Relationships Between Changes in Sustained Fronto-Striatal Connectivity and Positive Affect in Major Depression Resulting From Antidepressant Treatment
Abstract
Objective
Deficits in positive affect and their neural bases have been associated with major depression. However, whether reductions in positive affect result solely from an overall reduction in nucleus accumbens activity and fronto-striatal connectivity or the additional inability to sustain engagement of this network over time is unknown. The authors sought to determine whether treatment-induced changes in the ability to sustain nucleus accumbens activity and fronto-striatal connectivity during the regulation of positive affect are associated with gains in positive affect.
Method
Using fMRI, the authors assessed the ability to sustain activity in reward-related networks when attempting to increase positive emotion during performance of an emotion regulation paradigm in 21 depressed patients before and after 2 months of antidepressant treatment. Over the same interval, 14 healthy comparison subjects underwent scanning as well.
Results
After 2 months of treatment, self-reported positive affect increased. The patients who demonstrated the largest increases in sustained nucleus accumbens activity over the 2 months were those who demonstrated the largest increases in positive affect. In addition, the patients who demonstrated the largest increases in sustained fronto-striatal connectivity were also those who demonstrated the largest increases in positive affect when controlling for negative affect. None of these associations were observed in healthy comparison subjects.
Conclusions
Treatment-induced change in the sustained engagement of fronto-striatal circuitry tracks the experience of positive emotion in daily life. Studies examining reduced positive affect in a variety of psychiatric disorders might benefit from examining the temporal dynamics of brain activity when attempting to understand changes in daily positive affect.
A reduction in the ability to experience positive affect is a hallmark of major depressive disorder. Reduced positive affect is central to the concept of anhedonia, and symptoms of anhedonia or depressed mood are required for a DSM-IV diagnosis of depression. Despite the importance of reduced positive affect in depression, research has only recently begun to focus on this aspect of the disorder. Studies have found that patients with depression show reduced electrophysiological reactivity to positive stimuli (1, 2) and reduced striatal hemodynamic activity in response to monetary or visual rewards (3–6). However, these findings have not been consistently replicated (5, 7–17), suggesting that alternative models may be needed.
One possibility is that the attenuated positive affect characteristic of depression stems from difficulties sustaining affective responses to positive stimuli, rather than an attenuation of the overall affective response (18). Broadly consistent with this possibility is evidence that patients with depression have an impaired ability to integrate reward reinforcement history over consecutive trials (19) and fail to sustain normative response bias toward reward-predicting cues (20), and that mothers experiencing postpartum depression demonstrate a lack of within-trials sustained ventral striatal activity to financial rewards (21). (Note that we use the term “sustained activity” to refer to the temporal dynamics across trials. For more on this, see the data supplement that accompanies the online edition of this article.) More direct evidence comes from research showing that patients with depression demonstrate lack of sustained nucleus accumbens activity and fronto-striatal connectivity across trials when instructed to cognitively enhance their response to positive emotional images, whereas healthy comparison subjects do not show this effect (22). Notably, individual differences in the ability to sustain nucleus accumbens activity correlates with ratings of daily positive affect among depression patients, suggesting that sustained activity in this circuitry could generally support the experience of positive affect among patients. Theoretically speaking, these studies have generally examined the consummatory aspects of reward processing as opposed to the anticipatory aspects of reward (but see reference 23 for a discussion of both anticipatory and consummatory aspects of reward processing in depression).
In this study, we extend this line of work by testing whether treatment with antidepressants (fluoxetine or extended-release venlafaxine [hereafter referred to simply as venlafaxine]) strengthens activity in this circuitry and whether treatment-induced change in sustained activity accounts for treatment-related improvements in self-reported daily positive affect. Patients were randomly assigned to receive fluoxetine or venlafaxine in a double-blind design and followed for 6 months. The central circuitry underlying the ability to sustain positive affect was assayed at baseline and 2 months later in patients and comparison subjects using a well-validated emotion regulation paradigm (24). We predicted 1) that positive affect would increase and negative affect would decrease over 2 months of treatment and 2) that changes in sustained nucleus accumbens activity and fronto-striatal connectivity resulting from treatment would correlate with change in daily positive affect.
Method
Participants
At baseline, we assessed 29 medication-free right-handed adults who met DSM-IV criteria for major depressive disorder and 19 matched comparison subjects. Participants were recruited through community advertisements; volunteers were screened for standard MRI compatibility criteria, CNS medications, comorbid substance use disorders, other comorbid DSM-IV axis I or axis II diagnoses, and a personal or family history of bipolar disorder. Patients were required to have had depressive symptoms for at least 1 month prior to enrollment and a score >17 on the 21-item Hamilton Depression Rating Scale (HAM-D) (25) at enrollment and at the baseline functional MRI (fMRI) assessment (the patients’ mean baseline HAM-D score was 20.6 [SD=2.39]). All participants completed the 38-item version of the expanded form of the Positive and Negative Affect Schedule (26) (PANAS) to assess current state positive and negative affect, and all depressed participants except one completed the 90-item Mood and Anxiety Symptom Questionnaire (27). The PANAS was administered both at baseline and at the 2-month scanning session. On both scan days, the instrument was administered twice—immediately before and immediately after the scan. To create a reliable measure of current affective functioning, we computed the mean positive and negative affect for that participant across the pre- and postscan PANAS scores on that day.
Participants in this study are the same as those in previous studies we have reported on (22, 28, 29). This study was approved by the Institutional Review Board at the University of Wisconsin–Madison, and all participants provided written informed consent.
After the baseline assessment, patients were randomly assigned to 6 months of treatment with either fluoxetine or venlafaxine (for more details, see the online data supplement). For the first 2 months of treatment, patients had seven weekly medication visits with a physician associated with the study. During that period, the average daily dose for patients in the fluoxetine group was 37 mg (SD=8.7), and for those in the venlafaxine group, 118 mg (SD=36.6). At 2 months, participants returned for a second fMRI assessment. Eight patients and five comparison subjects had withdrawn from the study, leaving 21 patients and 14 comparison subjects. The participants who remained did not differ significantly in age, sex, or baseline positive or negative affect scores from those who withdrew. Baseline HAM-D score differed between those patients who completed the trial and those who did not, with a lower mean score for those who completed the trial, but the difference fell short of significance (t=1.93, df=27, p=0.06). After unblinding, it was revealed that 12 patients had been assigned to treatment with venlafaxine and nine patients to treatment with fluoxetine. Analyses incorporated medication responders and nonresponders.
fMRI Task
Participants underwent scanning while viewing a sequence of 72 positive and 72 negative images from the International Affective Picture System (30). The same images were used at both scan sessions, although the order of image presentation was randomized across the two sessions. Trials began with a 1-second fixation cross and auditory tone. Then an image was presented for 10 seconds, followed by a 6-second blank screen. At the onset of each image, participants used a button response pad to indicate whether they judged the image to be positive or negative. Four seconds after image onset, an auditory prompt instructed participants to increase (“enhance”) or decrease (“suppress”) their emotional response to the image or to continue to “attend” to the image. Participants had been trained on the use of cognitive reappraisal strategies to reevaluate the images as more or less emotional during a previous session while positioned inside a mock scanner. For the enhance condition, participants were trained either to imagine themselves or a loved one experiencing the situation being depicted or to imagine a more extreme outcome than the one depicted (e.g., in response to a picture of a stunning natural scene, a participant might imagine being in that scene or in one of their own choosing; see the online data supplement). Across six 380-second scans, there were 24 trials of the regulation conditions and 12 trials of the attend condition for each valence (the order was pseudorandomized; see the data supplement).
Image Acquisition and Analysis
The details of image acquisition are provided in the online data supplement. The analytic techniques we used were identical to those in our previous study (22). Briefly, data were slice-time and motion-corrected using AFNI (Analysis of Functional NeuroImages; http://afni.nimh.nih.gov/afni/). Single-subject general linear models were used to model each of the six trial types (positive/negative stimulus; enhance, attend, and suppress reappraisal instruction) separately, and for both the early phase (runs 1–3; denoted as “1st half” from here on) and the late phase (runs 4–6; denoted as “2nd half” from here on) of the scanning session as well as six motion nuisance covariates (see the data supplement). Because the process of sustaining nucleus accumbens activity during the increasing of positive affect was central to our previous report, contrasts for analyses presented here were the positive enhance(2nd half) – positive enhance(1st half) except as otherwise noted (see the data supplement). For analyses examining treatment response, the contrasts were positive enhance:2-month(2nd half – 1st half) – positive enhance:baseline(2nd half – 1st half). Single-subject contrasts were normalized to the 2-mm MNI152 template and smoothed (5-mm full width at half maximum).
Analyses examining sustained nucleus accumbens activity and nucleus accumbens-prefrontal cortex connectivity from baseline to the 2-month assessment used the contrast 2-month(2nd half – 1st half) – baseline(2nd half – 1st half) (see the data supplement). The resulting value was regressed on positive affect as well as negative affect. For control analyses examining reaction time (reaction time was successfully acquired for 19 of the 21 depressed patients) or pupil dilation (pupil data were successfully acquired for 15 of the 21 depressed patients at both time points), we performed the same analysis—2-month(2nd half – 1st half) – baseline(2nd half – 1st half). (For details on pupil dilation processing, see the data supplement.)
Results
Change in Symptom Severity
At 2 months, the mean HAM-D score was lower (t=–10.72, df=20, p<0.001; partial eta squared [pη2]=0.85) (Figure 1). Nine patients (43%) had achieved remission (defined as a HAM-D score ≤7). Of the remaining 12 patients, six (50%) were responders (defined as a decrease of ≥50% in HAM-D score), leaving six nonresponders. There was no difference between the fluoxetine and venlafaxine groups in change in HAM-D score.
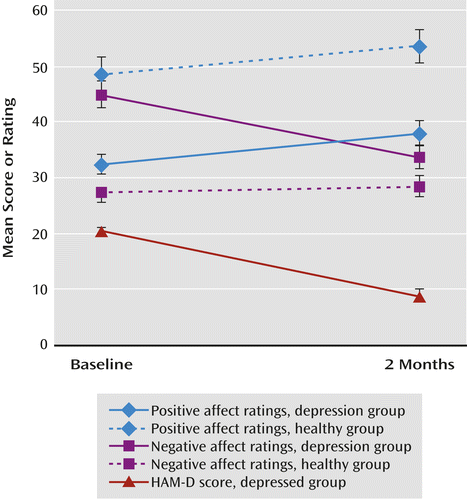
a The mean HAM-D score for healthy comparison subjects (not shown) was 1.07 at baseline and 1.64 at 2 months. There were significant differences between baseline and 2 months in positive affect ratings for both groups (p<0.01), in negative affect ratings for the depressed group (p<0.001), and in HAM-D score for the depressed group (p<0.001).
Participants in both the depressed and comparison groups provided ratings of positive affect and negative affect at both baseline and the 2-month assessment. We entered the change in positive affect and negative affect as dependent measures and performed a repeated-measures analysis of variance (change in negative affect was multiplied by –1 so that both scales were coded in the same direction. Results revealed that the group-by-scale interaction (depressed and comparison groups; positive and negative affect) was significant (F=18.36, df=1, 33, p<0.001; pη2=0.36). There was also a main effect of group on change in affect (F=6.19, df=1, 33, p=0.02; pη2=0.16), suggesting that affect changed more in the depressed group than in the comparison group. Follow-up tests revealed that in the depressed group, positive affect increased (pη2=0.22) and negative affect decreased (pη2=0.66) across assessments (p values, <0.03). In the comparison group, positive affect increased (p=0.03, pη2=0.30), whereas there was no change in negative affect (p=0.34, pη2=0.07). The groups did not differ in the magnitude of the change in positive affect. By contrast, the depressed group showed a steeper decrease in negative affect (p<0.001, pη2=0.44). Within the depressed group, change in positive affect and in negative affect did not differ significantly between patients receiving fluoxetine and those receiving venlafaxine.
We next examined the correlations between positive affect and negative affect measures. In the depression group, change in positive affect and negative affect scores from baseline to 2 months were significantly inversely correlated (r=–0.61, N=21, p=0.004; see Table S1 in the online data supplement), and in the comparison group, they were also inversely though not significantly correlated (r=–0.45, N=14, p=0.10). In the depression group, change on the anhedonia subscale of the Mood and Anxiety Symptom Questionnaire was also significantly correlated with change in positive affect (see Table S1; r=–0.68; t=–3.91, df=18, p=0.001)
Replication of Previous Findings
In these same patients, we previously reported that sustained nucleus accumbens activity correlated with positive affect ratings prior to treatment. After 2 months of treatment, we found that individual differences in sustained nucleus accumbens activity correlated with patient ratings of positive affect in daily life (peak, x=–12, y=18, z=–6; for positive affect at 2 months, unstandardized beta coefficient [B]=0.01; t=2.50, df=17, p=0.02; controlling for baseline positive affect and sustained nucleus accumbens activity at baseline, small volume corrected for multiple comparisons) (Table 1). This effect remained significant after controlling for drug type (B=0.01; t=2.38, df=16, p=0.03).
| Location (Brodmann’s Area) | Coordinates (x, y, z, in mm) | Cluster Size (Voxels) | Maximum t |
|---|---|---|---|
| Right insula (BA 48) | 60, 8, 4 | 605 | 4.33 |
| Right superior frontal gyrus (BA 45/46) | 34, 34, 20 | 342 | 5.37 |
| Supplementary motor area (BA 6) | 8, 0, 62 | 153 | 4.02 |
| Right occipital cortex | 20, –56, 38 | 122 | 4.73 |
| Right middle temporal gyrus | 70, –42, –4 | 104 | 6.10 |
| Left middle frontal gyrus (BA 46/47) | –40, 54, –4 | 97 | 4.32 |
| Left superior temporal gyrus (BA 21) | –60, –50, 4 | 81 | 4.24 |
| Superior occipital gyrus | –4, –96, 26 | 78 | 5.28 |
Change in Sustained Nucleus Accumbens Activity
Using the nucleus accumbens cluster described above, we next examined whether change in sustained nucleus accumbens activity correlated with gains in positive affect across assessments. This analysis showed that patients who exhibited larger improvements in sustained nucleus accumbens activity showed larger gains in positive affect (Figure 2) (B=0.01, r=0.54; t=2.76, df=19, p=0.01; R2=0.29; see Table S2 in the online data supplement). This finding remained significant after controlling for drug type (B=0.01; t=2.42, df=18, p=0.03).
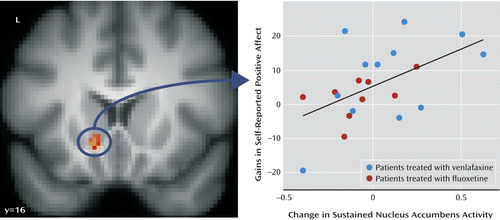
a For patients who completed the trial, those who exhibited the greatest improvement in sustained nucleus accumbens activity also had the largest gains in self-reported positive affect. In the graph, the x-axis reflects individual differences in changes in sustained nucleus accumbens activity from baseline to 2 months (2-months(2nd half – 1st half) – pretreatment baseline(2nd half – 1st half)). The y-axis reflects gains in self-reported positive affect. In this analysis, r=0.54.
We performed several control analyses to examine the specificity of this effect. First, we examined whether change in nucleus accumbens activity aggregated across the scan sessions (as is most commonly done in fMRI analyses) was associated with an increase in positive affect. We contrasted aggregated nucleus accumbens activity during the positive “enhance” condition at 2 months with aggregated activity during the same condition at baseline and correlated that activity with change in positive affect. There was no relationship between increases in nucleus accumbens activity aggregated across the scan session over the course of treatment and increases in positive affect over the same period. Additionally, we tested whether including both change in sustained nucleus accumbens activity and change in aggregated activity in the same regression model was associated with change in positive affect. We found that change in sustained nucleus accumbens activity was specifically associated with change in positive affect (B=24.01; t=2.90, df=18, p=0.01), whereas change in aggregated nucleus accumbens activity was not associated with change in positive affect. This provides further evidence that examination of changes in sustained activity over the scan sessions is important and uniquely associated with change in positive affect.
Second, we examined whether this effect could be explained by changes in reaction time or pupil dilation across the two scan sessions to examine whether these effects may be due to changes in engagement or fatigue after treatment. For reaction time, in the depressed group, there was no interaction of time (pretreatment versus 2-month) by session phase (2nd half versus 1st half), and individual differences in change in reaction time did not correlate with change in positive affect. For pupil dilation, in the depressed group, there was again no time-by-session phase interaction, and individual differences in change in pupil dilation did not correlate with change in positive affect. These data suggest that the relationship between changes in sustained nucleus accumbens activity and positive affect is unlikely to be due to changes in engagement or fatigue.
Third, we examined whether the relationship between changes in sustained nucleus accumbens activity and positive affect remained significant after controlling for change in negative affect. When controlling for change in negative affect, the association between change in positive affect and change in nucleus accumbens activity was no longer significant, suggesting that increases in sustained nucleus accumbens activity are associated with both increases in positive affect and decreases in negative affect among patients. (See the online data supplement for additional analyses addressing specificity.)
Change in Sustained Nucleus Accumbens Connectivity
We previously reported (22) that untreated patients have difficulty sustaining connectivity between the nucleus accumbens and the dorsolateral prefrontal cortex when instructed to enhance positive emotion elicited by positive images. In addition, since the nucleus accumbens is involved in processes other than reward (31), and since treatment-related changes in sustained nucleus accumbens activity were associated with changes in both positive and negative affect, it may be that change in nucleus accumbens-prefrontal cortex connectivity constitutes a more sensitive method for examining changes specific to positive affect. We therefore conducted a connectivity analysis (32) to examine whether treatment-related change in nucleus accumbens-prefrontal cortex connectivity is associated with gains in positive affect while controlling for change in negative affect. The analysis revealed a region of the left middle frontal gyrus (peak, x=–22, y=60, z=18; Brodmann’s area 10/46) where variation in sustained nucleus accumbens-middle frontal gyrus connectivity was associated with gains in positive affect (partial correlation [ρr]=0.64; t=3.61, df=18, p=0.002) (Figure 3, Table 2) when controlling for change in negative affect, corrected for multiple comparisons across the whole brain. This relationship was also significant when not controlling for change in negative affect (r=0.43; t=2.13, df=19, p=0.05). In addition, increases in sustained connectivity between the nucleus accumbens and the ventromedial prefrontal cortex were associated with gains in positive affect while controlling for negative affect (ρr=0.78; t=5.44, df=18, p<0.001) (Table 2). Similarly, this relationship was significant when not controlling for change in negative affect (r=0.56; t=2.94, df=19, p=0.008).
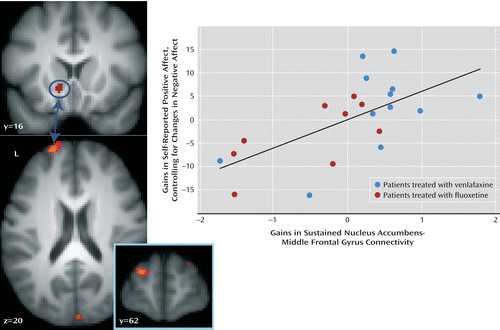
a In the images, increases in sustained nucleus accumbens-middle frontal gyrus (Brodmann’s area 10/46) connectivity are related to gains in self-reported positive affect after controlling for changes in self-reported negative affect. In the scatterplot, the x-axis reflects changes in sustained nucleus accumbens-middle frontal gyrus connectivity from baseline to 2 months (2-month(2nd half – 1st half) – baseline(2nd half – 1st half)), and the y-axis reflects gains in positive affect after controlling for changes in negative affect. In this analysis, ρr=0.64.
| Location (Brodmann’s Area) | Coordinates (x, y, z, in mm) | Cluster Size (Voxels) | Maximum t |
|---|---|---|---|
| Right anterior insula (BA 47) | 36, 22, 0 | 243 | 4.19 |
| Ventromedial prefrontal cortex (BA 11) | –4, 32, –14 | 76 | 4.81 |
| Left middle frontal gyrus (BA 10/46) | –22, 60, 18 | 66 | 3.90 |
As with the univariate analyses reported above, we conducted a control analysis examining whether change in sustained nucleus accumbens-middle frontal gyrus connectivity was uniquely associated with change in positive affect or whether aggregated nucleus accumbens-middle frontal gyrus connectivity across the scan session was additionally associated with change in positive affect (controlling for negative affect). Treatment-induced change in aggregated nucleus accumbens-middle frontal gyrus connectivity was not associated with change in positive affect (controlling for negative affect). We additionally ran a regression model including both change in sustained nucleus accumbens-middle frontal gyrus connectivity and change in aggregated nucleus accumbens-middle frontal gyrus connectivity (in addition to change in negative affect) in the same regression model to examine associations with change in positive affect. This analysis revealed that change in sustained connectivity was uniquely associated with change in positive affect when controlling for negative affect (B=6.25; t=3.51, df=17, p=0.002), whereas change in aggregated connectivity was not associated with change in positive affect. Similarly, with the ventromedial prefrontal cortex cluster, change in aggregated nucleus accumbens-ventromedial prefrontal cortex connectivity was not associated with change in positive affect, controlling for negative affect. We additionally included both change in sustained nucleus accumbens-ventromedial prefrontal cortex connectivity and change in aggregated nucleus accumbens-ventromedial prefrontal cortex connectivity (in addition to change in negative affect) in the same regression model to examine associations with change in positive affect. This analysis revealed that change in sustained connectivity was uniquely associated with change in positive affect when controlling for negative affect (B=13.00; t=5.47, df=17, p<0.001), whereas change in aggregated connectivity was not associated with change in positive affect. This result further supports the notion that examination of treatment-induced changes in sustained nucleus accumbens activity and connectivity capture unique variance in increases in positive affect.
Comparison of Venlafaxine and Fluoxetine
Next, we tested whether the effects we observed were due to differences driven by either one of the medications alone. The correlation between change in sustained nucleus accumbens activity and change in positive affect did not significantly differ between the two medication groups (fluoxetine: r=0.48, N=9; venlafaxine: r=0.50, N=12). The correlation between change in sustained nucleus accumbens-BA46 connectivity and change in positive affect, controlling for change in negative affect, also did not significantly differ for the two medication groups (fluoxetine: r=0.68, N=9; venlafaxine: r=0.54, N=12). Finally, the correlation between change in sustained nucleus accumbens-ventromedial prefrontal cortex connectivity and change in positive affect, controlling for change in negative affect, did not significantly differ between the medication groups (fluoxetine: r=0.72, N=9; venlafaxine: r=0.79, N=12). These results suggest that the mechanism of action in change in positive affect and change in sustained nucleus accumbens activity/fronto-striatal connectivity does not differ between these two antidepressants.
Anhedonic Symptoms
As the PANAS (current state version) is an acute state measure of positive affect, we additionally examined scores on the anhedonia scale of the Mood and Anxiety Symptom Questionnaire as a more traditional measure of anhedonia that asks participants to integrate their emotion over the previous week. The depressed group showed a significant decrease in anhedonic symptoms from baseline to 2 months (t=–2.41, df=20, p=0.026), whereas the comparison group showed no significant change in anhedonic symptoms over this period.
Within the depressed group, there was a significant correlation between change in anhedonic symptoms and change in sustained nucleus accumbens activity (r=–0.46, N=20, p=0.03), such that the patients who showed an increase in ability to sustain repeated engagement of nucleus accumbens activity were those who exhibited the greatest decrease in anhedonic symptoms. However, there was no relationship between change in sustained nucleus accumbens-BA46 connectivity during the positive enhance condition and anhedonic symptoms. Furthermore, including both change in anhedonic symptoms and change in current positive affect, increase in current positive affect was uniquely associated with change in nucleus accumbens-BA46 connectivity (B=0.055; t=2.55, df=18, p=0.02), while change in anhedonic symptoms was not uniquely associated with change in nucleus accumbens-BA46 connectivity. Similarly, we observed no relationship between change in nucleus accumbens-ventromedial prefrontal cortex connectivity during the positive enhance condition and change in anhedonic symptoms. In a simultaneous regression with change in current positive affect and change in anhedonic symptoms, change in current positive affect was uniquely associated with change in nucleus accumbens-ventromedial prefrontal cortex connectivity (B=0.034; t=2.97, df=18, p=0.008), whereas change in anhedonic symptoms was not (see the online data supplement).
Healthy Comparison Subjects
Using the same nucleus accumbens cluster identified in the depressed group, we found that the healthy comparison group showed no significant association between change in sustained nucleus accumbens activity and change in positive affect (r=–0.36, N=14, p=0.21; R2=0.13); note that the relationship in the comparison group was negative, whereas it was positive in the depressed group. Indeed, the strength of this relationship was greater in the depressed group than in the comparison group in the nucleus accumbens (z=2.54, p=0.01). When performing this correlation voxelwise in the comparison group, no region demonstrated a significant association between change in sustained activity and change in daily positive affect (after correcting for multiple comparisons).
With regard to the connectivity analyses presented above for the depressed group, the comparison group showed no significant relationship between change in nucleus accumbens-middle frontal gyrus connectivity and change in positive affect over the 2 months. Similarly, for nucleus accumbens-ventromedial prefrontal cortex connectivity, the comparison group showed no relationship between change in sustained connectivity and change in positive affect.
Discussion
This study provides novel evidence that improvements in daily positive affect in depressed patients after 2 months of antidepressant treatment can be explained in part by increases in patients’ ability to sustain activity in prefrontal-nucleus accumbens circuitry. In particular, we found that patients who exhibited the greatest improvement in sustained nucleus accumbens activity also had the largest gains in self-reported positive affect. Interestingly, the relationship between increases in sustained nucleus accumbens activity and gains in self-reported positive affect was partially accounted for by decreases in negative affect. Thus, change in sustained nucleus accumbens activity appears to be related to improvements in affect generally and may not be specific to positive affect. However, using a connectivity analysis strategy, we found that increases in sustained nucleus accumbens-middle frontal gyrus and nucleus accumbens-ventromedial prefrontal cortex connectivity when attempting to enhance positive emotion were specific to gains in self-reported positive affect, as this relationship was significant when controlling for change in negative affect. We also found that changes in sustained nucleus accumbens activity and nucleus accumbens-middle frontal gyrus as well as nucleus accumbens-ventromedial prefrontal cortex connectivity were associated with change in positive affect above and beyond changes in aggregated nucleus accumbens activity and nucleus accumbens-middle frontal gyrus connectivity. In addition, the fact that change in anhedonic symptoms was associated with change in sustained nucleus accumbens activity but not change in sustained nucleus accumbens-prefrontal cortex connectivity suggests that change in sustained nucleus accumbens activity may reflect both acute and longer-term state positive affect (as the questionnaire we used asks participants to integrate their affect over the previous week), whereas the ability to sustain top-down modulation of the nucleus accumbens by prefrontal cortex structures is particularly relevant for changes in current, acute positive affect following treatment. This provides further evidence that examining the temporal dynamics of striatal activity and fronto-striatal connectivity is important for understanding positive affect in depression.
It is potentially informative that when controlling for changes in negative affect, we observed no unique relationship between increases in sustained nucleus accumbens activity and increases in positive affect. This suggests that change in sustained nucleus accumbens activity may underlie alterations in both positive and negative affect (33). Indeed, a wealth of preclinical research demonstrates that the nucleus accumbens is not engaged solely during reward-related behaviors. In both rodents (34) and humans (35), studies demonstrate that the nucleus accumbens is involved in the processing of aversive stimuli. For example, Berridge et al. (36) reported that injection of a glutamate receptor antagonist in the shell of the nucleus accumbens can elicit either approach or avoidance behaviors, depending on whether the injection is made in the rostral or caudal portion of the nucleus accumbens shell, respectively. Unfortunately, the spatial coarseness of fMRI precludes distinguishing subcomponents of the nucleus accumbens. Furthermore, our findings suggest that the utilization of functional connectivity can provide additional sensitivity when examining changes in affect resulting from treatment (37), such that changes in sustained nucleus accumbens-prefrontal cortex connectivity underlie changes in positive affect in depression uniquely. These data suggest a role for connectivity analyses in helping to further uncover alterations in the pathophysiology of mood disorders with treatment.
Some of the densest direct connections between the ventral striatum and the prefrontal cortex lie within the medial portion of the prefrontal cortex (33, 38), including both the ventromedial prefrontal cortex and the middle frontal gyrus cluster identified in the connectivity analysis. These regions of the prefrontal cortex provide glutamatergic innervation to mostly GABA-ergic medium spiny neurons within the ventral striatum (33). This suggests that it is the afferents of the prefrontal cortex that affect the top-down regulation of affect. While the fMRI task we used does not allow dissociation of the relative contributions of the two prefrontal cortex clusters to changes in positive affect, speculation can be made based on the literature. Given the well-established role of the ventromedial prefrontal cortex in stimulus valuation and more dorsolateral areas of the prefrontal cortex in cognitive control, it may be that changes in sustained nucleus accumbens-ventromedial prefrontal cortex connectivity are related to changes in the evaluation of appetitive stimuli over time, whereas changes in sustained nucleus accumbens-middle frontal gyrus connectivity may underlie the effortful increasing of positive affect required in the experimental paradigm. Further studies, both in human and animal models, are required to elucidate the exact mechanisms by which interactions between the nucleus accumbens and prefrontal cortex underlie these phenomena.
Although the use of two separate drug treatments in our study constitutes a potential limitation, we found that controlling for drug treatment did not attenuate any of the effects, and the correlations were not significantly different from one another when run for each group separately. Furthermore, evidence suggests that fluoxetine and venlafaxine have similar striatal serotonin occupancy and mechanism of action (39). Meyer and colleagues (39), using positron emission tomography to assess 5-HT binding potential in the striatum in human subjects across five different antidepressants (citalopram, fluoxetine, sertraline, paroxetine, and venlafaxine), found that at therapeutic doses, the five agents had similar 5-HT binding potential specifically in the striatum. This suggests that there is a strong overlap in mechanism of action in the two treatments used in our study. Nonetheless, future research should seek to separate the distinct mechanism of action for all depression treatments (see reference 40, for example) and their effects on specific affective symptoms.
In summary, our findings suggest that sustained nucleus accumbens activity and fronto-striatal connectivity when attempting to enhance positive emotion track self-reported daily positive affect. Furthermore, individual differences in the magnitude of change in sustained nucleus accumbens activity and fronto-striatal connectivity correlate with gains in positive affect after antidepressant treatment. These findings are consistent with the hypothesis that reductions in state positive affect associated with depression may result in part from a loss of the ability to sustain nucleus accumbens activity and fronto-striatal connectivity over time. These findings underscore the need for studies to examine the mechanisms underlying the temporal dynamics of positive and negative affect in depression (41) and how these mechanisms are affected by antidepressant treatment.
1 : Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion 2007; 7:182–191Crossref, Medline, Google Scholar
2 : Looking at facial expressions: dysphoria and facial EMG. Biol Psychol 2002; 60:79–90Crossref, Medline, Google Scholar
3 : Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009; 166:702–710Link, Google Scholar
4 : Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 2006; 163:1784–1790Link, Google Scholar
5 : The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry 2005; 58:843–853Crossref, Medline, Google Scholar
6 : Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry 2010; 67:380–387Crossref, Medline, Google Scholar
7 : Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol Psychiatry 2006; 60:974–986Crossref, Medline, Google Scholar
8 : Neural responses to monetary incentives in major depression. Biol Psychiatry 2008; 63:686–692Crossref, Medline, Google Scholar
9 : Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport 2003; 14:177–182Crossref, Medline, Google Scholar
10 : Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry 2007; 12:767–775Crossref, Google Scholar
11 : Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety 2010; 27:859–863Crossref, Medline, Google Scholar
12 : Early- and late-onset startle modulation in unipolar depression. Psychophysiology 2004; 41:433–440Crossref, Medline, Google Scholar
13 : Neural systems of positive affect: relevance to understanding child and adolescent depression? Dev Psychopathol 2005; 17:827–850Crossref, Medline, Google Scholar
14 : Reduced facial expression and social context in major depression: discrepancies between facial muscle activity and self-reported emotion. Psychiatry Res 2000; 95:157–167Crossref, Medline, Google Scholar
15 : A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry 2005; 58:495–503Crossref, Medline, Google Scholar
16 : A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 2005; 57:201–209Crossref, Medline, Google Scholar
17 : Facial expressiveness in patients with schizophrenia compared to depressed patients and nonpatient comparison subjects. Am J Psychiatry 2005; 162:92–101Link, Google Scholar
18 : Anhedonia. Am J Psychiatry 1922; 2:87–103Link, Google Scholar
19 : Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 2008; 43:76–87Crossref, Medline, Google Scholar
20 : Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1045–1052Crossref, Medline, Google Scholar
21 : Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry 2011; 70:395–399Crossref, Medline, Google Scholar
22 : Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci USA 2009; 106:22445–22450Crossref, Medline, Google Scholar
23 : Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 2011; 35:537–555Crossref, Medline, Google Scholar
24 : Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology 2000; 37:515–522Crossref, Medline, Google Scholar
25 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
26 : Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54:1063–1070Crossref, Medline, Google Scholar
27 : Testing a tripartite model, I: evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 1995; 104:3–14Crossref, Medline, Google Scholar
28 : Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 2007; 27:8877–8884Crossref, Medline, Google Scholar
29 : Reduced right ventrolateral prefrontal cortex activity while inhibiting positive affect is associated with improvement in hedonic capacity after 8 weeks of antidepressant treatment in major depressive disorder. Biol Psychiatry 2011; 70:962–968Crossref, Medline, Google Scholar
30 Lang PJ, Bradley MM, Cuthbert BN: International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual (Technical Report A-6). Gainesville, University of Florida, Center for Emotion and Attention, 2005.Google Scholar
31 : Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010; 68:815–834Crossref, Medline, Google Scholar
32 : Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 2004; 23:752–763Crossref, Medline, Google Scholar
33 : The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35:4–26Crossref, Medline, Google Scholar
34 : Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 2005; 45:587–597Crossref, Medline, Google Scholar
35 : Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 2003; 40:1251–1257Crossref, Medline, Google Scholar
36 : Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci 2003; 17:2187–2200Crossref, Medline, Google Scholar
37 : Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry 2007; 61:729–730Crossref, Medline, Google Scholar
38 : Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci 2006; 26:8368–8376Crossref, Medline, Google Scholar
39 : Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry 2004; 161:826–835Link, Google Scholar
40 : Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci 2008; 9:788–796Crossref, Medline, Google Scholar
41 : Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology 2003; 40:655–665Crossref, Medline, Google Scholar



