Lack of Ventral Striatal Response to Positive Stimuli in Depressed Versus Normal Subjects
Abstract
Objective: Most of the functional neuroimaging studies of depression have focused primarily on the resting state or responses to negatively valenced stimuli. However, depression consists not only of an accentuation of negative affective processing but of an inability to experience pleasure or positive motivation. The authors tested the hypothesis that depressed subjects would show less activation than healthy comparison subjects, in response to positive stimuli, in ventral striatal regions associated with processing of reward and positive stimuli. Method: Positive, negative, and neutral words were presented to 10 unmedicated depressed patients and 12 healthy comparison subjects in the context of a 3T functional magnetic resonance imaging (MRI) paradigm. Image processing and analysis were performed using statistical parametric mapping with a mixed-effects model. Significant differences in neural responses were assessed, examining group, condition, and interaction effects of interest within the context of a general linear model. Results: Relative to comparison subjects, depressed patients demonstrated significantly less bilateral ventral striatal activation to positive stimuli, correlating with decreased interest/pleasure in and performance of activities. They also displayed decreased activation to positive stimuli in a dorsomedial frontal region associated with processing of self-related stimuli. Responses of depressed subjects to negative stimuli were consistent with the growing literature on frontolimbic dysfunction in depression. Conclusions: This finding 1) supports a pathophysiological model of depression that includes reward/motivational pathway dysfunction, 2) suggests a contributing neural substrate of the inability to experience pleasure or engage in rewarding activities, 3) provides greater specification of abnormalities of basal ganglia function in depression, and 4) may help guide treatment approaches.
Most of the previous functional neuroimaging studies of depression have focused, in their design or reporting of results, primarily on the resting state (1 – 3) or on responses to negative mood induction or other negatively valenced stimuli (4 – 6) at various stages of treatment. These approaches are valuable and have led to the development of neural circuit models of the disorder. However, in addition to an accentuation of negative affective processing, depression consists of an inability to experience pleasure or positive motivation. While abnormalities in the processing of positive stimuli by depressed subjects have been demonstrated (7 , 8) , less has been done to investigate the neural correlates of this core component of the illness.
Studies in both animals and humans demonstrate a central role for the ventral striatum—particularly the nucleus accumbens—a region receiving dopaminergic projections from the ventral tegmentum, in behavioral responses to, anticipation of, and/or monitoring of errors in the prediction of reward (9 – 11) . While classically studied in the context of drug addiction (12) or in response to stimuli closely associated with reward per se(9) , this role appears to extend to a broad range of positive stimuli (13) , including verbal stimuli (14) , which is consistent with the conceptualization of happiness as an approach emotion (15) . The nucleus accumbens also appears to respond to the emotional intensity and self-relatedness of a variety of stimuli, independent of their valence (16) , with both positive and negative valences possibly processed along a rostrocaudal gradient (17) .
Multiple neuroimaging studies of depression have demonstrated abnormalities of or changes in basal ganglia function with treatment, but generally these findings have not been specific to the ventral striatum and/or to positive or rewarding stimuli (e.g., 18 – 22) . Conversely, a number of functional neuroimaging studies of depression have included positive stimuli (23 – 28) but have not reported ventral striatal findings. Two previous studies have touched upon the relation between ventral or medial striatal activity and pleasure or reward in depressed subjects, although without specifically addressing ventral striatal responses to positive stimuli. Dunn and colleagues (29) found a correlation between a psychomotor-anhedonia symptom cluster and lower metabolism in the anteroventral caudate/putamen, among other regions, in depressed subjects performing an (nonemotional) auditory continuous performance task. In another study, Elliott and colleagues (24) reported significantly less activation in depressed patients relative to healthy comparison subjects to the presence of task-related feedback, regardless of its valence, in bilateral medial caudate nuclei and the tail of the left caudate, interpreted as differential activation associated with the expectation of reward. More recently, Surguladze and colleagues (30) found that depressed patients lack the linear increase in right putamen response to facial expressions of increasing happiness observed in healthy comparison subjects.
In our study, we used functional magnetic resonance imaging (fMRI) to examine neural responses to positive, negative, and neutral words in depressed patients relative to healthy comparison subjects. We hypothesized that depressed subjects would demonstrate decreased ventral striatal, particularly nucleus accumbens, activation in response to positive stimuli. While our study focused on the positive condition, we also predicted, based on the literature (31 , 32) , intergroup differences in the negative condition in the following regions: medial and lateral prefrontal cortex, anterior cingulate, amygdala and hippocampus.
Method
Subjects
Participants were 10 unmedicated subjects with DSM-IV major depression (nine women and one man; mean age=35.6 years, range=26–49; eight right-handed, two left-handed) and 12 healthy comparison subjects (seven women and five men; mean age=32.0 years, range=23–53; all right-handed). All subjects completed at least some education beyond high school, with level of education matched across subject groups. Apart from depression in the patient group, all participants were free of major psychiatric diagnoses, substance abuse, and significant neurological or medical disorders. After full explanation of the study to the participants, written informed consent was obtained. Hamilton Rating Scale for Depression (33) scores were obtained on the same day as the scan, before (but not immediately prior to) the imaging session.
Stimuli
Stimuli consisted of 24 positive words, 24 negative words, and 24 neutral words (adjectives, nouns, and verbs) balanced across categories for frequency, length and part of speech, with the exception that within the neutral list, verbs were substituted for adjectives. This was done because adjectives, which are important components of the valenced categories, are by nature generally not free of valence. Verbs were substituted rather than nouns, since their imageability is more similar to that of adjectives. Stimuli were designed to be relevant to depressive and counter-depressive themes, as defined by the literature and clinical experience, and were rated for suitability by a panel of three experienced clinicians. They were based on a similar list of words that were piloted on 34 healthy subjects who rated the three word types as significantly different in valence (p<0.001) and rated positive and negative words as not significantly different in intensity (p=0.26). Examples of such words are as follows: success, admired, heroic (positive words); worthless, bleak, burden (negative words); transfer, trunks, fasten (neutral words).
Paradigm
The three word types were visually presented on an MRI compatible Sharp LCD screen within a block design (six words per block, four blocks per valence), with blocks pseudorandomly balanced to control for order and time effects. Each word appeared for 2 seconds, followed by an interstimulus interval uniformly jittered around an average of 2.8 seconds for a total of 28.8 seconds per block. Each block was followed by 24 seconds of rest, with the paradigm as a whole preceded and followed by two additional 12-second rest periods. Stimulus presentation and response collection were performed within the Integrated Functional Imaging System SA/E-Prime environment (MRI Devices Corporation, Waukesha, Wisc.; Psychology Software Tools, Inc., Pittsburgh, Pa.). Subjects were instructed to read each word silently and then immediately press a button under their right index finger. During rest periods, they were instructed to look at a dash at the center of the screen, with their minds either blank or floating freely.
Immediately after imaging was completed and subjects were removed from the scanner, they were given a list of words consisting of the 72 stimuli seen during scanning (targets) and 36 other words (distractors), with which the targets were randomly interspersed. Distractor words were divided equally into positive, negative, and neutral categories and balanced for the same qualities as the target words. The subjects were instructed to read each word and to circle those words that they believed they had seen in the scanner. Following completion of this particular task, subjects were asked to rate the emotional valence and intensity of each word on a scale of –3 to +3.
Image Acquisition
All image data were acquired with a GE Signa 3 Tesla MRI scanner (max gradient strength 40mT/m, max gradient slew rate 150T/m/s, General Electric Company, Waukesha, Wisc.) using blood-oxygen-level-dependent fMRI, which measures hemodynamic and oxygenation changes associated with localized neuronal activity in the brain (34) . After shimming to maximize homogeneity, a series of fMRI scans was collected using gradient echo-planar imaging (TR=1200; TE=30; flip angle=70°; field of view=240 mm;15 slices; 5 mm thickness with 1 mm interslice space; matrix=64x64), with a single-shot version of a z-shimming algorithm to reduce susceptibility artifact at the base of the brain (35) . Echoplanar images were acquired in the axial plane parallel with the anterior commissure-posterior commissure (AC-PC) plane. The first five volumes of each session were discarded. A reference T1-weighted anatomical image with the same slice placement and thickness and a matrix of 256x256 was acquired immediately preceding the echo-planar imaging acquisition. A high-resolution T1-weighted anatomical image using a spoiled-gradient sequence with a resolution of 0.9375x0.9375x1.5 mm 3 was also acquired.
Image Processing and Data Analysis
SPM99 software (Wellcome Department of Imaging Neuroscience) was used for processing the data (36) , which included manual AC-PC reorientation of all anatomical and echo-planar images; realignment of functional echo-planar images based on intracranial voxels to correct for slight head movement between scans; coregistration of functional echo-planar images to the corresponding high-resolution anatomical image based on the transformation of the reference anatomical image to the latter for each individual subject; stereotactic normalization to the standardized coordinate space of Talairach and Tournoux (Montreal Neurological Institute [MNI] average of 152 T1 brain scans) based on the high-resolution anatomical image; and spatial smoothing of the normalized echo-planar images with an isotropic Gaussian kernel (7.5 mm, full width at half maximum). To perform image data analyses, a whole-brain voxel-by-voxel multiple linear regression model was employed at the individual subject level (37) . The temporal global fluctuation estimated as the mean intensity of intracranial voxels of each volume was removed through proportional scaling. The individual model comprised the regressor of interest (which consisted of the stimulus onset times convolved with a prototypical hemodynamic response function) and the covariates of no interest (which consisted of the first-order temporal derivative of the regressor of interest, global and physiological fluctuations [38] , realignment parameters, and scanning periods [39] ). An autoregressive model was also incorporated to accommodate the intrasubject temporal correlation pattern in residual. Effects at every brain voxel were estimated by a restricted maximum likelihood estimator using an expectation maximization algorithm, and regionally specific effects were then compared using linear contrasts. The resulting set of contrast-effect images and their corresponding standard deviation images was then entered into a set of second-stage group-level models.
For whole-brain group analyses, we used a mixed-effects model, which accounted for intra- and intersubject variabilities and allowed population-based inferences to be drawn (37) . Age, gender, and handedness were used as covariates of no interest in an analysis of covariance setting. A voxel-wise inference at the group level was then drawn according to Gaussian random field theory, focusing on the ventral striatum, considered as those regions of the nucleus accumbens, head of the caudate, and putamen where z≤–3mm and y≥0mm in MNI space. For the contrast of interest, a region of interest analysis was performed for the bilateral ventral striatum using a mask of the region as defined above. For the a priori regions of interest, the comparisons based on one-tailed t tests were considered significant if their initial voxel-wise p values were less than 0.001 and p<0.05 corrected for multiple comparisons within the bilateral nucleus accumbens, with a search volume of 1.8 cm 3 . For regions outside the ventral striatum, the comparisons were considered significant if their initial voxel-wise p values were less than 0.001, with a spatial extent of at least 50 mm 3 .
A set of supplementary analyses, based on the group level models, was performed to aid in the interpretation of the primary finding. A correlation analysis was performed to determine the association between activity in the ventral striatum and the Hamilton depression scale measure that quantifies interest and pleasure in, and subsequent performance of, activities. Analyses of behavioral data were performed using Mann-Whitney tests.
Results
Behavioral Data
Affective ratings
Within the group of healthy subjects, the three word types were rated as significantly different in valence (negative versus neutral: Z=–3.10, p<0.001; negative versus positive: Z=–3.03, p=0.001; positive versus neutral: Z=3.02, p=0.001), and the positive and negative words were rated as not significantly different in intensity (Z=1.06, p=0.29). Depressed subjects did not differ significantly from healthy comparison subjects on their ratings of positive or neutral words but did rate negative words as significantly more negative (Z=–2.74, p=0.003).
Reaction times
Within the group of normal subjects, reaction time was significantly faster for positive versus neutral words (Z=–2.85; p=0.003). Within the depressed group, there was no significant difference in reaction time across word categories. Depressed subjects did not show significantly different reaction times than healthy comparison subjects for any category of words.
Recognition memory
Recognition memory performance was calculated by adjusting the percentage of positive responses to target stimuli ( p ) by the rate of positive responses to distractor stimuli ( fp ) using the following formula: p′=(p–fp)/(1–fp). Within the group of healthy subjects, there were no significant differences in recognition memory performance among word types. Across groups, there were no differences in performance on positive words, but depressed subjects did recognize negative words at a significantly higher rate than healthy comparison subjects (Z=1.98, p=0.02). Within the depressed group, negative words were recognized at significantly higher rates than both neutral (Z=1.73, p=0.04) and positive (Z=1.73, p=0.04) words.
fMRI Data
Between-group contrasts revealed significantly less activation to positive stimuli in depressed patients relative to healthy subjects in bilateral ventral striatal regions, including ventral head of caudate and putamen and nucleus accumbens, with the left contrast maximum falling in the region of the nucleus accumbens (right:[15,6, –3], Z=–3.66, voxel-wise p=0.0001, p<0.05 [corrected]; left:[–15,15, –6], Z=–3.52,voxel-wise p=0.0002, p<0.05 [corrected]) ( Figure 1 A). Within-group analyses (reported at the statistical maxima of the between-group findings) revealed that these findings were due to a decrease in activation to positive stimuli in depressed subjects (right: [15, 6, –3], Z= –1.42, p=0.08; left: [–15, 15, –6], Z=–3.23, p=0.004), coupled with an increase in healthy comparison subjects (right:[15, 6, –3], Z=3.75, p<0.0001; left:[–15, 15, –6], z=1.82, p=0.04) ( Figure 1 B). Decreased activity in bilateral ventral striatum was also found in depressed patients relative to healthy comparison subjects in response to positive versus neutral stimuli (right:[15, 9, –3], Z=–2.43,voxel-wise p=0.008; left:[–18, 9, –6], Z=–3.23, voxel-wise p=0.0006, p<0.05 [corrected]). No significant between-group differences in ventral striatal activation were seen in any other contrast. The fMRI data are available as a supplement in the online version of this article.
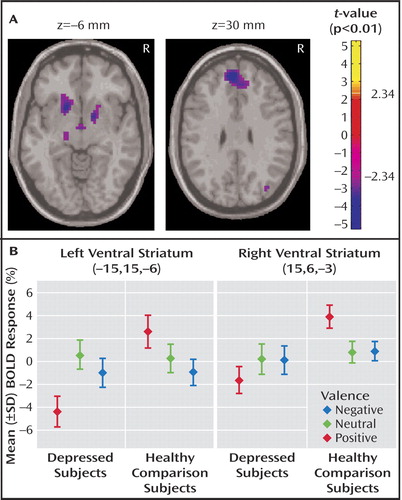
a In part A, axial slices reveal significant decreases in activation to positive stimuli in depressed patients compared with healthy subjects; left image: bilateral ventral striatum, with the left contrast maximum falling in the region of the nucleus accumbens (hypothalamic and thalamic decreases are also visible); right image: left dorsomedial frontal gyrus (Brodmann’s area 9). In part B, within-group, by condition barplots at the statistical maxima of the bilateral ventral striatal findings in the positive between-group condition, revealed these findings to be due to a decrease in activation to positive stimuli in depressed subjects coupled with an increase in healthy comparison subjects.
In order to determine whether the differential response to positive stimuli might be occurring in the setting of differential regional baseline activity, a between-group comparison of regional (relative to global) activity during rest (at the statistical maxima of the between-group finding for the positive condition) was performed by sampling two different time points in the experiment before subjects were exposed to valenced stimuli. The two time points are as follows: 1) before the blood-oxygen-level-dependent response to the initial stimulus began to emerge and 2) at the end of the 24-second rest period following the first neutral presentation block. No significant between-group differences were seen for either comparison (Time point 1: right: [15, 6, –3], Z=–0.27, p=0.40; left: [–15, 15, –6], Z=–0.83, p=0.20. Time point 2: right: [15, 6, –3], Z=–0.40, p=0.35; left: [–15, 15, –6], Z=–0.83, p=0.20). While the analysis does not constitute an integrated measure of baseline activity, it represents a relevant sampling of baseline activity in these subjects before valenced stimuli were delivered, which is useful for interpreting the main finding.
To determine whether the differences displayed between depressed patients and comparison subjects in positive blocks represented prolonged processing of negative stimuli (27 , 40) rather than a differential response to positive stimuli, we examined each block of positive stimuli individually to determine whether a lack of ventral striatal activation in depressed patients, compared with healthy subjects, was observed predominantly following blocks of negative stimuli. No such pattern was revealed. Similarly, because decreases in basal ganglia volumes have been reported in depressed patients (41) , a voxel-based morphometric comparison (42) of depressed patients relative to comparison subjects was performed. This revealed no significant differences in gray matter volume in ventral striatal regions (right: 15, 6, –3 [Z=–1.01, p=0.16]; left: –15, 15, –6 [Z=–0.33, p=0.37]) that might account for our findings.
A correlation analysis was performed within the depressed group to investigate the association between the blood-oxygen-level-dependent activation levels in depressed subjects in the positive condition and their day-of-scan scores on question 7 of the Hamilton depression scale, which assesses interest and pleasure in and subsequent performance of activities. A significant negative correlation was observed, with those subjects who had higher scores (i.e., less interest/pleasure/performance of activities) showing less activation in bilateral ventral striatal regions (right: 27, 6, –9 [Z=–2.20, p=0.01]; left: –18, 0, –6 [Z= –1.84, p=0.03]). In areas outside the region of interest (at the level of p<0.001), negative correlations with question 7 of the Hamilton depression scale were also found in the left dorsolateral prefrontal cortex (–33, 60, 18) and right frontal operculum (60, 9, 12), while a positive correlation was found in the left posterior cerebellum (–24, –72, –45).
Between-group differences outside the ventral striatum for positive, negative, neutral, positive versus neutral and negative versus neutral contrasts are shown in the Table (available in the online version of this article as a data supplement) and will be commented upon in the discussion section.
Discussion
The key finding of this study is the failure of depressed patients relative to healthy comparison subjects to activate ventral striatal regions in response to positive stimuli. This relative decrease is specific to the positive (as well as positive versus neutral) condition and is not due to medication effects. In addition, this decrease does not appear to reflect differential baseline activity or prolonged processing of negative stimuli by the depressed patients (27 , 40) , nor does it appear to reflect a failure to process the positive words, since there were no between-group differences in recognition performance for positive words. This relative decrease is also unlikely to be secondary to volume effects, given its condition-specific nature as well as the lack of significant between-group differences in ventral striatal gray matter volume found on voxel-based morphometric comparison.
A significant correlation was found between depressed subjects’ failure of activation to positive stimuli and their same-day scores on question 7 of the Hamilton depression scale, such that those with the least ventral striatal activation reported the least interest and pleasure in, and subsequent performance of activities. At the same time, behavioral data revealed that depressed subjects failed to display the significantly faster reaction time to positive versus neutral words shown by healthy comparison subjects, yet displayed no significant differences from healthy comparison subjects on either affective rating or recognition of positive words. Taken together, these correlational and behavioral findings suggest that the paucity of ventral striatal activation observed in the depressed patients relates more to the translation of motivational information into behavior than to affective evaluation or encoding per se, which is consistent with a model of the nucleus accumbens as the limbic-motor interface (43) .
Decreased activation in response to positive stimuli in depressed patients relative to comparison subjects was also found in regions outside the ventral striatum, including the (left) dorsomedial frontal gyrus (Brodmann’s area 9), a region associated with social cognition (44) and self-reflection (45) (see Figure 1A ). In healthy subjects, both this region and the nucleus accumbens have been shown to display increased activation to stimuli perceived as self-related (16 , 46) . In previous studies, dorsomedial frontal abnormalities have been found in depressed patients (31 , 32) , although not, to our knowledge, specifically in response to a positively valenced probe. In our study, decreased parahippocampal gyrus activation was also found in depressed subjects in response to positive as well as negative stimuli, an interesting finding in light of recent research on hippocampal abnormalities in depression, and their possible relevance to its pathophysiology and treatment (47) . Depressed patients also displayed decreased activation to positive stimuli in left dorsal head-of-caudate and bilateral dorsal thalamus, which is consistent with the literature and the role of frontal-subcortical-thalamic circuits in the regulation of emotion (48 , 49) . Finally, decreased activation to positive stimuli was found in depressed patients at a lower level of significance in the hypothalamus, a region associated with lower-level drive and motivational processing (3, –9, –6 [Z=–2.89, p=0.002]).
In the negative condition—in addition to the decreases in bilateral posterior paraphippocampal gyri previously discussed—depressed patients, relative to comparison subjects, displayed decreases in a region of right middle frontal gyrus (Brodmann’s area 9), which is consistent with earlier findings (31) , and in the left insula/claustrum, posterior to regions previously implicated in depression (49) . Increased activation to negative stimuli was found in depressed patients in midline subgenual anterior cingulate, which is consistent with the literature (49) , and in the right orbital gyrus, anterior to regions previously implicated in depression (31 , 32) .
In the positive versus neutral and negative versus neutral contrasts, depressed patients showed decreased activation to positive versus neutral stimuli and both increased and decreased activation to negative stimuli and negative versus neutral stimuli in right-sided regions involved in visual processing, suggesting a differential impact of valenced emotional content at the level of perceptual function. In the negative versus neutral contrast, depressed patients also displayed decreased activation in left extended amygdala. This decrease was due to a double dissociation wherein healthy subjects displayed increased activation to negative stimuli and decreased activation to neutral stimuli, while depressed patients showed the opposite pattern (reacting to neutral words as the comparison subjects reacted to negative words and to negative words as comparison subjects reacted to neutral words). This is consistent with the clinical/cognitive phenomenology of depression in which patients negatively interpret phenomena viewed by others as neutral. Depressed subjects also displayed decreased activation in both the positive versus neutral contrasts and negative versus neutral contrasts in the right superior temporal sulcus, which is a region associated with social processing (50) .
Our study provides greater specification of the potential localization and significance of previous reports, in depressed patients, of abnormalities of (19 , 21 , 24) or changes in (18 , 20) basal ganglia function with treatment, as well as the association of such abnormalities with anhedonia (29) . Along with the recent study by Surguladze and colleagues (30) , our study suggests that depressed patients lack the striatal activation to positive stimuli found in healthy subjects (13) , and it further correlates decreased ventral striatal response with a lack of interest and pleasure in and subsequent performance of activities. A variety of reasons may be posited for the absence of prior reports of this finding, despite the inclusion of positive stimuli in a number of previous functional neuroimaging studies of depression (23 – 28) . These include differences in a priori regions and comparisons of interest, modality and content of stimuli, task demands (including presence of a motor component), and image acquisition parameters (including those associated with susceptibility artifact at the base of the brain).
Our study has a number of limitations. The uneven distribution of men and women in our patient and comparison groups could be a factor in the results, although this is unlikely given that gender was used as a covariate of no interest in all between-group analyses and that our finding held up in a single-sex subanalysis. Another possible confound arises from the fact that neutral and valenced words were not completely balanced for part of speech, as described previously, or for imageability. This also appears unlikely, given the presence of our finding in the between-group positive (not only positive versus neutral) condition. Finally, although analysis of our data was performed with a mixed-effects model, which takes subject-by-subject variability into account and allows population-based inferences to be drawn (51) , we feel that additional subjects will be helpful to further assess this finding and its generalizability.
In conclusion, this study confirms our hypothesis of decreased activation to positive stimuli in depressed patients versus healthy subjects in ventral striatal regions, including the nucleus accumbens. Our findings support a pathophysiological model of depression that includes reward/motivational pathway dysfunction (32 , 52 , 53) and suggest a possible contributing neural substrate of the inability to experience pleasure and engage in rewarding activities. This line of investigation is thus likely to enhance our understanding of depression and to encourage further studies targeting ventral striatal regions for therapeutic modulation of affective symptoms (54 , 55) .
1. Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ: Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychol Med 1993; 23:579–590Google Scholar
2. Drevets WC: Functional neuroimaging studies of depression: the anatomy of melancholia. Ann Rev Med 1998; 49:341–361Google Scholar
3. Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT: Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997; 8:1057–1061Google Scholar
4. Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P: Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry 2002; 159:1830–1840Google Scholar
5. Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET: Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 2004; 61:877–889Google Scholar
6. Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, Stip E: The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. Neuroreport 1998; 9:3253–3258Google Scholar
7. Breslow R, Kocsis J, Belkin B: Contribution of the depressive perspective to memory function in depression. Am J Psychiatry 1981; 138:227–230Google Scholar
8. Dozois DJA, Dobson KS: Information processing and cognitive organization in unipolar depression: specificity and comorbidity issues. J Abnorm Psychol 2001; 110:236–246Google Scholar
9. Knutson B, Fong GW, Adams CM, Varner JL, Hommer D: Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 2001; 12:3683–3687Google Scholar
10. McGinty JF: Advancing from the ventral striatum to the extended amygdala: implications for neuropsychiatry and drug abuse. Ann N Y Acad Sci 1999; 877:xii–xvGoogle Scholar
11. McClure SM, York MK, Montague PR: The neural substrates of reward processing in humans: the modern role of fMRI. Neuroscientist 2004; 10:260–268Google Scholar
12. Koob GF: The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci 1999; 877:445–460Google Scholar
13. Phan KL, Wager T, Taylor SF, Liberzon I: Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002; 16:331–348Google Scholar
14. Hamann S, Mao H: Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport 2002; 13:15–19Google Scholar
15. Davidson RJ, Ekman P, Saron C, Senulis J, Friesen WV: Approach/withdrawal and cerebral asymmetry: emotional expression and brain physiology. J Pers Soc Psychol 1990; 58:330–341Google Scholar
16. Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I: Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage 2004; 21:768–780Google Scholar
17. Reynolds SM, Berridge KC: Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci 2003; 17:2187–2200Google Scholar
18. Goodwin GM, Austin MP, Dougall N, Ross M, Murray C, O’Carroll RE, Moffoot A, Prentice N, Ebmeier KP: State changes in brain activity shown by the uptake of 99mTc-exametazime with single photon emission tomography in major depression before and after treatment. J Affect Disord 1993; 29:243–253Google Scholar
19. Baxter LR Jr, Phelps ME, Mazziotta JC, Schwartz JM, Gerner RH, Selin CE, Sumida RM: Cerebral metabolic rates for glucose in mood disorders: studies with positron emission tomography and fluorodeoxyglucose F 18. Arch Gen Psychiatry 1985; 42:441–447Google Scholar
20. Martin SD, Martin E, Rai SS, Richardson MA, Royall R: Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Arch Gen Psychiatry 2001; 58:641–648Google Scholar
21. Mayberg HS, Lewis PJ, Regenold W, Wagner HN Jr: Paralimbic hypoperfusion in unipolar depression. J Nuclear Med 1994; 35:929–934Google Scholar
22. Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC: Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry 2004; 61:765–773Google Scholar
23. Davidson RJ, Irwin W, Anderle MJ, Kalin NH: The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 2003; 160:64–75Google Scholar
24. Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ: Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol Med 1998; 28:559–571Google Scholar
25. Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ: The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry 2002; 59:597–604Google Scholar
26. Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA: Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 2001; 50:651–658Google Scholar
27. Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS: Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 2002; 51:693–707Google Scholar
28. Mitterschiffthaler MT, Kumari V, Malhi GS, Brown RG, Giampietro VP, Brammer MJ, et al: Neural response to pleasant stimuli in anhedonia: an fMRI study. Neuroreport 2003; 14:177–182Google Scholar
29. Dunn RT, Kimbrell TA, Ketter TA, Frye MA, Willis MW, Luckenbaugh DA, Post RM: Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol Psychiatry 2002; 51:387–399Google Scholar
30. Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML: A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 2005; 57:201–209Google Scholar
31. Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S: Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 2004; 22:409–418Google Scholar
32. Drevets WC: Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol 2001; 11:240–249Google Scholar
33. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Google Scholar
34. Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K: Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging: a comparison of signal characteristics with a biophysical model. Biophys J 1993; 64:803–812Google Scholar
35. Gu H, Feng H, Zhan W, Xu S, Silbersweig DA, Stern E, Yang Y: Single-shot interleaved z-shim EPI with optimized compensation for signal losses due to susceptibility-induced field inhomogeneity at 3 T. Neuroimage 2002; 17:1358–1364Google Scholar
36. Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S, Friston K, Frith C, Dolan R, Price C (eds): Human Brain Function, Second Edition. San Diego, Elsevier Academic Press, 2004Google Scholar
37. Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC: A general statistical analysis for fMRI data. Neuroimage 2002; 15:1–15Google Scholar
38. Frank LR, Buxton RB, Wong EC: Estimation of respiration-induced noise fluctuations from undersampled multislice fMRI data. Mag Reson Med 2001; 45:635–644Google Scholar
39. McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RS, Holmes AP: Variability in fMRI: an examination of intersession differences. Neuroimage 2000; 11:708–734Google Scholar
40. Garrett AS, Maddock RJ: Time course of the subjective emotional response to aversive pictures: relevance to fMRI studies. Psychiatry Res 2001; 108:39–48Google Scholar
41. Beyer JL, Krishnan KR: Volumetric brain imaging findings in mood disorders. Bipolar Disord 2002; 4:89–104Google Scholar
42. Ashburner J, Friston KJ: Voxel-based morphometry–the methods. Neuroimage 2000; 11:805–821Google Scholar
43. Mogenson GJ, Jones DL, Yim CY: From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 1980; 14:69–97Google Scholar
44. Mitchell JP, Macrae CN, Banaji MR: Encoding-specific effects of social cognition on the neural correlates of subsequent memory. J Neurosci 2004; 24:4912–4917Google Scholar
45. Gusnard DA, Akbudak E, Shulman GL, Raichle ME: Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 2001; 98:4259–4264Google Scholar
46. Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, Mayberg H: In search of the emotional self: an fMRI study using positive and negative emotional words. Am J Psychiatry 2003; 160:1938–1945Google Scholar
47. Campbell S, Macqueen G: The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004; 29:417–426Google Scholar
48. Vataja R, Leppavuori A, Pohjasvaara T, Mantyla R, Aronen HJ, Salonen O, Kaste M, Erkinjuntti T: Poststroke depression and lesion location revisited. J Neuropsychiatry Clin Neurosci 2004; 16:156–162Google Scholar
49. Drevets WC: Neuroimaging studies of mood disorders. Biol Psychiatry 2000; 48:813–829Google Scholar
50. Gallagher HL, Frith CD: Functional imaging of “theory of mind.” Trend Cog Sci 2003; 7:77–83Google Scholar
51. Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ: Multisubject fMRI studies and conjunction analyses. Neuroimage 1999; 10:385–396Google Scholar
52. Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM: Neurobiology of depression. Neuron 2002; 34:13–25Google Scholar
53. Naranjo CA, Tremblay LK, Busto UE: The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry 2001; 25:781–823Google Scholar
54. De La Garza R 2nd, Jentsch JD, Verrico CD, Roth RH: Adaptation of monoaminergic responses to phencyclidine in nucleus accumbens and prefrontal cortex following repeated treatment with fluoxetine or imipramine. Brain Res 2002; 958:20–27Google Scholar
55. Zangen A, Hyodo K: Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport 2002; 13:2401–2405Google Scholar



