed attention in ADHD.
Limitations of this study include the relatively small study group size. In addition, despite the fact that we excluded one subject who met criteria for current major depression, a small percentage of individuals in the ADHD group met diagnostic criteria for other disorders, which may have confounded the findings. It should be noted that interpretation of fMRI group differences may reflect only performance impairments, as opposed to a deficit specific to diagnosis with ADHD. Future studies with sufficient power and errors to conduct a covariance analysis with error rates will be needed to disentangle this important question.
Overall, the results of this study demonstrate a relationship between impaired target detection and brain function in ADHD; more generally, these results contribute to our understanding of the neural correlates of impaired ability to direct attention in ADHD. Our findings suggest that individuals with ADHD have significant aberrations in the posterior parietal attentional system, which is known to play a critical role in maintaining and shifting attention. Prior imaging studies of ADHD have primarily focused on the role of frontal and striatal regions in executive functions, such as response inhibition (9 – 11) , since morphological abnormalities of these regions are commonly reported (21) . Our results, however, suggest that it is important to reconsider the notion of ADHD as primarily a disorder of frontal-striatal function, and consider the role of the parietal attentional system in the behavioral phenotype of ADHD. Additional studies are warranted to investigate the relationship and interconnections between frontal, striatal, and parietal regions in executive dysfunction in ADHD.
1. Stefanatos GA, Wasserstein J: Attention deficit/hyperactivity disorder as a right hemisphere syndrome. Selective literature review and detailed neuropsychological case studies. Ann N Y Acad Sci 2001; 931:172–195Google Scholar
2. Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A: Combined event-related fMRI and EEG evidence for temporal-parietal cortex activation during target detection. Neuroreport 1997; 8:3029–3037Google Scholar
3. Knight R: Contribution of human hippocampal region to novelty detection. Nature 1996; 383:256–259Google Scholar
4. Idiaz bal Alecha M, Palencia Taboada AB, Sangorr n J, Espadaler Gamissans JM: [Cognitive evoked potentials in the hyperactivity attention deficit disorder]. Rev Neurol 2002; 34:301–305Google Scholar
5. Kemner C, Verbaten MN, Koelega HS, Buitelaar JK, van der Gaag RJ, Camfferman G, van Engeland H: Event-related brain potentials in children with attention-deficit and hyperactivity disorder: effects of stimulus deviancy and task relevance in the visual and auditory modality. Biol Psychiatry 1996; 40:522–534Google Scholar
6. Lazzaro I, Anderson J, Gordon E, Clarke S, Leong J, Meares R: Single trial variability within the P300 (250–500 ms) processing window in adolescents with attention deficit hyperactivity disorder. Psychiatry Res 1997; 73:91–101Google Scholar
7. Lazzaro I, Gordon E, Whitmont S, Meares R, Clarke S: The modulation of late component event related potentials by pre- stimulus EEG theta activity in ADHD. Int J Neurosci 2001; 107:247–264Google Scholar
8. Jonkman LM, Kemner C, Verbaten MN, Van Engeland H, Camfferman G, Buitelaar JK, Koelega HS: Attentional capacity, a probe ERP study: differences between children with attention-deficit hyperactivity disorder and normal control children and effects of methylphenidate. Psychophysiology 2000; 37:334–346Google Scholar
9. Teicher MH, Anderson CM, Polcari A, Glod CA, Maas LC, Renshaw PF: Functional deficits in basal ganglia of children with attention- deficit/hyperactivity disorder shown with functional magnetic resonance imaging relaxometry. Nat Med 2000; 6:470–473Google Scholar
10. Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET: Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev 2000; 24:13–19Google Scholar
11. Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD: Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 1998; 95:14494–14499Google Scholar
12. Schulz KP, Fan J, Yang CK, Newcorn JH, Buchsbaum MS, Cheung AM, Halperin JM: Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related fMRI study. Am J Psychiatry 2004; 161:1650–1657Google Scholar
13. Tamm L, Menon V, Ringel J, Reiss AL: Event-related fMRI evidence for fronto-temporal involvement in aberrant response inhibition evidenced by adolescents with attention deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2004; 43:1430–1440Google Scholar
14. Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A: Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex 2001; 11:825–836Google Scholar
15. Stevens AA, Skudlarski P, Gatenby JC, Gore JC: Event-related fMRI of auditory and visual oddball tasks. Magn Reson Imaging 2000; 18:495–502Google Scholar
16. Sergeant JA, Oosterlaan J, van der Meere J: Information processing and energetic factors in attention-deficit/hyperactivity disorder, in Handbook of Disruptive Behavior Disorders. Edited by Hogan HCQAE. New York, Plenum Publishers, 1999, pp 75–104Google Scholar
17. Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF: Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology 2001; 38:133–142Google Scholar
18. Clark VP, Fannon S, Lai S, Benson R, Bauer L: Responses to rare visual target and distractor stimuli using event- related fMRI. J Neurophysiol 2000; 83:3133–3139Google Scholar
19. McCarthy G, Luby M, Gore J, Goldman-Rakic P: Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol 1997; 77:1630–634Google Scholar
20. Linden DE, Prvulovic D, Formisano E, Vollinger M, Zanella FE, Goebel R, Dierks T: The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex 1999; 9:815–823Google Scholar
21. Hendren RL, De Backer I, Pandina GJ: Review of neuroimaging studies of child and adolescent psychiatric disorders from the past 10 years. J Am Acad Child Adolesc Psychiatry 2000; 39:815–828Google Scholar
22. Lewin JS, Friedman L, Wu D, Miller DA, Thompson LA, Klein SK, Wise AL, Hedera P, Buckley P, Meltzer H, Friedland RP, Duerk JL: Cortical localization of human sustained attention: detection with functional MR using a visual vigilance paradigm. J Comput Assist Tomogr 1996; 20:695–701Google Scholar
23. Coull JT, Frackowiak RS, Frith CD: Monitoring for target objects: activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia 1998; 36:1325–1334Google Scholar
24. Sunshine JL, Lewin JS, Wu DH, Miller DA, Findling RL, Manos MJ, Schwartz MA: Functional MR to localize sustained visual attention activation in patients with attention deficit hyperactivity disorder: a pilot study. AJNR Am J Neuroradiol 1997; 18:633–637Google Scholar
25. Achenbach TM: Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, University of Vermont Department of Psychiatry Press, 1991Google Scholar
26. Menon V, Adleman NE, White CD, Glover GH, Reiss AL: Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 2001; 12:131–143Google Scholar
27. Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM: Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport 1998; 9:3735–3739Google Scholar
28. Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM: Stochastic designs in event-related fMRI. Neuroimage 1999; 10:607–619Google Scholar
29. Dale AM: Optimal experimental design for event-related fMRI. Hum Brain Mapp 1999; 8:109–114Google Scholar
30. Kruggel F, von Cramon DY: Temporal properties of the hemodynamic response in functional MRI. Hum Brain Mapp 1999; 8:259–271Google Scholar
31. Clark VP, Maisog JM, Haxby JV: An fMRI study of face perception and memory using random stimulus sequences. J Neurophysiol 1998; 79:3257–3265Google Scholar
32. Hayes C, Mathias C: Improved brain coil for fMRI and high resolution imaging, in ISMRM 4th Annual Meeting Proceedings. New York, ISMRM, 1996Google Scholar
33. Glover GH, Lai S: Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med 1998; 39:361–368Google Scholar
34. Holmes AP, Friston KJ: Generalizability, random effects, and population inference. Neuroimage 1998; 7:754Google Scholar
35. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Google Scholar
36. Poline JB, Worsley KJ, Evans AC, Friston KJ: Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 1997; 5:83–96Google Scholar
37. Duvernoy HM, Bourgouin P, Cabanis EA, Cattin F: The Human Brain: Surface, Three-Dimensional Sectional Anatomy With MRI, and Blood Supply. New York, Springer-Verlag, 1999Google Scholar
38. Tamm L, Menon V, Johnston CK, Hessl DR, Reiss AL: fMRI study of cognitive interference processing in females with fragile X syndrome. J Cognitive Neurosci 2002; 14:160–171Google Scholar
39. Kruggel F, Herrmann CS, Wiggins CJ, von Cramon DY: Hemodynamic and electroencephalographic responses to illusory figures: recording of the evoked potentials during functional MRI. Neuroimage 2001; 14:1327–1336Google Scholar
40. Kirino E, Belger A, Goldman-Rakic P, McCarthy G: Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study. J Neurosci 2000; 20:6612–6618Google Scholar
41. Yoshiura T, Zhong J, Shibata DK, Kwok WE, Shrier DA, Numaguchi Y: Functional MRI study of auditory and visual oddball tasks. Neuroreport 1999; 10:1683–1688Google Scholar
42. Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H: Functional anatomy of GO/NO-GO discrimination and response selection: a PET study in man. Brain Res 1996; 728:79–89Google Scholar
43. Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N, Toma K, Nakamura K, Hanakawa T, Konishi J, Fukuyama H, Shibasaki H: Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage 1999; 10:193–199Google Scholar
44. Herrmann CS, Knight RT: Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav Rev 2001; 25:465–476Google Scholar
45. Garavan H, Ross TJ, Stein EA: Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A 1999; 96:8301–8306Google Scholar
46. Liddle PF, Kiehl KA, Smith AM: Event-related fMRI study of response inhibition. Hum Brain Mapp 2001; 12:100–109Google Scholar
47. Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S: Novelty encoding networks in the human brain: positron emission tomography data. Neuroreport 1994; 5:2525–2528Google Scholar
48. Rossell SL, Bullmore ET, Williams SC, David AS: Brain activation during automatic and controlled processing of semantic relations: a priming experiment using lexical-decision. Neuropsychologia 2001; 39:1167–1176Google Scholar
49. Picard N, Strick PL: Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 1996; 6:342–353Google Scholar
50. Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H: Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex 2001; 11:85–92Google Scholar
51. Le TH, Pardo JV, Hu X: 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J Neurophysiol 1998; 79:1535–1548Google Scholar
52. Ford JM, Sullivan EV, Marsh L, White PM, Lim KO, Pfefferbaum A: The relationship between P300 amplitude and regional gray matter volumes depends upon the attentional system engaged. Electroencephalogr Clin Neurophysiol 1994; 90:214–228Google Scholar
53. Corbetta M, Shulman GL: Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002; 3:201–215Google Scholar
54. Downar J, Crawley AP, Mikulis DJ, Davis KD: The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage 2001; 14:1256–1267Google Scholar
55. Banich MT, Milham MP, Jacobson BL, Webb A, Wszalek T, Cohen NJ, Kramer AF: Attentional selection and the processing of task-irrelevant information: insights from fMRI examinations of the Stroop task. Prog Brain Res 2001; 134:459–470Google Scholar
56. Brass M, Zysset S, von Cramon DY: The inhibition of imitative response tendencies. Neuroimage 2001; 14:1416–1423Google Scholar
57. Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY: Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res 2000; 9:103–109Google Scholar
58. Clark VP, Fannon S, Lai S, Benson R: Paradigm-dependent modulation of event-related fMRI activity evoked by the oddball task. Hum Brain Mapp 2001; 14:116–127Google Scholar
59. Rushworth MF, Krams M, Passingham RE: The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci 2001; 13:698–710Google Scholar
60. Rushworth MF, Nixon PD, Renowden S, Wade DT, Passingham RE: The left parietal cortex and motor attention. Neuropsychologia 1997; 35:1261–1273Google Scholar
61. Perry RJ, Zeki S: The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain 2000; 123:2273–2288Google Scholar
62. Clark CR, Moores KA, Lewis A, Weber DL, Fitzgibbon S, Greenblatt R, Brown G, Taylor J: Cortical network dynamics during verbal working memory function. Int J Psychophysiol 2001; 42:161–176Google Scholar
63. Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A: Dissociating neural correlates of cognitive components in mental calculation. Cereb Cortex 2001; 11:350–359Google Scholar
64. Menon V, Rivera SM, White CD, Glover GH, Reiss AL: Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage 2000; 12:357–365Google Scholar
65. Gobel S, Walsh V, Rushworth MF: The mental number line and the human angular gyrus. Neuroimage 2001; 14:1278–1289Google Scholar
66. Herath P, Kinomura S, Roland PE: Visual recognition: evidence for two distinctive mechanisms from a PET study. Hum Brain Mapp 2001; 12:110–119Google Scholar
67. Faillenot I, Decety J, Jeannerod M: Human brain activity related to the perception of spatial features of objects. Neuroimage 1999; 10:114–124Google Scholar