Real-Time Electronic Ambulatory Monitoring of Substance Use and Symptom Expression in Schizophrenia
Abstract
Objective:
Despite evidence demonstrating elevated comorbidity between schizophrenia and substance use disorders, the underlying mechanisms of association remain poorly understood. The brief time intervals that characterize interactions between substance use and psychotic symptoms in daily life are inaccessible to standard research protocols. The authors used electronic personal digital assistants (PDAs) to examine the temporal association of diverse forms of substance use with psychotic symptoms and psychological states in natural contexts.
Method:
Of 199 community-dwelling individuals with schizophrenia or schizoaffective disorder who were contacted to participate in the study, 92% accepted and 73% completed the study. The 145 participants who completed the study provided reports of substance use, psychotic symptoms, mood, and event negativity multiple times per day over 7 consecutive days through PDAs.
Results:
Participants responded to 72% of the electronic interviews (N=2,737) across daily life contexts. Strong within-day prospective associations were observed in both directions between substance use and negative psychological states or psychotic symptoms, but considerable variation was observed by substance type. Consistent with the notion of self-medication, alcohol use was most likely to follow increases in anxious mood or psychotic symptoms. Cannabis and other illicit substances, demonstrating more complex patterns, were more likely to follow certain psychological states but were also associated with the later onset of psychotic symptoms.
Conclusions:
The dynamic interplay of substance use and psychotic symptoms is in many cases consistent with both causal and self-medication mechanisms, and these patterns of association should be considered in the design of treatment and prevention strategies.
Clinical and epidemiological research has extensively documented the high prevalence of alcohol and drug use disorders among individuals with schizophrenia (1–4). These forms of comorbidity have been shown to have severe consequences for clinical course and treatment response (3, 5–9), worsening the outcome of disorders that are already ranked among the leading causes of years lost due to disability when considered independently (10). Recent data from population surveys have also confirmed that a great majority of acts of violence committed by persons with schizophrenia are attributable to those with co-occurring substance abuse (11). Taken together, these findings underscore the public health necessity of reducing substance use among individuals with schizophrenia as well as the important treatment implications of understanding the nature of these associations.
Research on these forms of comorbidity has focused in large part on testing causal models of association whereby the use of certain substances may increase the risk of psychotic symptoms or where substances are consumed as a means of alleviating symptoms or negative emotional states (1, 2, 12, 13). However, a fundamental barrier to examining either hypothesis concerns the difficulties inherent in characterizing the time-limited effects of psychoactive substances relative to fluctuations in psychotic symptoms as they occur naturally in daily life. Computerized ambulatory monitoring techniques such as ecological momentary assessment or the experience sampling method have recently led to important advances in the study of temporal associations among daily life variables associated with use of alcohol or illicit drugs (14–18) as well as in persons with schizophrenia (19–21). These methods employ repeated within-day assessments capable of characterizing the association of behaviors and psychological states over brief time intervals, and the electronic nature of data collection overcomes errors in establishing the timing of frequent reports when using paper-based ambulatory monitoring methods (22, 23). Depending on the direction of risk, the confirmation of prospective within-day associations between substance use and psychotic symptoms would have important implications for understanding the development of substance use, abuse, or dependence in patients with schizophrenia or aid in understanding the biochemical mechanisms implicated in the expression of this disorder.
In this study, we examined the association of substance use with psychological states and psychotic symptoms in patients with schizophrenia. Participants were followed intensively for 1 week using computerized ambulatory monitoring techniques to provide reports of diverse psychological states and behaviors as they were experienced in real time and across diverse daily life contexts. We compared prospective associations of negative mood, perceived stress, and psychotic symptoms with the subsequent use of alcohol, cannabis, and other illicit substances, as well as prospective associations in the reverse direction. All analyses controlled for the status of the predicted outcome at the time of predictor variable assessment.
Method
Participants
Participants were recruited for a psychotherapy outcome study focused on improving community functioning. Recruitment sources included treatment and structured living settings (e.g., board and care) in San Diego County, Calif. The structured living settings provided supervision and medication reminders but did not administer treatment. Most facilities also provided partial or full assistance with daily living tasks, including meals and laundry services. All participants were receiving standard care from the Department of Veterans Affairs and community mental health centers, typically consisting of a combination of medications and psychosocial interventions (usually case management services). Participants had to be at least 18 years of age and physically and clinically stable enough to participate in outpatient group therapy. As participants were originally recruited for a clinical trial that involved novel cognitive-behavioral (CBT) interventions, patients who had received CBT in the past 5 years were excluded in order to avoid confounding treatment effects. A total of 199 community-dwelling patients with schizophrenia (N=144) or schizoaffective disorder (N=55) enrolled in the CBT study after receiving a complete description of the study and providing written informed consent. After enrollment, all participants were systematically presented with the opportunity to participate additionally in the ambulatory assessment study. Individuals who declined to participate (N=16, 8% of the original sample) did not differ from those who accepted in age, sex, or positive symptom score from the Positive and Negative Syndrome Scale (PANSS; 24), but they had higher negative symptom and total severity scores on the PANSS. Thirteen individuals who agreed to participate did not complete the study because of technical problems, and 25 participants were excluded for not achieving minimum compliance (defined as providing the equivalent of at least two full days of ambulatory monitoring). These individuals did not differ from those who completed the study in age, sex, or positive symptom score, but they had more negative symptoms and higher total severity scores on the PANSS. The final sample included 145 individuals with schizophrenia (N=98) or schizoaffective disorder (N=47) with a mean age of 46.5 years (SD=11.2) and a mean of 12.4 years of education (SD=2.1). These participants were 61% male, 60% white, 15% African American, 14% Hispanic, and 11% other ethnicities. The prevalence of lifetime substance use disorders other than caffeine or nicotine was 26.9%.
Procedures
After providing written informed consent, patients were interviewed with the Structured Clinical Interview for DSM-IV Axis I Disorders (25) to verify diagnoses of schizophrenia or schizoaffective disorder and to assess for substance use disorders. Patients then completed a battery of laboratory-based self-report and interview measures to assess mood, symptoms, and functioning in the preceding weeks. After these assessments, a 45-minute training session was provided on computerized ecological momentary assessment, including the operation of the electronic personal digital assistant (PDA) and the meaning of all questions and response choices that would be administered over the ambulatory monitoring week. The PDA was programmed using a modified version of the Purdue Momentary Assessment Tool, version 2.1.2 (26). The sampling schedules, electronic interview content, and question format were previously validated in a subsample (N=56) of the sample analyzed here (19). Participants were given PDAs to carry with them for 7 days, and each PDA was programmed to administer four electronic interviews per day. The PDA program permitted responses to be provided only within a 15-minute period following the interview signal, and all data entries were time-stamped. Investigation of fatigue effects and reactivity to this specific protocol revealed no correlation of time in the study with missing data or with the frequency or intensity of variables (27), and no participant indicated that PDA use prompted any change in usual activities. The signals occurred within each of the following daily periods: 9:00 a.m. to 12:00 noon, 12:00 noon to 3:00 p.m., 3:00 p.m. to 6:00 p.m., and 6:00 p.m. to 9:00 p.m. To allow individuals to participate without modifying their usual daily activities, the sampling windows were also adjusted to accommodate each participant's typical sleep and wake schedules. Participants were given the capacity to temporarily silence PDA alarms in case of social or personal constraints (e.g., during church, driving, naps). Two practice electronic interviews were completed in the laboratory under the supervision of the research staff in order to resolve any difficulties and address any questions from the participant. Individuals who demonstrated greater difficulty in understanding assessment questions or operating the device were given additional training. Information about sampling procedures, battery charging, and the pager number to call in case of questions was provided to participants in writing. All participants were contacted by telephone on the third day of sampling to resolve any questions or difficulties and to remind participants to charge the PDA. Participants received $35 for completing the weeklong electronic assessments. The study was approved by the institutional review board for the University of California, San Diego.
Electronic Ecological Momentary Assessment Interview
Psychotic symptoms.
Psychotic symptoms were assessed by six questions, patterned after the Psychotic Symptom Rating Scales (28), concerning the presence or absence of specific thoughts or perceptual experiences during the time elapsed since the last electronic interview (19). Five of these questions assessed delusions related to being spied on, mind reading, thought insertion, thought broadcasting, and having special powers. An additional question assessed the experience of visual or auditory hallucinations. The presence of any psychotic symptom at each electronic assessment was defined as a positive endorsement of at least one of these six questions.
Negative mood states and perceived stress.
Sad and anxious moods were assessed by separate 7-point Likert items that asked participants to evaluate their mood at that moment by checking the corresponding part of a continuous scale (19). The negativity of daily stressors was assessed by asking participants to select the event that had the greatest impact on them since the last electronic interview, and then to rate its negativity on a similar 7-point Likert scale. Higher scores indicated greater sad mood, anxious mood, or event negativity.
Substance use.
At each assessment, participants were asked if they had used different categories of substances during the time elapsed since the last electronic interview. The substance categories included alcohol, cannabis, and other illicit substances (cocaine, methamphetamine, other nonprescribed drugs).
Analysis
Prospective within-day associations among mood states, perceived event negativity, psychotic symptoms, and substance use were analyzed using hierarchical linear and nonlinear modeling in HLM, version 6.03 (29). The assessment data were time-lagged so that substance use or psychological states at any given assessment (T0) predicted the frequency or intensity of outcome variables at the subsequent assessment on the same day (T1). Multilevel linear models were used for continuous outcomes (mood and event negativity ratings), and Bernoulli models were used for dichotomous outcomes (substance use and psychotic symptoms). Analyses of this type are analogous to standard regression analyses but adjust for dependencies among observations generated by each individual. The γ coefficients from these models represent the pooled within-person association between a predictor (e.g., cannabis use) and the later-occurring outcome (e.g., psychotic symptoms), and the t-ratios are the test statistic values for the null hypotheses that corresponding parameters are equal to zero. All analyses adjusted for the effects of age and sex as well as for the status of the outcome variable as measured at the T0 assessment.
Results
The clinical and electronic interview variables for the study are presented in Table 1. On average, participants experienced moderate overall clinical severity (PANSS total score=66.77, SD=17.11), with individual scores ranging from mildly to severely ill (range=34-108). Participants responded to 72.1% (SD=19.1%) of the programmed electronic interviews over the assessment week, generating 2,737 valid observations. Electronic assessment reports of psychotic symptoms during this period were associated with higher levels of concurrent sad mood (γ=0.307, SE=0.071, t=4.306, p<0.001), anxious mood (γ=0.489, SE=0.086, t=5.658, p<0.001), and perceived event negativity (γ=0.453, SE=0.109, t=4.151, p<0.001). Substance use was more frequent among individuals with a lifetime substance use disorder (γ=0.872, SE=0.373, t=2.339, p<0.05) but did not vary by age or sex. Of all positive substance use reports (N=189), 13.8% involved more than one class of substance. The number of assessments per day in which psychotic symptoms, substance use, or missing data were observed was unrelated to day in study (Figure 1).However, participants required less time to respond to electronic interviews as the study progressed (γ=−17.189, SE=2.133, t=−8.058, p<0.001). Approximately 8% of the sample (N=12) reported no use of prescribed medication over the assessment week. However, 76% of the sample (N=110) reported medication use for the majority of assessment days, and 41% (N=59) reported medication use across all days of the study.
| Characteristic | ||
|---|---|---|
| N | % | |
| Female | 57 | 39.3 |
| Assisted living | 66 | 45.5 |
| Lifetime substance use disorder | 39 | 26.9 |
| Mean | SD | |
| Age (years) (range=18.5–79.3) | 46.50 | 11.16 |
| Education (years) (range=4–20) | 12.44 | 2.13 |
| Positive and Negative Syndrome Scale | ||
| Total score (range=34–108) | 66.77 | 17.11 |
| Positive symptom score (range=7–35) | 18.26 | 6.04 |
| Negative symptom score (range=7–32) | 15.26 | 5.69 |
| Assessment reports (2,737 observations) | ||
| Psychotic symptoms (N=1,230, 44.9%) | 0.45 | 0.50 |
| Substance use (any) (N=189, 6.9%) | 0.07 | 0.25 |
| Alcohol use (N=89, 3.3%) | 0.03 | 0.18 |
| Cannabis use (N=59, 2.2%) | 0.02 | 0.15 |
| Other drug use (N=67, 3.4%) | 0.02 | 0.16 |
| Sad mood | 2.55 | 1.64 |
| Anxious mood | 2.69 | 1.70 |
| Perceived event negativity | 2.98 | 1.70 |
TABLE 1. Clinical and Demographic Characteristics and Assessment Data for Participants (N=145) in an Electronic Ambulatory Monitoring Study of Substance Use and Psychiatric Symptoms in Schizophrenia
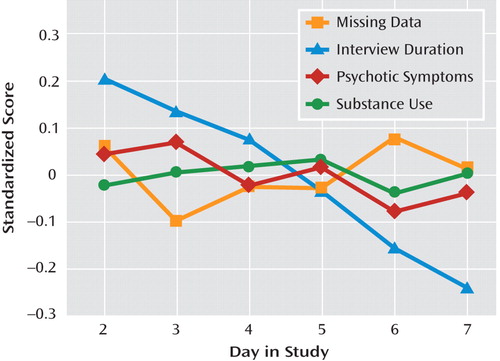
Table 2 presents the associations of negative mood states, event negativity, and psychotic symptoms measured at any given assessment with the onset of substance use at the subsequent assessment of the same day. For the aggregate category of any psychoactive substance, both sad mood (γ=0.311, SE=0.112, t=2.786, p<0.01) and psychotic symptoms (γ=1.374, SE=0.354, t=3.878, p<0.001) were significant risk factors for later substance use. These patterns of significance were similar for the category of other illicit or nonprescribed psychoactive substances. Concerning predictors of alcohol use, psychotic symptoms remained a significant risk factor (γ=1.022, SE=0.343, t=2.983, p<0.01), but prospective effects were also observed for anxious mood (γ=0.206, SE=0.056, t=3.712, p<0.001). This latter association is illustrated in Figure 2 as a function of baseline (T0) alcohol consumption, suggesting a proportionally greater role for anxiety in the continuation of alcohol use, as opposed to its initial onset. The table also demonstrates that cannabis use was more likely following anxious mood (γ=0.104, SE=0.043, t=2.408, p<0.05) and perceived negative events (γ=0.367, SE=0.045, t=8.165, p<0.001), but it was less likely following sad mood (γ=−0.297, SE=0.047, t=−6.324, p<0.001).
| Assessment Variable | γ Coefficient | SE | df | t ratio |
|---|---|---|---|---|
| Any psychoactive substance use | ||||
| Sad mood | 0.311 | 0.112 | 1551 | 2.786** |
| Anxious mood | 0.013 | 0.053 | 1550 | 0.240 |
| Event negativity | −0.123 | 0.111 | 1549 | −1.116 |
| Psychotic symptoms | 1.374 | 0.354 | 1539 | 3.878*** |
| Alcohol use | ||||
| Sad mood | 0.073 | 0.119 | 1551 | 0.614 |
| Anxious mood | 0.206 | 0.056 | 1550 | 3.712*** |
| Event negativity | −0.084 | 0.105 | 1549 | −0.802 |
| Psychotic symptoms | 1.022 | 0.343 | 1539 | 2.983** |
| Cannabis use | ||||
| Sad mood | −0.297 | 0.047 | 1551 | −6.324*** |
| Anxious mood | 0.104 | 0.043 | 1550 | 2.408* |
| Event negativity | 0.367 | 0.045 | 1549 | 8.165*** |
| Psychotic symptoms | 0.355 | 0.370 | 1539 | 0.958 |
| Other illicit or nonprescribed use | ||||
| Sad mood | 0.558 | 0.184 | 1551 | 3.037** |
| Anxious mood | −0.210 | 0.124 | 1550 | −1.697 |
| Event negativity | 0.037 | 0.132 | 1549 | 0.281 |
| Psychotic symptoms | 0.754 | 0.325 | 1540 | 2.324* |
TABLE 2. Mood, Perceived Stress, and Psychotic Symptoms as Predictors of Later Substance Use (Within–Day Lagged Models) in an Electronic Ambulatory Monitoring Study of Substance Use and Psychiatric Symptoms in Schizophrenia (N=145)
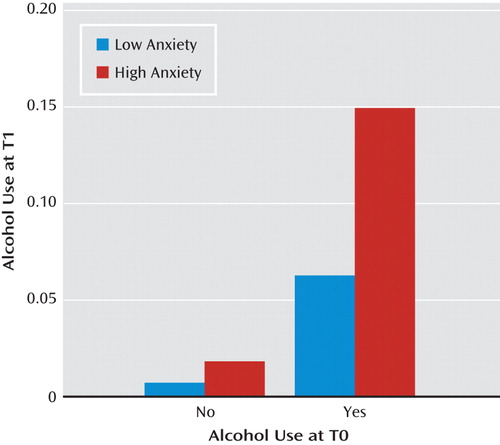
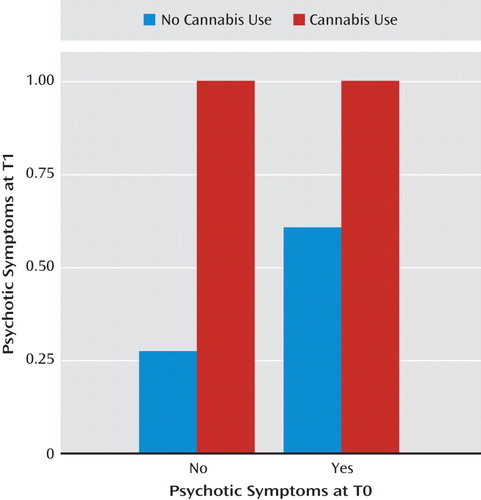
Table 3 presents the reverse prospective associations, where substance use was examined as a predictor of later psychotic symptoms, negative moods, or perceived event negativity. The aggregate analyses demonstrate that the use of any psychoactive substance was generally associated with increases in anxiety (γ=0.583, SE=0.244, t=2.385, p<0.05) as well as with an increased likelihood of psychotic symptom onset (γ=1.092, SE=0.350, t=3.119, p<0.01). Cannabis use was associated with later decreases in perceived event negativity (γ=<0.484, SE=0.218, t=−2.222, p<0.05) but with the strongest increases observed in the risk of psychotic symptoms (γ=3.043, SE=0.455, t=6.683, p<0.001). As demonstrated in Figure 3, use of cannabis was associated both with initial psychotic symptom onset among individuals who did not report symptoms at baseline and with a greater risk for the continuation of symptoms among those who already reported hallucinations or delusions at baseline. An increase in psychotic symptoms (Table 3) was also associated with use of other illicit or nonprescribed substances (γ=0.913, SE=0.335, t=2.730, p<0.01), as was an increase in anxious mood (γ=0.549, SE=0.243, t=2.256, p<0.05). No significant prospective effects were observed for alcohol use relative to later psychotic symptoms, mood states, or perceived event negativity.
| Assessment Variable | γ Coefficient | SE | df | t Ratio |
|---|---|---|---|---|
| Any psychoactive substance use | ||||
| Sad mood | 0.280 | 0.278 | 1562 | 1.009 |
| Anxious mood | 0.583 | 0.244 | 1558 | 2.385* |
| Event negativity | 0.243 | 0.237 | 1555 | 1.029 |
| Psychotic symptoms | 1.092 | 0.350 | 1539 | 3.119** |
| Alcohol use | ||||
| Sad mood | 0.396 | 0.371 | 1562 | 1.067 |
| Anxious mood | 0.119 | 0.433 | 1558 | 0.275 |
| Event negativity | −0.140 | 0.245 | 1555 | −0.571 |
| Psychotic symptoms | 0.259 | 0.212 | 1539 | 1.223 |
| Cannabis use | ||||
| Sad mood | 0.491 | 0.591 | 1562 | 0.831 |
| Anxious mood | 0.604 | 0.968 | 1558 | 0.623 |
| Event negativity | −0.484 | 0.218 | 1555 | −2.222* |
| Psychotic symptoms | 3.043 | 0.455 | 1539 | 6.683*** |
| Other illicit or nonprescribed use | ||||
| Sad mood | 0.088 | 0.307 | 1562 | 0.286 |
| Anxious mood | 0.549 | 0.243 | 1558 | 2.256* |
| Event negativity | 0.324 | 0.286 | 1555 | 1.131 |
| Psychotic symptoms | 0.913 | 0.335 | 1539 | 2.730** |
TABLE 3. Substance Use as a Predictor of Later Mood, Perceived Stress, and Psychotic Symptoms (Within–Day Lagged Models) in an Electronic Ambulatory Monitoring Study of Substance Use and Psychiatric Symptoms in Schizophrenia (N=145)
All models were examined for potential biases associated with prescribed medication use, systematic variation in missing data, time-of-day effects, and polysubstance use. These time-lagged analyses demonstrated that medication use did not influence the direction or significance of any of the observed associations, and the presence of psychotic symptoms or use of psychoactive substances was not associated with the frequency of missing data at the subsequent assessment. In order to examine whether the observed effects may reflect differences in variable frequency at different times of the day rather than true associations among the variables, time was included as a covariate in all models. The effects of specific substances were also examined by excluding all cases of polysubstance use. These secondary analyses demonstrated that time of day and polysubstance use had little effect on the significance of findings, except that anxious mood no longer predicted later cannabis use and the association of any substance use with later psychotic symptoms now fell short of statistical significance.
Discussion
Despite a large body of literature documenting the comorbidity of schizophrenia and substance use disorders (1, 3–9, 11), the order of onset of these conditions and their underlying mechanisms remain poorly understood (2). The principal causal explanations for these associations, including the possibility of self-medication or the induction of psychotic symptoms through substance use, assume that disorders fulfilling diagnostic criteria result from far more dynamic or “micro” clinical phenomena. Investigating these causal associations therefore requires methods that allow assessment of the time-limited effects of psychoactive substances and within-day fluctuations in psychotic symptoms. This study is the first to examine these dynamic associations in community-dwelling persons with schizophrenia using methods capable of verifying order of onset in daily life. In aggregate analyses, both sad mood and psychotic symptoms experienced at a given moment during the day increased the risk of substance use over subsequent hours. In turn, the use of psychoactive substances was associated with later increases in the risk of psychotic symptom onset and with greater feelings of anxiety. All analyses controlled for the status of predicted outcomes as they existed at the moment of predictor variable assessment, thereby modeling change (as opposed to persistence) of the outcome variables. In this way, the findings provide strong support for reciprocal risks between psychotic symptoms and substance use in individuals with schizophrenia who demonstrate moderate overall impairment (30).
A 40-year-old African American single female agreed to participate in the study in May of 2008. In addition to a primary diagnosis of schizoaffective disorder (total PANSS score=68; Beck Depression Inventory score=24), the patient met criteria for current alcohol abuse. During the training session addressing the ambulatory monitoring procedures, the patient stated that she had no previous experience in operating a PDA, but she was able to independently complete a trial computerized ecological momentary assessment without assistance by the research staff. At the end of the weeklong ambulatory monitoring period, the patient returned the PDA and participated in a brief interview concerning her impressions of the study. She rated her overall experience as a participant as being pleasant and stated, “At first, I was a bit nervous, but once I started doing it I saw it was fun.” She also described the procedures for responding to questions as being “self-explanatory.” No difficulties were reported in using the PDA, and she felt that it did not interfere with her regular daily activities. However, the patient stated that she would have preferred to have been able to choose the assessment times herself, rather than being asked to respond at times that were preprogrammed into the PDA device.
In addition to these general associations, highly specific patterns were observed by substance class. Alcohol use was found to increase after reports of both anxious mood states and psychotic symptoms. However, no prospective association was observed in the reverse direction. These findings are similar to those of previous ambulatory monitoring studies of heavy drinkers (18) and suggest that the pharmacological properties of alcohol as a CNS depressant may explain its increased or continued use after periods of anxiety or other strongly correlated symptoms. The unidirectional patterns of association for alcohol use are therefore more consistent with self-medication than with the alleviation of dysphoria more generally (2). By contrast, cannabis use showed more complex patterns of association with psychotic symptoms and psychological states. Sad mood was associated with reduced risk of later cannabis use, while both anxious mood and perceived event negativity increased the risk. Predictors of cannabis use are therefore partially consistent with self-medication, but the reduced risk observed for sad mood may also reflect recreational use or specific social contexts associated with cannabis (31). After the use of cannabis, the perception of event negativity decreased, but strong increases in psychotic symptoms were observed. These findings are consistent notably with cohort studies demonstrating that cannabis use is associated prospectively with an increased risk of psychotic symptoms or episodes (32–36). However, the findings confirm for the first time the within-day directionality of this association in a manner that is inaccessible to standard clinical research protocols or to noncomputerized ambulatory methods (12, 37). Secondary analyses of potential biases confirmed that these associations were generally independent of time-of-day effects, medications, and polysubstance use.
Although the development of psychosocial interventions for individuals with schizophrenia and substance use problems has been slow, recent treatment strategies have demonstrated success in reducing substance use as well as in improving functioning in community settings in this population (38). The present findings should contribute to these strategies by identifying precise targets in the management of momentary risk factors for substance use, as well as by providing an empirical basis for psychoeducation addressing the immediate consequences of substance use. Evidence that certain psychoactive substances have an immediate influence on psychotic symptoms is also essential for basic neuroscience research on the biochemical processes implicated in these interactions. While the momentary effects of cannabis on psychotic symptom expression have been attributed specifically to delta-9-tetrahydrocannabinol (39), an important finding is that -psychotic symptoms were also more likely to follow the use of other illicit substances. This association was not observed in initial cohort studies that have implicated cannabis in the etiology of schizophrenia (32), but it is consistent with research suggesting that substances other than cannabis may be associated with the onset of psychotic symptoms (40).
The strengths of this study include the use of state-of-the-art ambulatory monitoring techniques allowing for the collection of data in the daily lives of community-dwelling individuals with schizophrenia. To our knowledge, the sample is the largest to date to have participated in an ambulatory monitoring study of this disorder, and the study is the first to use computerized methods capable of confirming the temporal nature of associations between different forms of substance use and psychotic symptoms in daily life.
In interpreting the findings, it is important to note the study's focus was on substance use in persons already diagnosed with schizophrenia, not on the potential etiological role of substance use in the onset of this disorder. Ambulatory data collection raises concerns for participant burden and therefore limits the breadth of specific information that can be collected. Thus, in this study we focused on examining the risk of positive but not negative symptoms, and we did not examine certain prevalent psychoactive substances, notably nicotine. The contribution of nicotine to our findings cannot be quantified, and this issue should be considered in formulating conclusions. As the effect of anxiety predicting later cannabis use was no longer significant after we controlled for potential confound variables, this finding should be interpreted with caution. Finally, the assessment of substance use in this study did not include information on quantity of use, and the general category of other illicit substances does not permit conclusions concerning effects for specific types of substances. Substance use reports could not be confirmed with biological assays and may be influenced by social desirability bias. Further research is needed to investigate additional psychological and contextual variables associated with substance use in this population, as well as to examine the applications of ambulatory monitoring and novel technologies as a means of clinical intervention in daily life.
1. : Psychiatric comorbidities and schizophrenia. Schizophr Bull 2009; 35:383–402Crossref, Medline, Google Scholar
2. : Reasons for increased substance use in psychosis. Clin Psychol Rev 2007; 27:494–510Crossref, Medline, Google Scholar
3. : Illicit substance use and its correlates in first episode psychosis. Acta Psychiatr Scand 2010; 121:351–358Crossref, Medline, Google Scholar
4. : Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007; 370:319–328Crossref, Medline, Google Scholar
5. : Association of pre-onset cannabis, alco-hol, and tobacco use with age at onset of prodrome and age at onset of psychosis in first-episode patients. Am J Psychiatry 2009; 166:1251–1257Link, Google Scholar
6. : Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry 1994; 51:273–279Crossref, Medline, Google Scholar
7. : Comorbidity of schizophrenia and substance abuse: implications for treatment. J Consult Clin Psychol 1992; 60:845–856Crossref, Medline, Google Scholar
8. : Impact of persistent substance misuse on 1-year outcome in first-episode psychosis. Br J Psychiatry 2009; 195:242–248Crossref, Medline, Google Scholar
9. : Cannabis use and age at onset of schizophrenia. Am J Psychiatry 2004; 161:501–506Link, Google Scholar
10.
11. : Schizophrenia, substance abuse, and violent crime. JAMA 2009; 301:2016–2023Crossref, Medline, Google Scholar
12. : COMT ValMet moderation of cannabis-induced psychosis: a momentary assessment study of “switching on” hallucinations in the flow of daily life. Acta Psychiatr Scand 2009; 119:156–160Crossref, Medline, Google Scholar
13. : Cannabinoid self-administration attenuates PCP-induced schizophrenia-like symptoms in adult rats. Eur Neuropsychopharmacol 2010; 20:25–36Crossref, Medline, Google Scholar
14. : Feasibility and validity of computerized ambulatory monitoring in drug-dependent women. Drug Alcohol Depend 2009; 99:322–326Crossref, Medline, Google Scholar
15. : Cell phones for ecological momentary assessment with cocaine-addicted homeless patients in treatment. J Subst Abuse Treat 2006; 30:105–111Crossref, Medline, Google Scholar
16. : Patterns of urges during early abstinence in alcohol-dependent subjects. Am J Addict 2005; 14:248–255Crossref, Medline, Google Scholar
17. : An in vivo study of the relationship between craving and reaction time during alcohol detoxification using the ecological momentary assessment. Alcohol Clin Exp Res 2005; 29:2135–2143Crossref, Medline, Google Scholar
18. : Mood and alcohol consumption: an experience sampling test of the self-medication hypothesis. J Abnorm Psychol 2000; 109:198–204Crossref, Medline, Google Scholar
19. : Feasibility and validity of computerized ecological momentary assessment in schizophrenia. Schizophr Bull 2008; 34:507–514Crossref, Medline, Google Scholar
20. : Concurrent measurement of “real world” stress and arousal in individuals with psychosis: assessing the feasibility and validity of a novel methodology. Schizophr Bull (Epub ahead of print, May 8, 2009) Google Scholar
21. : Computerized experience sampling method (ESMc): assessing feasibility and validity among individuals with schizophrenia. J Psychiatr Res 2006; 40:221–230Crossref, Medline, Google Scholar
22. : Signaling does not adequately improve diary compliance. Ann Behav Med 2003; 26:139–148Crossref, Medline, Google Scholar
23. : Patient compliance with paper and electronic diaries. Control Clin Trials 2003; 24:182–199Crossref, Medline, Google Scholar
24. : The Positive and Negative -Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
25. : Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995 Google Scholar
26. : Constructing EMA studies with PMAT: the Purdue Momentary Assessment Tool user's manual. West Lafayette, Ind, Purdue University, Military Family Research Institute, 2004 Google Scholar
27. : Computerized ambulatory monitoring in psychiatry: a multi-site collaborative study of acceptability, compliance, and reactivity. Int J Methods Psychiatr Res 2009; 18:48–57Crossref, Medline, Google Scholar
28. : Scales to measure dimensions of hallucinations and delusions: the Psychotic Symptom Rating Scales (PSYRATS). Psychol Med 1999; 29:879–889Crossref, Medline, Google Scholar
29. : HLM for Windows, Version 6.03. Lincolnwood, Ill, Scientific Software International, 2005 Google Scholar
30. : What does the PANSS mean? Schizophr Res 2005; 79:231–238Crossref, Medline, Google Scholar
31. : Mood and personality-based models of substance use. Psychol Addict Behav 2010; 24:129–136Crossref, Medline, Google Scholar
32. : Cannabis and schizophrenia: a longitudinal study of Swedish conscripts. Lancet 1987; 2:1483–1486Crossref, Medline, Google Scholar
33. : Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 2002; 325:1212–1213Crossref, Medline, Google Scholar
34. : Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ 2002; 325:1183–1184Crossref, Medline, Google Scholar
35. : Cannabis use predicts future psychotic symptoms, and vice versa. Addiction 2005; 100:612–618Crossref, Medline, Google Scholar
36. : Cannabis use and psychosis: a longitudinal population-based study. Am J Epi-demiol 2002; 156:319–327Crossref, Medline, Google Scholar
37. : Effects of cannabis and psychosis vulnerability in daily life: an experience sampling test study. Psychol Med 2003; 33:23–32Crossref, Medline, Google Scholar
38. : A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental illness. Arch Gen Psychiatry 2006; 63:426–432Crossref, Medline, Google Scholar
39. : Modulation of mediotemporal and ventrostriatal function in humans by delta9-tetrahydrocannabinol: a neu-ral basis for the effects of cannabis sativa on learning and psychosis. Arch Gen Psychiatry 2009; 66:442–451Crossref, Medline, Google Scholar
40. : Stimulant psychosis: systematic review. Br J Psychiatry 2004; 185:196–204Crossref, Medline, Google Scholar



