Failure of Anterior Cingulate Activation and Connectivity With the Amygdala During Implicit Regulation of Emotional Processing in Generalized Anxiety Disorder
Abstract
Objective
Clinical data suggest that abnormalities in the regulation of emotional processing contribute to the pathophysiology of generalized anxiety disorder, yet these abnormalities remain poorly understood at the neurobiological level. The authors recently reported that in healthy volunteers the pregenual anterior cingulate regulates emotional conflict on a trial-by-trial basis by dampening activity in the amygdala. The authors also showed that this process is specific to the regulation of emotional, compared to nonemotional, conflict. Here the authors examined whether this form of noninstructed emotion regulation is perturbed in generalized anxiety disorder.
Method
Seventeen patients with generalized anxiety disorder and 24 healthy comparison subjects underwent functional MRI while performing an emotional conflict task that involved categorizing facial affect while ignoring overlaid affect label words. Behavioral and neural measures were used to compare trial-by-trial changes in conflict regulation.
Results
Comparison subjects effectively regulated emotional conflict from trial to trial, even though they were unaware of having done so. By contrast, patients with generalized anxiety disorder were completely unable to regulate emotional conflict and failed to engage the pregenual anterior cingulate in ways that would dampen amygdalar activity. Moreover, performance and brain activation were correlated with symptoms and could be used to accurately classify the two groups.
Conclusions
These data demonstrate that patients with generalized anxiety disorder show significant deficits in the noninstructed and spontaneous regulation of emotional processing. Conceptualization of anxiety as importantly involving abnormalities in emotion regulation, particularly a type occurring outside of awareness, may open up avenues for novel treatments, such as by targeting the medial prefrontal cortex.
Generalized anxiety disorder is characterized by frequent and difficult-to-control episodes of free-floating anxiety or worry (1). Cognitive models suggest that worry reflects an overlearned compensatory strategy for dulling emotional experience (2). However, it is unclear why emotional experiences in patients with generalized anxiety disorder necessitate the use of this cognitively costly regulatory strategy. Seen from the perspective of emotion regulation, patients with generalized anxiety disorder may resort to worry because of an underlying abnormality in regulating emotional processing (3–5).
Studies of other anxiety disorders predict that the amygdala in patients with generalized anxiety disorder would be hyperreactive to negative emotional stimuli (6). Two studies of adolescents with generalized anxiety disorder support this prediction (7, 8), and two similar studies of adults do not (9, 10). One study of adults with generalized anxiety disorder even found amygdalar hyporesponsiveness to faces with fearful expressions (9), although another study found nonspecifically exaggerated amygdalar reactivity to warning cues preceding either aversive or neutral pictures (11). We recently reported on an intra-amygdalar perturbation at a subregional level in adults with generalized anxiety disorder (12).
Studies of generalized anxiety disorder also point to an important role for the prefrontal cortex. Paulesu et al. (13) found that patients were unable to normalize activation in the dorsal anterior cingulate cortex and dorsomedial prefrontal cortex after a worry induction. Pediatric studies have noted exaggerated ventrolateral prefrontal activation to emotional stimuli in patients (7, 14), with the degree of hyperactivation negatively correlated with anxiety scores (14), which suggests a compensatory role for the hyperactivation. We also found evidence for compensatory coupling of a lateral prefrontal cortical executive network with the amygdala in patients (12). Thus, while abnormalities appear to exist within both limbic and prefrontal regions in generalized anxiety disorder, the nature of these abnormalities remains poorly understood.
Use of an experimental paradigm in which emotion regulation can be tracked from trial to trial may be a fruitful approach to understanding generalized anxiety disorder. We recently reported (15, 16) on a facial affect identification emotional conflict task in which healthy volunteers were asked to identify the expression of a face (fearful or happy) while ignoring an overlying emotion word ("fear" or "happy") that either matched (congruent) or conflicted (incongruent) with the facial expression. Reaction time interference by emotionally incongruent stimuli was seen in nearly every participant (15, 16). Interestingly, there is less conflict, indexed by faster reaction times, for incongruent trials if they are preceded by an incongruent trial than if they are preceded by a congruent trial (15–20), which suggests that the emotional conflict generated by incongruency on the previous trial activates a regulatory mechanism that leads to improved emotional conflict regulation on the current incongruent trial (16, 21–23), thus optimizing task performance. We termed this across-trial effect "emotional conflict adaptation" (15, 16), in reference to the label previously applied to similar congruency sequence effects observed in nonemotional conflict tasks (21). Likewise, performance on postcongruent congruent trials is often superior to that on postincongruent congruent trials (21).
To date, the cognitive model that best accounts for the conflict adaptation effect, after eliminating potential confounders (24), is the "conflict monitoring hypothesis" (17–23, 25, 26). According to this model, conflict is continuously evaluated, such that greater conflict regulation can be flexibly recruited as required by the amount of conflict. Thus, the conflict-monitoring hypothesis distinguishes between two important functions—conflict evaluation and conflict regulation. Many studies have examined the regions associated with these functions during adaptation to nonemotional conflict (e.g., color-word Stroop or flanker tasks) by comparing activity during incongruent trials that differ only with respect to whether they were preceded by a congruent or an incongruent trial (17, 18, 20–23, 25, 26). Regions whose activity tracks the amount of conflict (i.e., postcongruent incongruent trials > postincongruent incongruent trials) have been interpreted as conflict evaluation regions (17, 18, 20–23, 25, 26). Regions showing the opposite effect (postincongruent incongruent trials > postcongruent incongruent trials) have been interpreted as conflict regulation regions (17, 18, 20–23, 25, 26), as activity in these regions is greatest when conflict is minimized through regulation. Because these contrasts compare physically identical incongruent trials, the behavioral and neural effects differ only by virtue of expectation created by conflict on the previous trial (17–23, 25, 26).
In previous studies (15, 16) we applied this logic to the analysis of adaptation in a novel emotional conflict task. Greater activity during postincongruent incongruent trials (i.e., regulation-related) was seen in the pregenual anterior cingulate, and this was accompanied by strong negative coupling between the pregenual cingulate and the amygdala. These findings are consistent with other contexts in which emotion regulation is observed (27–29). By contrast, greater activity during postcongruent incongruent trials (i.e., evaluation-related) was seen in the amygdala and the dorsal anterior cingulate/dorsomedial prefrontal cortex.
We also compared activations during emotional conflict adaptation with those during nonemotional conflict adaptation (gender identification with the same emotional faces, while ignoring gender words overlaid on the faces) to determine the specificity of activations for emotion. Pregenual cingulate activation and coupling with the amygdala was specific to emotional conflict adaptation, whereas dorsal anterior cingulate/dorsomedial prefrontal cortex activation was shared by emotional and nonemotional conflict (15, 16), consistent with the role of these latter regions in the evaluation of conflict in many other studies of nonemotional conflict adaptation (17, 20, 25, 26).
Considering that the clinical phenomenology of generalized anxiety disorder suggests that a deficit in the regulation of emotional processing is at the core of this disorder, we hypothesized that patients would show abnormalities in adapting to emotional conflict in our task. Additionally, to better understand emotional conflict adaptation more generally, and thus enhance interpretation of abnormalities in patients, we investigated in a separate cohort of healthy volunteers whether they were aware of these trial-to-trial adaptation effects and thus whether conscious attention is required for this process. We hypothesized that participants would not be aware of the adaptation effect and thus that this process is carried out at an implicit level.
Method
Participants
A total of 41 individuals, recruited locally through online advertisements, participated in the functional MRI (fMRI) component of this study; all provided informed consent. DSM-IV-based psychiatric diagnoses were determined through both an informal clinical interview with a psychiatrist and the Mini-International Neuropsychiatric Interview, a structured diagnostic interview (30, 31). Generalized anxiety disorder was the primary diagnosis for all patients, in terms of both onset and severity. Exclusion criteria were major depressive disorder, bipolar disorders, psychotic disorders, substance abuse, and posttraumatic stress disorder; a history of a neurological disorder, head trauma, or loss of consciousness; claustrophobia; or regular use of benzodiazepines, opioids, or thyroid medications. No patient was taking regular psychiatric medications or had used a benzodiazepine within 48 hours of the scan. No patient had ever received an evidence-based structured psychotherapy, and only five patients had ever received antidepressant medication. Nine patients had no comorbid disorders, five had one comorbid disorder (two with dysthymia and three with social anxiety), three had two comorbid disorders (two with social anxiety and panic disorder, and one with social anxiety and obsessive-compulsive disorder), and none had more than two comorbid disorders. All comparison subjects were free of any current or past axis I conditions or psychiatric medications. All participants completed the Spielberger State-Trait Anxiety Inventory (32), the Penn State Worry Questionnaire (33), the Beck Anxiety Inventory (34), the Beck Depression Inventory (35), and the Mood and Anxiety Symptoms Questionnaire (36, 37), from which the anxious arousal and anhedonic depression subscales were used. Resting state data from nine of the healthy comparison subjects and 10 of the patients were included in a previous study (12). The behavior-only study was conducted on a group of 19 healthy volunteers (mean age=25.2 years [SD=1.0]; 13 of them women) that did not overlap with the healthy comparison group involved in the fMRI study.
Experimental Paradigm
The emotional conflict task was performed as previously described (15, 16). Stimuli were presented with the Presentation software package (Neurobehavioral Systems, http://nbs.neuro-bs.com) during fMRI scanning and displayed through a custom-built MRI-compatible projection system. The task consisted of 148 presentations of happy or fearful facial expression photographs drawn from the set of Ekman and Friesen (38), overlaid with the words "FEAR" or "HAPPY." Stimuli were presented for 1,000 msec, with a varying interstimulus interval of 3000–5000 msec (mean=4,000 msec), in a pseudorandom order, counterbalanced across trial types for expression, word, response button, and gender. Participants indicated facial affect with a button press response. Behavioral data were analyzed in SPSS (SPSS, Inc., Chicago). For the behavior-only task, a questionnaire was administered after the task to assess participants' awareness of the conflict adaptation effect.
fMRI Data Acquisition
Images were acquired on a 3-T GE Signa scanner using a custom-built head coil. Twenty-nine axial slices (4.0 mm thickness with a 0.5 mm gap) were acquired across the whole brain using a T2*-weighted gradient echo spiral pulse sequence (repetition time=2,000 msec, echo time=30 msec, flip angle=80°, interleave=1, field of view=22 cm, matrix=64×64) (39). To reduce blurring and signal loss arising from field inhomogeneities, an automated high-order shimming method based on spiral acquisitions was used before acquisition of functional MRI scans (40). A high-resolution T1-weighted three-dimensional inversion recovery spoiled gradient-recalled acquisition in the steady state MRI sequence was used with the following parameters: inversion time=300 msec, repetition time=8 msec; echo time=3.6 msec; flip angle=15°; field of view=22 cm; 124 slices in coronal plane; matrix=256×192; number of excitations=2; acquired resolution=1.5×0.9×1.1 mm. The images were reconstructed as a 124×256×256 matrix.
fMRI Data Analysis
The first five volumes were not analyzed to allow for signal equilibration effects. A linear shim correction was applied separately for each slice during reconstruction using a magnetic field map acquired automatically by the pulse sequence at the beginning of the scan (39). Functional MRI data were then preprocessed using the SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (MathWorks, Inc., Natick, Mass.). Images were realigned to correct for motion, slice timing-corrected, spatially transformed to the Montreal Neurologic Institute coordinate system (41), resampled every 2 mm, and smoothed with a 6 mm full-width at half-maximum Gaussian kernel. During preprocessing, the effects of global signal were also removed separately for each voxel (42). A 128-second temporal high-pass filter was applied to the data, and temporal autocorrelation was estimated using a first-order autoregressive model. Separate regressors for the stimulus events (convolved with a canonical hemodynamic response function) were created for postcongruent incongruent trials, postincongruent incongruent trials, postcongruent congruent trials, and postincongruent congruent trials, with error and posterror trials modeled separately. Additional regressors of no interest corresponding to the six motion parameters were also included. This model was applied to normalized data in the context of a generalized linear model (43) and submitted to group-level random-effects analyses using two-sample t tests. As described previously and above (15, 16), our contrasts took advantage of the conflict adaptation effect to compare activity during incongruent (or congruent) trials for which behavior differs by virtue only of expectation created by the previous trial type (e.g., postincongruent incongruent trials minus postcongruent incongruent trials).
For the psychophysiologic interaction analyses (44), we extracted for each participant a deconvolved time course from the healthy comparison group-level contrast of postincongruent incongruent trials minus postcongruent incongruent trials (p=0.01). Activity within the amygdala was then regressed on a voxel-wise basis against the product of this time course and the vector of the psychological variable of interest, with the physiological and the psychological variables serving as regressors of no interest, along with the six motion parameters. The results were then taken to a random-effects group analysis using two-sample t tests.
We report results within independently defined a priori regions of interest based only on our prior data with the emotional conflict task (15, 16) using small-volume corrections (45) (p<0.05, family-wise error-corrected). Specifically, to determine the optimal center coordinates for spherical regions of interest, we averaged the medial prefrontal or anterior cingulate peak coordinates from our previous studies of healthy volunteers scanned with the identical task and created spheres (intersected with a dilated gray matter mask) of 12 mm radius around these coordinates for the dorsomedial prefrontal cortex (x=5, y=33, z=31; 6,848 mm3) and the pregenual cingulate (x=–10, y=42, z=0; 5,696 mm3). In this way, our statistical inferences in this study are directly driven by a priori hypotheses about spatial location of effects of interest from our previous studies. The amygdala region of interest corresponded to the left and right amygdala in the Wake Forest University PickAtlas (left: 12×10×18 mm, 1,264 mm3; right: 14×12×16 mm, 1,288 mm3) (46). Results are displayed within these regions of interest only.
Results
Behavior
Our patient and comparison groups were well matched for age, gender, handedness, and education (Table 1). No group difference was observed in either overall reaction times or accuracy (comparison group: reaction time=793 msec [SD=22], accuracy=94.9% [SD=0.8]; patient group: reaction time=872 msec [SD=58], accuracy=93.4% [SD=1.4]). Emotional conflict slowed reaction times similarly in both groups (incongruent minus congruent trial difference), including in all healthy comparison subjects and in all but one patient (comparison group: t=6.77, df=23, p<0.000001; Cohen's d=1.4; patient group: t=5.82, df=16, p<0.00005; d=1.4); group comparison: t=0.09, df=39, p>0.9; see Figure 1B). There was a significant group difference in across-trial reaction time adjustment related to emotional conflict adaptation during incongruent trials (t=2.39, df=39, p<0.05; d=0.8; see Figure 1B). This effect was driven by the predicted faster performance of healthy comparison subjects on postincongruent incongruent trials than on postcongruent incongruent trials (t=2.19, df=23, p<0.05; d=0.45, see Figure 1B). Patients with generalized anxiety disorder failed to show this effect. By contrast, for congruent trials, exposure to an immediately preceding congruent trial produced similarly significant reaction time facilitation in both groups (comparison group: t=3.26, df=23, p<0.005; d=0.66; patient group: t=2.87, df=16, p=0.01; d=0.7; group comparison: t=0.93, df=39, p>0.35; see Figure 1B; see also Table S1 in the data supplement that accompanies the online edition of this article).
 |

aPanel A shows a sample task time course illustrating the contrasts made to examine adaptation during congruent or incongruent trials. Panel B shows reaction time difference scores reflecting the overall effect of emotional conflict (incongruent minus congruent trials), the facilitation in reaction times during emotional conflict adaptation (postincongruent incongruent trials [iI] faster than postcongruent incongruent trials [cI], resulting in a negative reaction time difference score), and similar adaptation on congruent trials (postcongruent congruent trials [cC] faster than postincongruent congruent trials [iC]). A group difference was observed only during adaptation on incongruent trials. The inset in panel B shows reaction times for each condition (a detailed table is presented in the online data supplement).
bOne-sample t test, p<0.001.
cTwo-sample t test, p<0.05.
dOne-sample t test, p<0.05.
eOne-sample t test, p<0.01.
Finally, we asked a separate group of healthy volunteers whether they were aware of any pattern across trials that might help or hinder their performance. No participant mentioned previous trial conflict. In addition, discrimination in a forced-choice question of whether performance on a current incongruent trial was improved by a previous incongruent trial compared to a previous congruent trial did not differ from chance (p>0.25), which suggests that conscious awareness of the adaptation phenomenon is not required for successful adaptation.
Abnormal Medial Prefrontal Responses to Emotional Conflict in Patients
We first examined overall responses to emotional conflict (i.e., incongruent > congruent). As shown in Figure 2A, healthy comparison subjects exhibited greater activation to emotional conflict than did patients with generalized anxiety disorder in the dorsomedial prefrontal cortex (x=0, y=36, z=38; z=3.96; d=1.22; 2,832 mm3; x=6, y=44, z=34; z=3.33; d=1.14). This difference resulted from activation by emotional conflict within this cluster in comparison subjects (t=3.9, df=23, p=0.001; d=0.8) but not in patients (t=1.84, df=16, p>0.05; see Figure 2B). No group differences were observed in the pregenual cingulate or the amygdala.
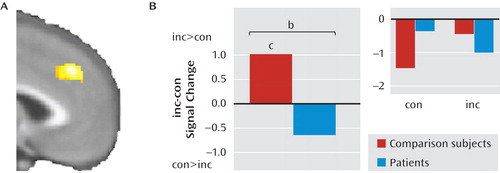
aPanel A shows the healthy comparison > patient contrast for the incongruent (inc) minus congruent (con) trial difference within the dorsomedial prefrontal region of interest. Panel B shows each group's data extracted for the cluster, for both difference scores and individual trial types (inset). Only healthy comparison subjects were found to activate the dorsomedial prefrontal cortex in response to emotional conflict.
bTwo-sample t test, p<0.001.
cOne-sample t test, p<0.01.
Next, we explored the neural correlates of group differences in emotional conflict adaptation, guided by our behavioral results. Based on our previous findings with the emotional conflict task in healthy volunteers (15, 16), we examined the contrast of postincongruent incongruent trials minus postcongruent incongruent trials in the pregenual cingulate in patients and healthy comparison subjects and found a significant cluster (x=–12, y=32, z=–4; z=3.49; 376 mm3; d=1.2; see Figure 3A). Average signal within this cluster was extracted for each group to further describe the effect. As predicted, in this cluster, healthy comparison subjects had greater activity during postincongruent incongruent trials (t=3.34, df=23, p<0.005; d=0.68), whereas in patients no difference was observed (t=1.6, df=16, p>0.1; see Figure 3B).

aPanel A shows the healthy comparison subject > patient contrast for the postincongruent incongruent trial (iI) minus postcongruent incongruent trial (cI) difference within the pregenual cingulate region of interest; panel B shows each group's data extracted for the cluster, for both difference scores and individual trial types (inset). The pregenual cingulate was activated only in healthy comparison subjects. Panel C shows the healthy comparison subject > patient contrast for the postcongruent incongruent trial (cI) minus postincongruent incongruent trial (iI) difference within the dorsomedial prefrontal region of interest; panel D shows each group's data extracted for the cluster, for both difference scores and individual trial types (inset). Healthy comparison subjects were found to exhibit less dorsomedial prefrontal activity in postincongruent incongruent trials (hence a positive difference score). By contrast, in patients, there was inappropriately greater activity in the dorsomedial prefrontal cortex in response to postincongruent incongruent trials (i.e., negative difference scores).
bTwo-sample t test, p<0.01.
cOne-sample t test, p<0.01.
dOne-sample t test, p<0.05.
Next, we examined the contrast of postcongruent incongruent trials minus postincongruent incongruent trials in the dorsomedial prefrontal cortex and amygdala in both groups and found a significant cluster in the dorsomedial prefrontal cortex (x=–1, y=36, z=38; z=3.26; 568 mm3; d=1.15; see Figure 3C) but not in the amygdala. Extraction of average signal within this cluster revealed that the group difference was driven by the expected greater activity in postcongruent incongruent trials in healthy comparison subjects (t=2.36, df=23, p<0.05; d=0.48) and by the opposite effect in patients (t=2.66, df=16, p<0.05; d=0.64; see Figure 3D). Note that the inability of patients to decrease dorsomedial prefrontal activity in postincongruent incongruent trials paralleled patients' inability to improve reaction times during these trials compared to healthy comparison subjects. No group differences were observed in any of the regions of interest for the contrast of postcongruent congruent trials with postincongruent congruent trials. Finally, comparing across all trial types, we found significantly greater activation in patients than in comparison subjects in the left amygdala (x=–22, y=–2, z=–18; z=3.04; 232 mm3; d=1.04). Using cytoarchitectonic probability maps of the basolateral, centromedial, and superficial amygdalar subregions (47, 48), we found that 78% of this cluster corresponded to the superficial amygdala and 21.1% to the basolateral amygdala.
Absent Pregenual Cingulate-Amygdala Connectivity in Patients
We next examined differential functional connectivity between the pregenual cingulate and the amygdala during postincongruent incongruent trials compared with postcongruent incongruent trials using psychophysiologic interaction analyses, with the pregenual cingulate as the seed and the amygdala as the target, while controlling for task-related activations in both regions and task-nonspecific connectivity (44). As shown in Figure 4A, we found a significant group difference in both the left (x=–20, y=–4, z=–22; z=3.54; 536 mm3; d=1.15) and right amygdala (x=30, y=–4, z=–22; z=3.4; 168 mm3; d=1.15). Extraction of average connectivity strength within these clusters revealed that the group effect resulted from the predicted significant negative pregenual cingulate-amygdala connectivity in healthy comparison subjects during postincongruent incongruent trials, compared with postcongruent incongruent trials (left side: t=4.14, df=23, p<0.001; d=0.85; right side: t=3.08, df=23, p=0.005; d=0.63), but not in patients (see Figure 4B). We did not pursue further characterization of differential group cingulate-amygdala connectivity using effective connectivity methods such as dynamic causal modeling, as we had in a previous study of healthy volunteers (16), since we did not think it would add significant new information beyond the result from the functional connectivity analysis above. Finally, we found that the majority of the left amygdala differential connectivity cluster was in the basolateral amygdala (55%), with 44.3% in the superficial amygdala and only 0.4% in the centromedial amygdala. One hundred percent of the right amygdala cluster was in the basolateral amygdala.
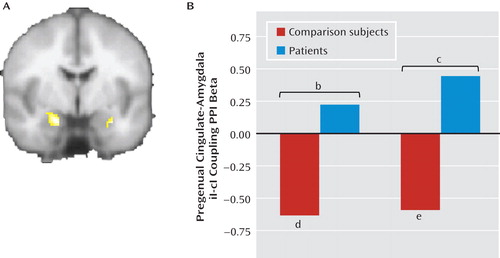
aPanel A shows the healthy comparison > patient contrast (left=left) for the psychophysiologic interaction functional connectivity analysis between the pregenual cingulate during postincongruent incongruent trials compared with postcongruent incongruent trials (iI-cI) and the amygdala; panel B shows each group's clusters. Only healthy comparison subjects showed robust negative connectivity between the pregenual cingulate and the amygdala during postincongruent incongruent trials.
bTwo-sample t test, p<0.001.
cTwo-sample t test, p<0.01.
dOne-sample t test, p<0.001.
eOne-sample t test, p<0.01.
Additional Findings
We conducted several additional analyses to better understand the group differences reported above. First, for the patients, we correlated symptom scale scores with behavior and brain activity within the group difference clusters. We found that the impairment in emotional conflict adaptation was greatest, in terms of both reaction times and dorsomedial prefrontal modulation, for the most anxious patients (see the online data supplement). Second, we conducted multivariate pattern classification to determine whether behavior and brain activation could be used to determine participants' diagnostic group. Significant classification of patients and healthy comparison subjects could be achieved with both behavior and brain activation data, reaching 95% when whole-brain data were used (see the online data supplement).
Discussion
In this study, we investigated emotional conflict adaptation using a paradigm in which emotional processing is regulated spontaneously and in the absence of explicit instruction. We found that patients with generalized anxiety disorder were unable to adapt to emotional conflict through engagement of this regulatory process. By contrast, adaptation during congruent trials was similar in both groups, as was the overall reaction time interference due to emotional conflict, demonstrating the specificity of the deficit.
At the neural level, patients with generalized anxiety disorder failed to activate the pregenual cingulate and demonstrate negative top-down (16) pregenual cingulate-amygdala connectivity during the regulation of emotional conflict. As in previous studies of emotion regulation (27, 28), regulation-related changes in activity were seen in the context of overall task-independent medial prefrontal deactivation from an implicitly modeled baseline, and this deactivation did not differ between our groups (data not shown). Moreover, since the critical contrast involves only incongruent trials, the many processes that differ between incongruent and congruent stimuli are controlled for, as are nonspecific responses to task demands, leaving only the effect of previous trial conflict on processing of emotional conflict on the current trial.
We suggest that patients' failure to show the neural effects related to previous trial conflict accounts for their behavioral regulatory deficit during emotional conflict adaptation, in accordance with predictions made by the conflict monitoring hypothesis about brain activity during conflict adaptation—a cognitive model supported by an extensive neuroimaging literature (17, 18, 20–23, 25, 26). These conclusions are also consistent with emotion regulatory roles attributed to ventromedial prefrontal regions through connectivity with the amygdala in other studies (27–29, 49). Moreover, we recently found functional connectivity and structural evidence for an intra-amygdalar abnormality at a subregional level in generalized anxiety disorder (12). Thus, it appears that patients have deficits both in activating relevant control regions (pregenual cingulate) and in the connectivity required for such regions to exert control over limbic structures. It is therefore interesting to speculate that the lateral prefrontal hyperactivation (7, 9, 14) or increased connectivity with the amygdala (12) previously reported in patients with generalized anxiety disorder may reflect the compensatory engagement of worry, an attention-demanding cognitive process, to regulate the effects of emotional stimuli in the absence of patients being otherwise able to recruit pregenual cingulate-based regulatory mechanisms.
Finally, there is controversy regarding overall emotional responsiveness in generalized anxiety disorder, indexed largely through activation in the amygdala. Pediatric studies have shown hyperactivity to negative emotional expression faces (7, 8), and adult studies have shown either no difference (10) or hypoactivation (9) to similar stimuli, although one adult study showed nonspecifically exaggerated amygdalar reactivity to both negative emotional and neutral cues (11). Consistent with the latter study, we found greater amygdalar activity in patients during both congruent and incongruent trials.
Our data also highlight an emerging theme in affective neuroscience, namely, that there are many ways by which emotional processing is regulated and that deficits in these functions contribute importantly to psychopathology. To date, the neurobiology underlying the regulation of emotional processing has been primarily studied by asking participants to deliberately alter their emotional responses to defined stimuli (i.e., "explicit" regulation) (50). Much of the normal regulation of emotional processing, however, probably occurs in the absence of explicit effort (51, 52). Far less is known about these "implicit" forms of regulation.
Based on the fact that behavioral performance in our task indicates the engagement of a mechanism for regulating emotional processing that occurs in the absence of specific regulation instructions, we and others have argued that emotional conflict adaptation is a type of implicit regulation of emotional processing (51, 52). In this study, we provide direct behavioral evidence for this in healthy participants. The striking deficit in emotional conflict regulation in patients, in the context of otherwise intact task performance, provides the strongest evidence to date linking abnormalities in a defined form of implicit regulation and a type of psychopathology whose clinical presentation suggests emotion regulatory abnormalities.
Several limitations are important to note. First, we are unable to report on subjective ratings of emotion during emotional conflict adaptation, as asking participants to report on subjective emotional states might itself lead to emotion regulation (53–57). Thus, we inferred the effects of emotion from behavioral indices, such as reaction times, and patterns of brain activation. Second, although we focused in this study primarily on the neural effects we previously found to be specific to emotional conflict (15), it would be useful in future experiments also to examine adaptation to nonemotional conflict. Finally, it is unknown whether medial prefrontal dysfunction during emotional conflict adaptation reflects a disorder-specific abnormality or a more general endophenotype of affective disorders, such as major depression. Nonetheless, the robust group differences seen at both the behavioral and neural levels suggest that the inability of patients to adapt to emotional conflict is an important aspect of the pathophysiology of generalized anxiety disorder—and potentially of other psychiatric disorders—and thus merits continued, deeper, study.
1 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV). Washington, DC, American Psychiatric Association, 1994 Google Scholar
2 : Avoidance theory of worry and generalized anxiety disorder, in Generalized Anxiety Disorder: Advances in Research and Practice. Edited by Heimberg RGTurk CLMennin DS. New York, Guilford, 2004, pp 77–108 Google Scholar
3 : The contributory role of worry in emotion generation and dysregulation in generalized anxiety disorder. Behav Res Ther 2007; 45:1735–1752 Crossref, Medline, Google Scholar
4 : Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behav Res Ther 2005; 43:1281–1310 Crossref, Medline, Google Scholar
5 : Delineating components of emotion and its dysregulation in anxiety and mood psychopathology. Behav Ther 2007; 38:284–302 Crossref, Medline, Google Scholar
6 : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488 Link, Google Scholar
7 : Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 2007; 64:97–106 Crossref, Medline, Google Scholar
8 : Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 2008; 65:568–576 Crossref, Medline, Google Scholar
9 : Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry 2008; 165:1193–1202 Link, Google Scholar
10 : A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry 2007; 63:858–863 Crossref, Medline, Google Scholar
11 : Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry 2009; 166:302–310 Link, Google Scholar
12 : Disrupted amygdalar subregion functional connectivity and evidence for a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry (in press) Google Scholar
13 : Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychol Med 2010; 40:117–124 Crossref, Medline, Google Scholar
14 : Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry 2006; 163:1091–1097 Link, Google Scholar
15 : Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex 2008; 18:1475–1484 Crossref, Medline, Google Scholar
16 : Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 2006; 51:871–882 Crossref, Medline, Google Scholar
17 : Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 1999; 402:179–181 Crossref, Medline, Google Scholar
18 : Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci 2005; 8:1784–1790 Crossref, Medline, Google Scholar
19 : Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen 1992; 121:480–506 Crossref, Medline, Google Scholar
20 : Anterior cingulate conflict monitoring and adjustments in control. Science 2004; 303:1023–1026 Crossref, Medline, Google Scholar
21 : Conflict monitoring and cognitive control. Psychol Rev 2001; 108:624–652 Crossref, Medline, Google Scholar
22 : Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 2004; 8:539–546 Crossref, Medline, Google Scholar
23 : Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci 2009; 10:141–152 Crossref, Medline, Google Scholar
24 : Conflict adaptation effects in the absence of executive control. Nat Neurosci 2003; 6:450–452 Crossref, Medline, Google Scholar
25 : Parsing executive processes: strategic vs evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA 2000; 97:1944–1948 Crossref, Medline, Google Scholar
26 : The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage 2005; 24:539–547 Crossref, Medline, Google Scholar
27 : Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43:897–905 Crossref, Medline, Google Scholar
28 : Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 2008; 59:829–838 Crossref, Medline, Google Scholar
29 : Placebo in emotional processing-induced expectations of anxiety relief activate a generalized modulatory network. Neuron 2005; 46:957–969 Crossref, Medline, Google Scholar
30 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33 Medline, Google Scholar
31 : Reliability and validity of the MINI International Neuropsychiatric Interview (MINI): according to the SCID-P. Eur Psychiatry 1997; 12:232–241 Crossref, Google Scholar
32 : Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1970 Google Scholar
33 : Development and validation of the Penn State Worry Questionnaire. Behav Res Ther 1990; 28:487–495 Crossref, Medline, Google Scholar
34 : Beck Anxiety Inventory. San Antonio, Tex, Psychological Corp, 1993 Google Scholar
35 : Manual for Beck Depression Inventory II (BDI-II). San Antonio, Tex, Psychological Corp, 1996 Google Scholar
36 : Testing a tripartite model, II: exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol 1995; 104:15–25 Crossref, Medline, Google Scholar
37 : Testing a tripartite model, I: evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 1995; 104:3–14 Crossref, Medline, Google Scholar
38 : Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologists Press, 1976 Google Scholar
39 : Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med 1998; 39:361–368 Crossref, Medline, Google Scholar
40 : Regularized higher-order in vivo shimming. Magn Reson Med 2002; 48:715–722 Crossref, Medline, Google Scholar
41 : Spatial registration and normalization of images. Hum Brain Mapp 1995; 2:165–189 Crossref, Google Scholar
42 : A method for removal of global effects from fMRI time series. Neuroimage 2004; 22:360–366 Crossref, Medline, Google Scholar
43 : Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210 Crossref, Google Scholar
44 : Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997; 6:218–229 Crossref, Medline, Google Scholar
45 : A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996; 4:58–73 Crossref, Medline, Google Scholar
46 : An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239 Crossref, Medline, Google Scholar
47 : Cytoarchitectonic mapping of the human amygdala, hippocampal region, and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005; 210:343–352 Crossref, Medline, Google Scholar
48 : A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005; 25:1325–1335 Crossref, Medline, Google Scholar
49 : From fear to safety and back: reversal of fear in the human brain. J Neurosci 2008; 28:11517–11525 Crossref, Medline, Google Scholar
50 : The cognitive control of emotion. Trends Cogn Sci 2005; 9:242–249 Crossref, Medline, Google Scholar
51 : Functional neuroanatomy of anxiety: a neural circuit perspective, in Behavioral Neurobiology of Anxiety and Its Treatment. Edited by Stein MBSteckler T. New York, Springer (in press) Google Scholar
52 : A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008; 13:829, 833–857 Crossref, Medline, Google Scholar
53 : Affective flexibility: evaluative processing goals shape amygdala activity. Psychol Sci 2008; 19:152–160 Crossref, Medline, Google Scholar
54 : Amygdala activation and facial expressions: explicit emotion discrimination versus implicit emotion processing. Neuropsychologia 2007; 45:2369–2377 Crossref, Medline, Google Scholar
55 : Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 2000; 11:43–48 Crossref, Medline, Google Scholar
56 : Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci 2007; 18:421–428 Crossref, Medline, Google Scholar
57 : Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 2003; 18:650–659 Crossref, Medline, Google Scholar



