Response to Emotional Expressions in Generalized Social Phobia and Generalized Anxiety Disorder: Evidence for Separate Disorders
Abstract
Objective: Generalized social phobia involves fear/avoidance, specifically of social situations, whereas generalized anxiety disorder involves intrusive worry about diverse circumstances. It remains unclear the degree to which these two, often comorbid, conditions represent distinct disorders or alternative presentations of a single, core underlying pathology. Functional magnetic resonance imaging assessed the neural response to facial expressions in generalized social phobia and generalized anxiety disorder. Method: Individuals matched on age, IQ, and gender with generalized social phobia without generalized anxiety disorder (N=17), generalized anxiety disorder (N=17), or no psychopathology (N=17) viewed neutral, fearful, and angry expressions while ostensibly making a simple gender judgment. Results: The patients with generalized social phobia without generalized anxiety disorder showed increased activation to fearful relative to neutral expressions in several regions, including the amygdala, compared to healthy individuals. This increased amygdala response related to self-reported anxiety in patients with generalized social phobia without generalized anxiety disorder. In contrast, patients with generalized anxiety disorder showed significantly less activation to fearful relative to neutral faces compared to the healthy individuals. They did show significantly increased response to angry expressions relative to healthy individuals in a lateral region of the middle frontal gyrus. This increased lateral frontal response related to self-reported anxiety in patients with generalized anxiety disorder. Conclusions: These results suggest that neural circuitry dysfunctions differ in generalized social phobia and generalized anxiety disorder.
Generalized social phobia and generalized anxiety disorder are two highly prevalent, chronic, and disabling anxiety disorders (1 , 2) that are sometimes comorbid. Generalized social phobia involves fear/avoidance, specifically of social situations, whereas generalized anxiety disorder involves intrusive worry about a broader array of everyday life circumstances. Although both have considerable social and economic costs, disagreement exists concerning the degree to which the conditions result from a shared or unique pathophysiology. For example, high rates of comorbidity in cross-sectional and longitudinal studies suggest that the distinction between the two conditions may be relatively subtle at the descriptive level (3 , 4) . On the other hand, data from family-based and therapeutic research suggest the two conditions can be dissociated. Specifically, such dissociation is reflected in patterns of disorder aggregation within families (5) , as well as by the fact that generalized anxiety disorder, but not social phobia, responds to most tricyclic antidepressants and to buspirone (6 – 8) . Because no brain-imaging study has directly compared the two conditions, it remains unclear whether the two disorders have dissociable neural correlates.
The principal goal of the current study was to investigate whether generalized social phobia and generalized anxiety disorder are associated with different, or similar, pathological neural responses to expression stimuli. Both disorders have been related to increased amygdala responsiveness. Thus, a core feature of generalized social phobia is atypically increased responses to other individuals’ emotional expressions in regions of the brain, including the amygdala, the anterior cingulate cortex, and the extrastriate cortex (9 – 17) . The empirical picture is less clear in generalized anxiety disorder. Three studies have investigated responses to emotional expressions in adolescent generalized anxiety disorder, a condition linked to adult generalized anxiety disorder in family and outcome studies (18 – 20) . Monk and colleagues found that the adolescents with anxiety showed greater blood-oxygen-level-dependent (BOLD) responses to angry faces than healthy adolescents within the right ventrolateral frontal cortex (19) . Although two other studies implicated amygdala pathology in adolescent generalized anxiety disorder, both found that perturbed amygdala functioning occurred in the context of associated ventral frontal cortex dysfunction (18 , 20) . Thus, taken together, the three studies implicated frontal cortex pathology most consistently in adolescent generalized anxiety disorder, consistent with several theoretical positions emerging largely from work with adults that also attribute the condition’s central feature to frontal pathology (21 , 22 ; see also references 23 , 24) .
A secondary goal is to determine whether disorder-specific patterns of association emerge between any pathological neural response and severity of clinical symptoms. Level of amygdala activation to harsh faces has been related to intensity of social anxiety symptoms in patients with generalized social phobia (15) . On the other hand, both Monk et al. (19) and McClure et al. (25) found associations between anxiety severity and perturbed ventral frontal engagement to faces in adolescent generalized anxiety disorder, at least when faces were presented for 500 msec or longer. These and other data implicating frontal pathology in generalized anxiety disorder (21 , 22) suggest that activity in this region might predict symptom-severity levels in patients with generalized anxiety disorder.
Although generalized social phobia often presents as an isolated condition, generalized anxiety disorder typically occurs with comorbid anxiety disorders, particularly generalized social phobia, complicating attempts to compare the two disorders. Prior functional magnetic resonance imaging (fMRI) studies from our group combined subjects with generalized anxiety disorder with or without generalized social phobia into a single group (19 , 20 , 25) . These prior studies informed decisions about classification in the current study, which separated participants into three groups: 1) patients with generalized social phobia but no other current anxiety disorder (referred to in the article as patients with generalized social phobia without generalized anxiety disorder), 2) patients with generalized anxiety disorder including those with and those without generalized social phobia (referred to in the article as patients with generalized anxiety disorder), and 3) healthy comparison individuals. This patient-grouping scheme in the current and prior studies considers the possibility that development of comorbid generalized social phobia in the context of co-occurring generalized anxiety disorder reflects a process whereby generalized worry extends to social settings. This process might be distinct from the development of isolated, or “pure,” generalized social phobia, in the context of minimal worries in nonsocial settings.
Method
Subjects
Patients with generalized social phobia without generalized anxiety disorder (N=17), generalized anxiety disorder (N=17), and no psychopathology (N=17) participated in the study. Study groups were matched on age, gender, and IQ ( Table 1 ). The patients were required to meet criteria for either current generalized social phobia or generalized anxiety disorder according to the DSM-IV criteria based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (26) and a confirmatory clinical interview by a board-certified psychiatrist (D.S.P.). All patients with any other co-occurring anxiety disorder were excluded, as were patients with severe major depressive disorder. Other patients with major depressive disorder were included if the evaluating psychiatrist determined the severity of the major depressive disorder to be milder than that of the anxiety disorder, such that the anxiety disorder was deemed the primary condition in need of clinical attention. Only one of 34 patients exhibited comorbid major depressive disorder. As in our prior work, the seven patients with generalized anxiety disorder identified with ongoing comorbid generalized social phobia were placed in the same group as subjects with noncomorbid generalized anxiety disorder. Beyond one major depressive disorder case, in the generalized social phobia group, none of the 33 other patients had any other axis I diagnosis.
All participants were required to be currently medication-free (no regular use of psychotropic medication within 2 weeks or fluoxetine/benzodiazepine within 8 weeks of the study). Comparison individuals were excluded if they had current/past history of any psychiatric illness. All subjects were in good physical health, as confirmed by a complete physical examination, and provided written informed consent. The patients with generalized social phobia without generalized anxiety disorder and those with generalized anxiety disorder reported significantly greater depression, social anxiety, and general anxiety than the healthy comparison individuals. However, the patient groups did not differ on these measures ( Table 1 ).
Task
The subjects viewed static grayscale images of emotionally expressive faces from the empirically valid and reliable Pictures of Facial Affect (27) . Ten different faces were selected for use in the study. Each face was digitally morphed to represent seven different expressions: 50% fear, 100% fear, 150% fear, 50% anger, 100% anger, 150% anger, and neutral. Since it is conventional to signal approval in normal social interaction, 100% neutral faces can appear slightly cold and threatening, and a slightly happy expression (25% happy) was used for neutral stimuli. This resulted in 70 different facial stimuli. The decision to use morphed faces reflected our desire to increase the variability in displayed levels of emotion, beyond that displayed in tasks relying on repeated presentations of prototypical 100% face-emotion displays. This aims to reduce the habituation associated with repeatedly viewing identical face emotions. In the data analysis, face-viewing events were categorized with weights reflecting these levels of emotion.
Each face stimulus was presented for 2500 msec with a 500-msec interstimulus interval. An event-related design whereby all events occurred randomly throughout the task was used. The subjects were required to judge stimulus gender and respond by pressing a left or right response button. Each run consisted of 29 3000-msec fixation point trials, 20 neutral faces, and 10 of each intensity of both expressions; i.e., there were 80 face stimuli per run and 109 trials in total.
fMRI Parameters
Whole-brain BOLD fMRI data were acquired using a 1.5 T GE MRI scanner (Milwaukee). Following sagittal localization, functional T 2 *-weighted images were acquired using an echoplanar single-shot gradient-echo pulse sequence with a matrix of 64×64 mm, TR=3000 msec, TE=30 msec, field of view=240 mm, and voxels of 3.75×3.75×4 mm. Images were acquired in 31 contiguous 4-mm axial slices per brain volume. The functional data were acquired over four runs, each lasting 5 minutes and 27 seconds. In the same session, a high-resolution T 1 -weighted anatomical image was acquired to aid with spatial normalization (three-dimensional spoiled gradient-recalled acquisition in a steady state, TR=8.1 msec, TE=3.2 msec, flip angle=20°, field of view=240 mm, 124 axial slices, thickness=1.0 mm, 256×256 acquisition matrix).
fMRI Analysis
Data were analyzed within the framework of the general linear model with Analysis of Functional Neuroimages (28) . Both individual- and group-level analyses were conducted. The first four volumes in each scan series, collected before equilibrium magnetization was reached, were discarded. Motion correction was performed by registering all volumes in the echoplanar imaging data set to a volume collected shortly before acquisition of the anatomical data set.
The echoplanar imaging data sets for each subject were spatially smoothed (with an isotropic 6-mm Gaussian kernel) to reduce the influence of anatomical variability among the individual maps in generating group maps. Next, the time series data were normalized by dividing the signal intensity of a voxel at each time point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percent signal change from the mean. Following this, regressors depicting each of the three emotions (fearful, angry, and neutral) were created by convolving the train of stimulus events with a gamma-variate hemodynamic response function to account for the slow hemodynamic response. The fearful and angry regressors were weighted according to the proportion of the emotional prototypes (intensity) in the presented faces. With this approach, the regressor for each face-emotion type effectively models activation associated with all of the gradations of one or another face-emotion type. Linear regression modeling was performed with the regressors described above plus regressors to model a first-order baseline drift function. This produced for each voxel and each regressor a beta coefficient and its associated t statistic.
Voxelwise group analyses involved transforming single subject beta coefficients into the stereotaxic spatial array of Talairach and Tournoux (29) . Subsequently, a 3 (group: generalized social phobia without generalized anxiety disorder, generalized anxiety disorder, and healthy comparison individuals) by 3 (emotion: fearful, angry, and neutral) analysis of variance (ANOVA) was performed to produce statistical maps of the two main effects and the group-by-emotion interaction. To correct for multiple comparisons, we performed a spatial clustering operation using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) with 1,000 Monte Carlo simulations taking into account the entire echoplanar imaging matrix. This procedure yielded a minimum cluster size of six voxels (337.5 mm 3 ) to generate a map-wise, whole-brain-corrected false positive probability of p<0.05.
This initial, omnibus statistical approach produced regions of interest in which activation clusters surpassed the map-wise threshold. To facilitate interpretation of these results, the average percent signal change was measured within each region of interest so that mean values for each event type in each subject could be extracted and group-level statistics performed within SPSS (SPSS, Chicago).
Results
Behavioral and Movement Data
Because the error rate was extremely low (less than 2%), with no significant difference across groups, no analyses were conducted on the behavioral data. The three groups did not differ significantly in their movement across runs (F<1.0, 2, 48, n.s.).
fMRI Data
The BOLD response data were analyzed by a 3 (group: generalized social phobia without generalized anxiety disorder, generalized anxiety disorder, and healthy comparison individuals) by 3 (emotion: fearful, angry, and neutral) ANOVA. A secondary 3 (group: generalized social phobia without generalized anxiety disorder, generalized anxiety disorder, and healthy comparison individuals) by 3 (emotion: fearful, angry, and neutral) ANOVA was performed with exclusion of the generalized social phobia patient that also presented with major depressive disorder. The exclusion of that patient resulted in only minimal differences, which did not significantly change the main findings. This revealed significant group-by-emotion interactions as well as main effects for group and emotion.
Group-by-Emotion Interaction
Regions showing a group-by-emotion interaction included the middle frontal gyrus/frontal polar cortex (Brodmann’s area 10), the lateral frontal cortex (Brodmann’s area 46), the rostral anterior cingulate cortex (Brodmann’s area 32/Brodmann’s area 10), the inferior (Brodmann’s area 20) and superior (Brodmann’s area 38) temporal cortex, the culmen, and the amygdala ( Table 2 ). To clarify factors that contributed to these interactions, follow-up analyses were implemented on data extracted from functional regions of interest identified as interactions.
In all regions, the patients with generalized social phobia without generalized anxiety disorder showed significantly greater activity to fearful relative to neutral expressions (df=1,16, p<0.05). In addition, in all regions, they showed significantly greater activity to fearful relative to neutral expressions compared to the generalized anxiety disorder (df=1, 32, p<0.05) and the healthy comparison groups (df=1, 32, p<0.05, except in the amygdala, where F=2.89, df=1, 32, p<0.10).
In contrast, the patients with generalized anxiety disorder and the healthy comparison individuals did not show significantly different responses for fearful relative to neutral expressions in these regions, except the amygdala. Within the amygdala, unlike in the other two groups, the patients with generalized anxiety disorder showed similar responses to fearful and neutral expressions (F<1.0, df=1, 16, n.s.). Moreover, they showed significantly less activity to fearful relative to neutral expressions, compared to the group with generalized social phobia without generalized anxiety disorder (F=6.94, df=1–32, p<0.05) and relative to the healthy comparison groups (F=4.30, df=1, 32, p<0.05). Thus, patients with generalized anxiety disorder exhibited a unique response relative to the other two groups. Of importance, this result within the amygdala was not due to group differences in the response to neutral stimuli; these were not significant (F<1.0, df=2, 48, n.s.) ( Figure 1 ). Rather, it reflected significant group differences in the response to fearful stimuli (F=3.47, df=2, 48, p<0.05).

a Blood-oxygen-level-dependent responses within the right amygdala, the left middle frontal gyrus, and the left middle frontal gyrus.
Patients with generalized anxiety disorder did show significantly elevated responses to angry relative to neutral expressions relative to healthy comparison individuals in the lateral region of the middle frontal gyrus (Brodmann’s area 10: x, y, z=42, 51, 15), the inferior temporal cortex, and the culmen (F=10.13, 11.07, 4.11, respectively, df=1, 32, p<0.05). However, unlike for the amygdala, their responses within these three regions did not significantly differ from those shown by the patients with generalized social phobia without generalized anxiety disorder, who also showed elevated responses to angry relative to neutral expressions relative to the healthy comparison individuals in the lateral region of the middle frontal gyrus and the inferior temporal gyrus (F=13.97, 4.90, respectively, df=1, 32, p<0.05) (see Figure 2 ).

a Blood-oxygen-level-dependent responses within the right middle frontal gyrus and the inferior temporal gyrus.
Main Effect of Group
The regions that showed a differential BOLD response for group included the bilateral inferior frontal cortex (Brodmann’s area 47) and the medial orbital frontal cortex (Brodmann’s area 11). In the lingual gyrus, healthy comparison individuals showed significantly greater activation than both anxiety groups (F=5.00, df=1, 32, p<0.05, for generalized social phobia without generalized anxiety disorder, and F=18.48, df=1, 32, p=0.001, for generalized anxiety disorder, respectively). In all other regions, however, the generalized social phobia without generalized anxiety disorder group showed significantly greater activation than both the generalized anxiety disorder and healthy comparison groups (df=1, 32, p<0.05) ( Table 2 ).
Main Effect of Emotion
The regions that showed a differential BOLD response for emotion included bilateral regions of the middle frontal gyrus (Brodmann’s areas 6 and 9) and the left inferior frontal cortex (Brodmann’s area 47). In all cases, fearful and angry expressions were associated with greater BOLD responses than neutral expressions (df=1, 50, p<0.05) ( Table 2 ).
Patients With Generalized Anxiety Disorder and Generalized Social Phobia
Seven of the patients with generalized anxiety disorder also presented with generalized social phobia. The parameter estimates for these seven patients are also depicted in Figures 1 and 2 for the functional regions of interest generated from the ANOVA above. As can be seen, their data parallels that shown by the patients with generalized anxiety disorder rather than those with generalized social phobia without generalized anxiety disorder. To confirm this impression, we conducted a secondary 2 (group: generalized anxiety disorder with generalized social phobia and generalized social phobia without generalized anxiety disorder) by 3 (emotion: fearful, angry, and neutral) ANOVA on the BOLD response data. The results of this ANOVA were broadly consistent with the results from our main ANOVA. Significant group-by-emotion interactions emerged in the right amygdala and in the middle frontal gyrus/frontal polar cortex (Brodmann’s area 10) (x, y, z=–25, 59, 25 and 23, 71, 4, compared to –19, 61, 14, from our main ANOVA; F=10.97, 11.52, respectively). In both regions, the patients with generalized social phobia without generalized anxiety disorder showed elevated responses to fearful relative to neutral expression (F=5.88, 4.91, df=1, 16, p<0.05 and 0.06, respectively), whereas the patients with generalized anxiety disorder with generalized social phobia did not. In addition, in all regions, the patients with generalized social phobia without generalized anxiety disorder showed significantly greater activity to fearful relative to neutral expressions compared to the patients with generalized anxiety disorder with generalized social phobia (df=1, 22, p<0.05).
Functional Correlational Analysis
Our correlational analyses focused on the atypical neural responses most likely to be related to generalized social phobia and generalized anxiety disorder symptom profiles. For the generalized social phobia symptom profile, this was the heightened amygdala response to emotional expressions previously related to the anxiety symptom profile in this population (15) . With respect to generalized anxiety disorder, we were interested in investigating the implications of either heightened amygdala (cf. reference 25 ) or lateral regions of the orbital/inferior frontal cortex (Brodmann’s area 10 or 47; cf. reference 19 ) responsiveness. For completion, the data involving the amygdala and generalized anxiety disorder and Brodmann’s area 10 and generalized social phobia without generalized anxiety disorder are also reported.
In generalized social phobia without generalized anxiety disorder, the increase in activation within the right amygdala to both fearful and angry, relative to neutral, expressions significantly correlated with anxiety symptoms on the Beck Anxiety Inventory (Pearson’s r=0.530, 0.551, p<0.05) ( Figure 3 ). However, no relationships emerged with the Liebowitz Social Anxiety Scale or the State-Trait Anxiety Inventory (Pearson’s r=0.288, 0.093, and 0.332, 0.082, respectively; n.s.).
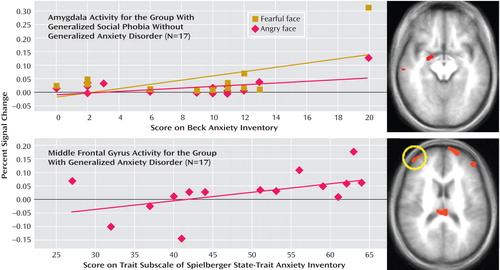
a The significant correlation shown for the generalized social phobia without generalized anxiety disorder group for amygdala activity and Beck Anxiety Inventory scores and the generalized anxiety disorder group for middle frontal gyrus activity and State-Trait Anxiety Inventory scores.
In generalized anxiety disorder, no correlation involving the amygdala was significant (Pearson’s r range=–0.036 to 0.174, n.s.). For both fearful- and angry-face responses, the amygdala-Beck Anxiety Inventory correlation tended to be greater in patients with generalized social phobia than in patients with generalized anxiety disorder (z=1.81, 2.10, p<0.10 and 0.05).
We next examined the correlations between anxiety severity and engagement within the region of the lateral frontal cortex (Brodmann’s area 10), which had shown perturbed engagement to angry versus neutral expressions in generalized anxiety disorder. The State-Trait Anxiety Inventory exhibited a significant positive relationship with neural engagement in this region (Pearson’s r=0.506, p<0.05) ( Figure 3 ); no associations emerged in generalized anxiety disorder for this region, however, with scores on the Liebowitz Social Anxiety Scale or the Beck Anxiety Inventory (Pearson’s r=0.200 and 0.137, respectively, n.s.). In generalized social phobia without generalized anxiety disorder, no correlation involving frontal regions was significant (Pearson’s r range=–0.122 to 0.302, n.s.). However, for the Brodmann’s area 10 frontal region exhibiting a correlation with State-Trait Anxiety Inventory in generalized anxiety disorder, the difference in the activation-symptom association between the two patient groups was only significant at a liberal statistical threshold (z=1.67, p<0.10).
Discussion
The current study compared BOLD responses to fearful, angry, and neutral expressions among healthy subjects, patients with generalized social phobia without generalized anxiety disorder, and patients with generalized anxiety disorder. These data were used to address two questions, both related to the nature of relationships between neural architecture and these two intricately related anxiety disorders. First, to what extent do these two disorders exhibit similar or different patterns of perturbed neural responses? Second, to what extent does symptom severity in the two conditions exhibit overlapping or distinct associations with patterns of perturbed neural responses?
Our results provide provocative, initial evidence that these two conditions might be dissociable. Generalized social phobia without generalized anxiety disorder was particularly associated with increased responses to fearful expressions, relative to the healthy individuals, in several regions including the amygdala. Generalized anxiety disorder, however, was not. In fact, within the amygdala, patients with generalized anxiety disorder showed an anomalously reduced response to fearful expressions relative to both the generalized social phobia without generalized anxiety disorder and healthy individual groups. Of interest, at least in some attention states, although not in others, prior research on adolescent generalized anxiety disorder also finds evidence of such an anomalously reduced amygdala response (18) . Generalized anxiety disorder was marked by increased responses to angry expressions in the frontal cortex, particularly a lateral region of the middle frontal gyrus, another finding observed previously in adolescents (19) . Moreover, in both instances, region-specific patterns of atypical neural responding were associated with anxiety severity levels.
The current data suggest that the neural correlates of generalized social phobia without generalized anxiety disorder differ from those of generalized anxiety disorder. The group-by-emotion interactions revealed an exaggerated response to fearful relative to neutral expressions in generalized social phobia without generalized anxiety disorder relative to the healthy individuals. This was seen in the middle frontal gyrus/frontal polar cortex (Brodmann’s area 10), the lateral frontal cortex (Brodmann’s area 46), the superior temporal cortex (Brodmann’s area 38), and the right amygdala. In contrast, for fearful expressions, the patients with generalized anxiety disorder showed no indication in any brain region of significantly elevated responses relative to the two other groups. However, they did show an exaggerated response to angry relative to neutral expressions in relation to healthy comparison individuals in a lateral region of the middle frontal gyrus (Brodmann’s area 10), the inferior temporal cortex, and the culmen. Their response within these regions did not differ from that in patients with generalized social phobia without generalized anxiety disorder.
Generalized social phobia, particularly when it occurs with comorbid generalized anxiety disorder, might be viewed as the more severe expression of perturbations in the same neural regions linked to generalized anxiety disorder (30 , 31) . We suggest that two findings clearly show that this is not the case; rather, the neural responding in these two anxiety disorders can be dissociated.
First, patients with generalized anxiety disorder showed an anomalously reduced amygdala response to fearful expressions, relative to both the healthy comparison individuals and the patients with generalized social phobia without generalized anxiety disorder. Moreover, the seven patients with generalized anxiety disorder with comorbid generalized social phobia resembled other patients with noncomorbid generalized anxiety disorder, with reduced amygdala responses. Although such data in this small group remain far from definitive, a view of anxiety disorders as amygdala-based conditions lying on a severity continuum would not predict anomalously reduced responding in any generalized anxiety disorder group , either with or without generalized social phobia. Parenthetically, these data also raise important broader questions on the degree to which distinct neurocognitive processes may give rise to social anxiety in patients with, relative to those without, broader patterns of worry.
Second, symptom severity correlated with the amygdala response to fearful expressions only in generalized social phobia without generalized anxiety disorder but not in generalized anxiety disorder. Rather, in this latter group, symptom level correlated significantly with the lateral frontal cortex response to angry expressions. Consistent with prior data on family aggregation and treatment response (5 – 8) , these data suggest that generalized anxiety disorder and generalized social phobia represent dissociable conditions.
Although considerable previous research implicates the amygdala in generalized social phobia, relatively few imaging studies on generalized anxiety disorder examine function in the amygdala or any other brain region. In general, studies do not report significant amygdala response to emotional stimuli in the disorder (19 , 23 , 24) . Of interest, two recent studies in adolescent generalized anxiety disorder did find enhanced amygdala response to emotional faces, in patients both with and without generalized social phobia, with no differences between the comorbid and noncomorbid forms of the condition (18 , 20) . However, in each instance, this pattern emerged against a background of associated perturbations in the frontal cortex. As such, these data suggest that when amygdala dysfunction manifests in generalized anxiety disorder, it represents an expression of broader perturbations in amygdala-frontal circuitry. When considered in light of the current and prior data on amygdala involvement in generalized social phobia, enhanced frontal engagement in the current study and in Monk et al. (19) , in the absence of perturbed amygdala function, might be viewed as suggesting that frontal pathology, specifically, plays a relatively more important role in generalized anxiety disorder than in generalized social phobia.

1. Schneier FR: Social anxiety disorder. BMJ 2003; 327:515–516Google Scholar
2. Stein MB, Kean YM: Disability and quality of life in social phobia: epidemiologic findings. Am J Psychiatry 2000; 157:1606–1613; correction 157:2075Google Scholar
3. Pine DS, Cohen P, Gurley D, Brook J, Ma Y: The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry 1998; 55:56–64Google Scholar
4. Bruce SE, Machan JT, Dyck I, Keller MB: Infrequency of “pure” GAD: impact of psychiatric comorbidity on clinical course. Depress Anxiety 2001; 14:219–225Google Scholar
5. Coelho HF, Cooper PJ, Murray L: A family study of co-morbidity between generalized social phobia and generalized anxiety disorder in a non-clinic sample. J Affect Disord 2007; 100:103–113Google Scholar
6. Rickels K, Rynn M: Pharmacotherapy of generalized anxiety disorder. J Clin Psychiatry 2002; 63 (suppl 14):9–16Google Scholar
7. Liebowitz MR, Ninan PT, Schneier FR, Blanco C: Integrating neurobiology and psychopathology into evidence-based treatment of social anxiety disorder CNS Spectr 2005; 10 (suppl 13):1–12Google Scholar
8. Blanco C, Schneier FR, Schmidt A, Blanco-Jerez CR, Marshall RD, Sanchez-Lacay A, Liebowitz MR: Pharmacological treatment of social anxiety disorder: a meta-analysis. Depress Anxiety 2003; 18:29–40Google Scholar
9. Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG: Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 2002; 59:1027–1034Google Scholar
10. Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS: Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry 2005; 57:975–981Google Scholar
11. Straube T, Mentzel HJ, Glauer M, Miltner WH: Brain activation to phobia-related words in phobic subjects. Neurosci Lett 2004; 372:204–208Google Scholar
12. Straube T, Mentzel HJ, Miltner WH: Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology 2005; 52:163–168Google Scholar
13. Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH: Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry 2004; 56:921–930Google Scholar
14. Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H: fMRI reveals amygdala activation to human faces in social phobics. Neuroreport 1998; 9:1223–1226Google Scholar
15. Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME: Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry 2006; 59:424–429Google Scholar
16. Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL: Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-tesla functional MRI study. Psychiatry Res 2007; 154:93–98Google Scholar
17. Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH: Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res 2006; 148:55–59Google Scholar
18. McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS: Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 2007; 64:97–106Google Scholar
19. Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJ, Chen G, Charney DS, Ernst M, Pine DS: Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry 2006; 163:1091–1097Google Scholar
20. Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, Chen G, McClure-Tone EB, Ernst M, Pine DS: Amygdala and ventromedial prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry (in press)Google Scholar
21. Nutt DJ: Neurobiological mechanisms in generalized anxiety disorder. J Clin Psychiatry 2001; 62(suppl 11):22–27Google Scholar
22. Stein DJ, Westenberg HG, Liebowitz MR: Social anxiety disorder and generalized anxiety disorder: serotonergic and dopaminergic neurocircuitry. J Clin Psychiatry 2002; 63(suppl 6):12–19Google Scholar
23. Wu JC, Buchsbaum MS, Hershey TG, Hazlett E, Sicotte N, Johnson JC: PET in generalized anxiety disorder. Biol Psychiatry 1991; 29:1181–1199Google Scholar
24. Hoehn-Saric R, Schlund MW, Wong SH: Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Res 2004; 131:11–21Google Scholar
25. McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Leibenluft E, Ernst M, Pine DS: fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl) 2007; 191:97–105Google Scholar
26. First M, Spitzer R, Gibbon M, Williams J: Structured Clinical Interview for DSM-IV. Washington, DC, American Psychiatric Press, 1997Google Scholar
27. Ekman P, Friesen WV: Pictures of Facial Affect. Palo Alto, Calif, Consulting Psychologists Press, 1976Google Scholar
28. Cox RW: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Google Scholar
29. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany, Thieme, 1988Google Scholar
30. Buckley PJ, Michels R, Mackinnon RA: Changes in the psychiatric landscape. Am J Psychiatry 2006; 163:757–760Google Scholar
31. Hyman SE: Can neuroscience be integrated into the DSM-V? Nat Rev Neurosci 2007; 8:725–732Google Scholar





