Deficits in Social Cognition and Response Flexibility in Pediatric Bipolar Disorder
Abstract
OBJECTIVE: Little is known about neuropsychological and social-cognitive function in patients with pediatric bipolar disorder. Identification of specific deficits and strengths that characterize pediatric bipolar disorder would facilitate advances in diagnosis, treatment, and research on pathophysiology. The purpose of this study was to test the hypothesis that youths with bipolar disorder would perform more poorly than matched healthy comparison subjects on measures of social cognition, motor inhibition, and response flexibility. METHOD: Forty outpatients with pediatric bipolar disorder and 22 comparison subjects (no differences in age, gender, and IQ) completed measures of social cognition (the pragmatic judgment subtest of the Comprehensive Assessment of Spoken Language, facial expression recognition subtests of the Diagnostic Analysis of Nonverbal Accuracy Scale, the oral expression subtest of the Test of Language Competence), inhibition and response flexibility (stop and stop-change tasks), and motor inhibition (continuous performance tasks). RESULTS: Pediatric bipolar disorder patients performed more poorly than comparison subjects on social-cognitive measures (pragmatic judgment of language, facial expression recognition) and on a task requiring response flexibility. These deficits were present in euthymic patients. Differences between patients and comparison subjects could not be attributed to comorbid attention deficit hyperactivity disorder. CONCLUSIONS: Findings of impaired social cognition and response flexibility in youths with pediatric bipolar disorder suggest continuity between pediatric bipolar disorder and adult bipolar disorder. These findings provide a foundation for neurocognitive research designed to identify the neural mechanisms underlying these deficits.
For adults with bipolar disorder, research has begun to delineate a neuropsychological and social-cognitive profile characterized by deficits in verbal memory and sustained attention (1–6) and by differences from healthy comparison subjects in facial expression processing (7–11). Less is known about neuropsychological and social-cognitive features of pediatric bipolar disorder. In particular, the delineation of trait markers of vulnerability, or potential endophenotypes, for pediatric bipolar disorder could facilitate diagnosis and treatment. Neuropsychological testing provides one means for identifying such markers, which can then be studied by using neuroimaging to identify dysfunction in neural circuitry. Based on preliminary data, clinical observation, and findings in adults, two sets of cognitive skills—social cognition and response flexibility (flexible alternation between motor inhibition and execution)—represent candidate neuropsychological correlates of narrow-phenotype pediatric bipolar disorder, in which the full DSM-IV criteria for bipolar disorder are met (12).
Social Cognition
Youths with pediatric bipolar disorder demonstrate marked social impairments (13), despite normal social functioning before illness onset (14). These impairments remain imprecisely characterized, however, and examination of social-cognitive skills may enhance understanding of the mechanisms underlying functional outcomes in pediatric bipolar disorder.
Children and adults with bipolar disorder perform differently from comparison subjects and individuals with other disorders on social-cognitive measures, particularly facial expression processing tasks (7, 8, 15). Symptomatic adults with bipolar disorder also have difficulty using contextual cues to infer others’ mental states (3). Less research has focused on expressive aspects of social cognition; clinical observation of youths with pediatric bipolar disorder, however, suggests that language pragmatics (i.e., appropriate social use of verbal/nonverbal language) may be problematic.
This pattern of aberrant social-cognitive skill suggests dysfunction in neural structures thought to mediate social, emotional, and possibly pragmatic linguistic processing (16–18). These structures include the ventrolateral and medial prefrontal cortices and the amygdala (19–21), which exhibit structural and/or functional anomalies in bipolar disorder (22–27).
Inhibition and Response Flexibility
Studies demonstrate differences between individuals with bipolar disorder and comparison subjects on tasks involving motor inhibition or shifting. These measures require either simple inhibition of a prepotent response or substitution of an alternate response for an inhibited behavior. Although studies using continuous performance tasks have yielded consistent findings of discrimination deficits, they have provided mixed evidence of impaired inhibition in adults with symptomatic bipolar disorder (4, 28, 29) and no evidence of inhibitory deficits in adolescents with remitted bipolar disorder (30).
Research regarding inhibition with alternate responding, or response flexibility, in bipolar disorder is limited. However, performance on related measures involving reward-related response reversal or attentional set-shifting showed impairment in adults (29) and youths with bipolar disorder (31, 32). These findings suggest diminished flexibility in response to environmental contingencies.
Converging evidence from neuroimaging and lesion studies indicates that the inferior/ventrolateral prefrontal cortices modulate both motor inhibition and execution of alternative responses (33–35). In light of the structural and functional aberrations observed in the ventral prefrontal cortex in individuals with bipolar disorder (22–27), further characterization of both inhibition and response flexibility in pediatric bipolar disorder is warranted.
We hypothesized that patients with pediatric bipolar disorder would perform more poorly than comparison subjects on social-cognitive, inhibition, and response flexibility tasks. In addition, we explored the effects on patients’ performance of current mood state and comorbid attention deficit hyperactivity disorder (ADHD). As noted earlier, we were particularly interested in identifying trait-related deficits.
Method
Subjects
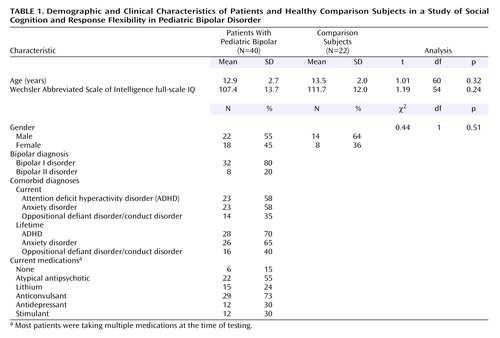
Patients consisted of 40 youths who met the DSM-IV diagnostic criteria for pediatric bipolar disorder. Table 1 shows demographic data. Best-estimate diagnoses were based on data integrated from several sources, including separate child and parent interviews by clinicians with master’s-level or higher-level credentials using the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (36), treating physicians’ assessments, and medical records. Diagnoses were generated in consensus conferences that included all interviewers and were chaired by two psychiatrists (K.E.T., E.L.) with extensive experience assessing children with pediatric bipolar disorder. All children exhibited narrow phenotype pediatric bipolar disorder (each had experienced at least one hypomanic or manic episode that met the full DSM-IV duration criterion and included expansive, elevated mood) (12). Comorbid disorders were common (Table 1); 83% met the criteria for at least one additional current disorder, generally an anxiety disorder, ADHD, or oppositional defiant disorder. ADHD and oppositional defiant disorder diagnoses frequently overlapped. Of the 23 patients with ADHD, 48% (N=11) had comorbid oppositional defiant disorder; only three (12%) of the 14 youths with oppositional defiant disorder did not have current ADHD. Patients with severe pervasive developmental disorder, substance use within the past 3 months, or IQ <70 were excluded.
Mood state was assessed at the time of each task. Youths were classified as euthymic or symptomatic at each time point on the basis of parent/child summary scores on the Young Mania Rating Scale (37) and the Children’s Depression Rating Scale (38). Symptomatic youths had Young Mania Rating Scale scores >12 and/or Children’s Depression Rating Scale scores >40. For euthymic youths, Young Mania Rating Scale scores ranged from 0 to 11 (mean=3.7 to mean=5.4) and Children’s Depression Rating Scale scores ranged from 18 to 40 (mean=22.9 to mean=26.1). For symptomatic youth, Young Mania Rating Scale scores ranged from 0 to 30 (mean=15.9 to mean=18.0) and Children’s Depression Rating Scale scores ranged from 18 to 76 (mean=29.1 to mean=33.4). All but six patients were receiving medication (Table 1).
The comparison subjects consisted of 22 youths identified as diagnosis-free according to the K-SADS-PL (36). Comparison subjects were excluded if they or a first-degree relative met the criteria for any DSM-IV diagnosis (family history was ascertained by a clinical interview of the parent), if they reported substance use within the past 3 months, or if they had an IQ <70.
The groups did not differ in age or Wechsler Abbreviated Scale of Intelligence (39) IQ (Table 1). No group differences in gender were evident; however, because the male-to-female ratio in the two groups was unequal, we examined effects of gender on task performance. The results were significant for only one measure; for analyses of this measure, gender was covaried. The National Institute of Mental Health (NIMH) Institutional Review Board approved the study; each participant and a parent provided written informed consent/assent.
Procedures
Subjects completed testing during outpatient visits to NIMH. Verbal language tasks were administered by licensed psychologists; other tasks were administered by trained research assistants.
Measures
Social cognition
The pragmatic judgment subtest of the Comprehensive Assessment of Spoken Language (40) assesses awareness of appropriate language for varied social situations. Participants respond verbally to social vignettes that elicit polite interruptions, introductions, etc. The total standard score was the dependent variable.
Two subtests from the Diagnostic Analysis of Nonverbal Accuracy Scale (41) were administered to evaluate identification of emotional facial expressions. Participants viewed standardized photographs of children (N=24) and adults (N=24) displaying high- and low-intensity expressions of happiness, sadness, anger, or fear. After viewing each picture for 2 seconds, the participant identified which of the four expressions was displayed. For both the child and adult facial expression subtests, the total number of errors was the dependent variable. Post hoc analyses were also conducted to examine discriminability for each emotion. Discriminability (hit rate minus false-alarm rate) provides a measure of sensitivity in discriminating among expression types (42). Higher values indicate greater sensitivity.
To examine contributions of other aspects of expressive language to group differences in social cognition, we administered the oral expression subtest of the Test of Language Competence (43). This subtest requires participants to assemble verbal concepts into grammatically correct sentences; the total T score from this measure was covaried in analyses of social-cognitive variables.
Inhibition and response flexibility
Participants completed the stop and stop-change tasks (44, 45). Whereas the stop task indexes simple motor inhibition, the stop-change task measures ability to inhibit a prepotent response and substitute an alternative response. The stop task consists of go trials (participants are instructed to press “1” as quickly as possible when “X” appears or to press “2” when “O” appears) and stop trials (participants are instructed not to respond when the “X” or “O” appears followed by a change to a red background [the stop signal]). In the stop-change task, change trials were substituted for stop trials. In the change trials, subjects were instructed to press “3” rather than “1” or “2” when the background changed to blue (change signal) after the “X” or “O” appeared. On the first stop or change trial in a block, the signal appeared 250 msec after the “X” or “O.” If the subject responded correctly, the next signal appeared 50 msec later, making inhibition more difficult. If the subject responded incorrectly, the next signal appeared 50 msec earlier, making inhibition easier. The timing of each subsequent “signal” trial was based on the previous trial of the same type.
The stop signal reaction time (speed of inhibition) and the change signal reaction time (speed of inhibition plus execution of an alternate response) were calculated by using an interpolation algorithm (45, 46). Stop signal reaction time and change signal reaction time served as dependent measures.
Motor inhibition
Indices from three continuous performance tasks served as measures of motor inhibition. The AX Continuous Performance Test requires participants to sustain attention while making conditional discriminations of targets and nontargets (47). Response bias, or failure to inhibit response to a nontargeted cue, served as the predictor variable. We also report data regarding a measure of discriminability, consistent with the literature on adult bipolar disorder.
The Flanker Continuous Performance Test is a test of selective attention that requires inhibition of a prepotent response when “incongruent” distractor stimuli appear (48, 49). The degree to which the presence of distractors during the incongruent trials impaired performance, relative to neutral trials (reaction time cost), served as the dependent variable.
The Identical Pairs Continuous Performance Test taxes both attention and working memory (50). Subjects are required to press a button whenever two identical stimuli appear consecutively within a sequence of rapidly flashed trials. The task includes 30 such target pairs; 29 “catch” trials, in which two successive stimuli are similar, but not identical; and 90 trials in which stimuli are randomly organized and dissimilar. Response bias, or failure to inhibit response to nontargeted cues, was the dependent variable.
Statistical Analysis
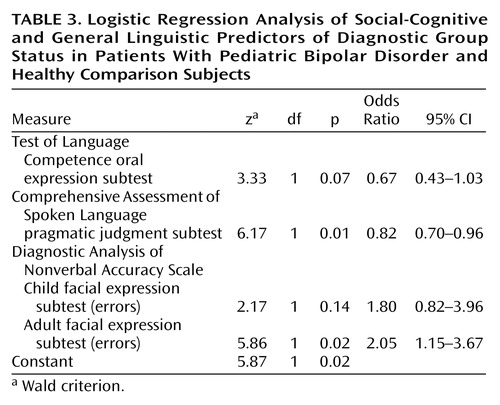
Data for variables with significantly skewed distributions according to Shapiro-Wilk statistics were analyzed with nonparametric statistical tests. Data for normally distributed variables were examined by using parametric tests. We first performed Student’s t tests or Mann-Whitney U tests for each target measure to identify significant differences between pediatric bipolar disorder patients and comparison subjects. To examine whether group differences on social-cognitive measures could be accounted for by general language competence, we followed the unprotected comparisons with a logistic regression analysis. Group (pediatric bipolar disorder group, comparison group) was the outcome variable, and the Comprehensive Assessment of Spoken Language pragmatic judgment score and Diagnostic Analysis of Nonverbal Accuracy Scale adult and child facial expression total error scores were the predictors. Test of Language Competence oral expression score was covaried.
To study effects of mood state, we conducted post hoc analyses of the variance of normally distributed variables between pediatric bipolar disorder patients (euthymic versus symptomatic) and comparison subjects, followed by pairwise comparisons with Tukey’s honestly significant difference test where omnibus effects were significant. For nonnormally distributed variables, we conducted Kruskal-Wallis H tests, followed by post hoc Wilcoxon tests. We then used the previously described procedures to compare results on each measure for the healthy subjects with those for the pediatric bipolar disorder patients with and without current ADHD and with those for the pediatric bipolar disorder patients with and without current anxiety disorders. We set alpha at 0.05 for all tests.
Because of time constraints or symptom severity during testing, not all participants completed every task.
Results
Social Cognition
The pediatric bipolar disorder group scored significantly lower than the comparison subjects on the Comprehensive Assessment of Spoken Language pragmatic judgment test (p<0.001) and made significantly more errors on the Diagnostic Analysis of Nonverbal Accuracy Scale child facial expression (p=0.01) and adult facial expression (p<0.01) subtests (Table 2). Scores on the Comprehensive Assessment of Spoken Language pragmatic judgment test and the Diagnostic Analysis of Nonverbal Accuracy Scale child and adult facial expression subtests were entered into a logistic regression model predicting group membership (pediatric bipolar disorder group, comparison group). Test of Language Competence oral expression score was covaried. The predictors, as a set, reliably distinguished between youths with pediatric bipolar disorder and healthy comparison subjects (χ2=36.6, df=4, N=49, p<0.001); 84% of the pediatric bipolar disorder group, 89% of the comparison group, and 86% of all participants were classified correctly (Table 3). According to the Wald criterion, only the Comprehensive Assessment of Spoken Language pragmatic judgment score (z=6.17, p=0.01) and the Diagnostic Analysis of Nonverbal Accuracy Scale adult facial expression total errors score (z=5.86, p=0.02) reliably predicted group status.
Post hoc Mann-Whitney U tests of discriminability scores for each emotion within each Diagnostic Analysis of Nonverbal Accuracy Scale subtest indicated that youths with pediatric bipolar disorder, relative to the comparison subjects, were less sensitive to happiness (z=–2.07, p=0.04) and anger (z=–2.78, p<0.01) in children and to sadness (z=–3.62, p<0.001) and anger (z=–2.43, p=0.02) in adults (Table 2).
Response Flexibility/Inhibition
The groups did not differ on inhibition measures, including the AX, Flanker, and Identical Pairs Continuous Performance Tests and stop signal reaction time (p>0.05) but did differ significantly on the AX Continuous Performance Test discriminability measure (p=0.004) (Table 2). The pediatric bipolar disorder group also had a significantly longer change signal reaction time than did the comparison subjects, whether gender was covaried (p=0.03) or not (p=0.02), suggesting a deficit in response flexibility.
Post Hoc Analyses: Mood State
Social-cognitive tasks
Exploratory Kruskal-Wallis H tests comparing the healthy subjects, the euthymic pediatric bipolar disorder patients, and the symptomatic pediatric bipolar disorder patients yielded significant omnibus differences among the three groups on the Comprehensive Assessment of Spoken Language pragmatic judgment test score (p<0.001) and the total errors scores on the Diagnostic Analysis of Nonverbal Accuracy Scale child facial expression (p=0.02) and adult facial expression (p=0.02) subtests (Table 2). Post hoc pairwise comparisons indicated that both pediatric bipolar disorder patient groups performed significantly more poorly than the healthy comparison subjects on all three measures (p<0.05).
Response-flexibility/inhibition tasks
Comparisons of the healthy subjects, euthymic pediatric bipolar disorder patients, and symptomatic pediatric bipolar disorder patients indicated significant omnibus differences on change signal reaction time, whether gender was covaried (p=0.05) or not (p=0.02), but not on any other motor inhibition measures (all p>0.05). Post hoc contrasts showed that only euthymic pediatric bipolar disorder patients performed more poorly than the healthy comparison subjects on change signal reaction time (gender covaried: p<0.05; no covariates: p=0.01).
Post Hoc Analyses: Comorbid Diagnoses
Exploratory comparisons indicated significant omnibus differences among the pediatric bipolar disorder patients with ADHD, the pediatric bipolar disorder patients without ADHD, and the comparison subjects on the social-cognitive measures (Comprehensive Assessment of Spoken Language pragmatic judgment: χ2=16.72, df=2, N=52, p<0.001; Diagnostic Analysis of Nonverbal Accuracy Scale adult facial expressions: χ2=8.92, df=2, N=60, p=0.01; Diagnostic Analysis of Nonverbal Accuracy Scale child facial expressions: χ2=6.30, df=2, N=58, p=0.04), but not on the inhibition/response flexibility measures (AX Continuous Performance Test response bias: χ2=3.31, df=2, N=55, p=0.19; Flanker Continuous Performance Test reaction time cost: χ2=3.01, df=2, N=55, p=0.21; Identical Pairs Continuous Performance Test response bias: χ2=1.58, df=2, N=55, p=0.45; stop signal reaction time: F=1.55, df=2, 59, p=0.22), except for change signal reaction time (F=3.25, df=2, 55, p<0.05, with gender covaried). Post hoc comparisons indicated that the patients with and without ADHD performed worse than the comparison subjects on all three social-cognitive measures (p<0.05 for each comparison) and that the patients without ADHD had poorer change signal reaction time scores than the comparison subjects (p<0.05). There were no significant effects of current anxiety disorder on any measure (all p>0.05).
Discussion
Results indicate deficits in social cognition and motor flexibility in patients with narrow-phenotype pediatric bipolar disorder. Relative to comparison subjects of comparable age, gender, and IQ, youths with pediatric bipolar disorder performed more poorly on tasks involving facial emotion identification and formulation of socially appropriate responses to interpersonal situations. Indeed, performance on social-cognitive tasks correctly classified 86% of the study participants. Although the patients’ motor inhibition was not clearly impaired, they showed deficient response flexibility when required both to inhibit a prepotent behavior and to execute an alternate response (the stop-change task). No group differences were attributable to comorbid ADHD.
The patients all had “narrow-phenotype” pediatric bipolar disorder, which has a clinical presentation similar to that of adult bipolar disorder (12, 51). Like symptomatic adults with bipolar disorder, the patients in our study showed deficits in recognition of facial affect (9). The patients also showed significant deficits in expressive pragmatic language. Although adults with bipolar disorder have difficulty interpreting cues about others’ mental states (3, 7, 9), little research has examined expressive language pragmatics in this population. Thus, although both children and adults with bipolar disorder have social-cognitive deficits, further study is needed to clarify these deficits and their continuity across development. Use of measures that can be implemented consistently across development would facilitate such research. In addition, comparison of performance across individuals with varied psychiatric disorders will be important to determine if the observed deficits are specific to bipolar disorder.
The results regarding response flexibility and inhibition showed some consistency with reports in the literature on adult bipolar disorder. In particular, the group differences on change signal reaction time, a measure of response flexibility, resemble reports in adult bipolar disorder and pediatric bipolar disorder of deficits on tasks that require both inhibition and flexible responding (29, 31). Also like other studies, our study showed no clear deficits on simple motor inhibition tasks (4, 28–30). Both the literature and our data suggest that, across the developmental spectrum of bipolar disorder, deficits may be more marked on tasks that pair inhibition and execution of alternative behaviors than on tasks that involve inhibition alone. This pattern of performance could reflect many factors, ranging from differences in task complexity to differences in neural mechanisms mediating the two task types.
The present study yields evidence of some behavioral continuity across narrow-phenotype pediatric bipolar disorder and adult bipolar disorder. An important next step will be to elucidate the neural mechanisms underlying social-cognitive and response-flexibility deficits in bipolar disorder. Evidence indicates that regions including the ventral prefrontal cortex and amygdala mediate facial affect processing and that the ventral prefrontal cortex and other prefrontal areas contribute to complex inhibitory functions (16, 18, 19, 33, 34, 52). Less is known about the neural mechanisms underlying pragmatic language, although research has implicated the frontal and temporal cortical regions (17). Given findings of structural and functional anomalies in the prefrontal cortex and amygdala in bipolar disorder (22–24, 26), neuroimaging studies implementing measures of social cognition and response flexibility in pediatric bipolar disorder patients are warranted. Optimally, such studies should include clinical comparison groups to evaluate the specificity of observed anomalies.
Our inclusion of euthymic and symptomatic pediatric bipolar disorder patients allowed us to examine performance in the context of both normal and clinically significant mood states. Both euthymic and symptomatic patients performed more poorly than comparison subjects on measures of expressive pragmatic language and facial expression identification. This pattern of findings raises the possibility that social-cognitive deficits may represent trait-based vulnerability markers and possibly endophenotypes for pediatric bipolar disorder. On a measure of response flexibility, the one other task where pediatric bipolar disorder patients exhibited deficits, only euthymic patients differed significantly from comparison subjects. This finding suggests that response-flexibility deficits may represent an additional trait vulnerability marker for pediatric bipolar disorder. However, the lack of difference between the symptomatic patients and the comparison subjects is puzzling. This negative finding, which may reflect type II error, requires further study. Replication in proband and high-risk groups, as well as longitudinal study of patients across both euthymic and symptomatic states, is warranted.
This study had several limitations. The number of subjects was relatively small, and most of the pediatric bipolar disorder patients were receiving medication. However, given that group differences were selective, it appears unlikely that they were entirely related to medication. Nonetheless, examination of social cognition and response inhibition/flexibility in unmedicated youths with bipolar disorder and in children at risk for bipolar disorder is indicated. In addition, direct comparison of youths with pediatric bipolar disorder and those with other disorders, particularly ADHD/oppositional defiant disorder and the broad phenotype of pediatric bipolar disorder (12), would provide further evidence about the specificity of our findings. We are currently conducting studies to address these limitations.
In summary, our findings, along with those from other recent studies (31), begin to delineate a neuropsychological and social-cognitive profile for narrow-phenotype pediatric bipolar disorder. These results offer evidence of continuity between pediatric bipolar disorder and adult bipolar disorder, highlighting the need for further study using comparable measures in both populations. In addition, our findings of impairment among pediatric bipolar disorder patients on tasks involving pragmatic language, facial affect recognition, and response flexibility lay the groundwork for neurocognitive research aimed at elucidating neural mediators of these deficits.
 |
 |
 |
Received April 12, 2004; revision received Aug. 23, 2004; accepted Sept. 24, 2004. From the Mood and Anxiety Disorders Program, National Institute of Mental Health. Address correspondence and reprint requests to Dr. McClure, Mood and Anxiety Disorders Program, NIMH, 15K North Dr., Room 102, MSC 2670, Bethesda, MD 20892; [email protected] (e-mail). The authors thank the study participants and their families and the NIMH Mood and Anxiety Disorders Program staff who contributed to this work.
1. Cavanagh J, Van Beck M, Muir W, Blackwood D: Case-control study of neurocognitive function in euthymic patients with bipolar disorder: an association with mania. Br J Psychiatry 2002; 180:320–326Crossref, Medline, Google Scholar
2. Clark L, Iversen SD, Goodwin GM: A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry 2001; 158:1605–1611Link, Google Scholar
3. Kerr N, Dunbar RIM, Bentall RP: Theory of mind deficits in bipolar affective disorder. J Affect Disord 2003; 73:253–259Crossref, Medline, Google Scholar
4. Liu SK, Chiu C-H, Chang C-J, Hwang T-J, Hwu H-G, Chen WJ: Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. Am J Psychiatry 2002; 159:975–982Link, Google Scholar
5. Quraishi S, Frangou S: Neuropsychology of bipolar disorder: a review. J Affect Disord 2002; 72:209–226Crossref, Medline, Google Scholar
6. Van Gorp W, Altshuler L, Theberge D, Mintz J: Declarative and procedural memory in bipolar disorder. Biol Psychiatry 1999; 46:525–531Crossref, Medline, Google Scholar
7. Harmer CJ, Grayson L, Goodwin GM: Enhanced recognition of disgust in bipolar illness. Biol Psychiatry 2002; 51:298–304Crossref, Medline, Google Scholar
8. Lembke A, Ketter TA: Impaired recognition of facial emotion in mania. Am J Psychiatry 2002; 159:302–304Link, Google Scholar
9. Getz GE, Shear PK, Strakowski SM: Facial affect recognition deficits in bipolar disorder. J Int Neuropsychol Soc 2003; 9:623–632Crossref, Medline, Google Scholar
10. Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML: Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 2004; 55:578–587Crossref, Medline, Google Scholar
11. Phillips ML, Drevets WC, Rauch SL, Lane R: Neurobiology of emotion perception, II: implications for major psychiatric disorders. Biol Psychiatry 2003; 54:515–528Crossref, Medline, Google Scholar
12. Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS: Defining clinical phenotypes of juvenile mania. Am J Psychiatry 2003; 160:430–437Link, Google Scholar
13. Geller B, Bolhofner K, Craney JL, Williams M, DelBello MP, Gundersen K: Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. J Am Acad Child Adolesc Psychiatry 2000; 39:1543–1548Crossref, Medline, Google Scholar
14. Kutcher S, Robertson HA, Bird D: Premorbid functioning in adolescent onset bipolar I disorder: a preliminary report from an ongoing study. J Affect Disord 1998; 51:137–144Crossref, Medline, Google Scholar
15. McClure EB, Pope K, Hoberman AJ, Pine DS, Leibenluft E: Facial expression recognition in adolescents with mood and anxiety disorders. Am J Psychiatry 2003; 160:1172–1174Link, Google Scholar
16. Haxby JV, Hoffman EA, Gobbini MI: Human neural systems for face recognition and social communication. Biol Psychiatry 2002; 51:59–67Crossref, Medline, Google Scholar
17. Kuperberg GR, Holcomb PJ, Sitnikova T, Greve D, Dale AM, Caplan D: Distinct patterns of neural modulation during the processing of conceptual and syntactic anomalies. J Cogn Neurosci 2003; 15:272–293Crossref, Medline, Google Scholar
18. Adolphs R: The neurobiology of social cognition. Curr Opin Neurobiol 2001; 11:231–239Crossref, Medline, Google Scholar
19. Adolphs R, Tranel D, Damasio AR: The human amygdala in social judgment. Nature 1998; 393:470–474Crossref, Medline, Google Scholar
20. Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS: Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage 2003; 20:420–428Crossref, Medline, Google Scholar
21. Wood JN, Romero SG, Makale M, Grafman J: Category-specific representations of social and nonsocial knowledge in the human prefrontal cortex. J Cogn Neurosci 2003; 15:236–248Crossref, Medline, Google Scholar
22. Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T, Epstein J, Isenberg N, McBride PA, Kemperman I, Emmerich S, Dhawan V, Eidelberg D, Kocsis JH, Silbersweig DA: Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999; 156:1986–1988Abstract, Google Scholar
23. Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck PE Jr, Hawkins JM: Frontosubcortical neuroanatomy and the Continuous Performance Test in mania. Am J Psychiatry 1999; 156:139–141Link, Google Scholar
24. Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD: fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord 2000; 2(3, part 2):237–248Google Scholar
25. DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM: Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord 2004; 6:43–52Crossref, Medline, Google Scholar
26. Blumberg HP, Martin A, Kaufman J, Leung H-C, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH, Peterson BS: Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry 2003; 160:1345–1347Link, Google Scholar
27. Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS: Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry 2003; 60:1201–1208Crossref, Medline, Google Scholar
28. Sax KW, Strakowski SM, McElroy SL, Keck PE Jr, West SA: Attention and formal thought disorder in mixed and pure mania. Biol Psychiatry 1995; 37:420–423Crossref, Medline, Google Scholar
29. Clark L, Iversen SD, Goodwin GM: Sustained attention deficit in bipolar disorder. Br J Psychiatry 2002; 180:313–319Crossref, Medline, Google Scholar
30. Robertson HA, Kutcher SP, Lagace DC: No evidence of attentional deficits in stabilized bipolar youth relative to unipolar and control comparators. Bipolar Disord 2003; 5:330–339Crossref, Medline, Google Scholar
31. Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E: Neuropsychological performance in bipolar disorder. Biol Psychiatry 2004; 55:32–39Crossref, Medline, Google Scholar
32. Gorrindo T, Blair RJR, Budhani S, Dickstein D, Pine DS, Leibenluft E: Probabilistic response reversal deficits in pediatric bipolar disorder. Am J Psychiatry (in press)Google Scholar
33. Menon V, Adleman NE, White CD, Glover GH, Reiss AL: Error-related brain activation during a go/no-go response inhibition task. Hum Brain Mapp 2001; 12:131–143Crossref, Medline, Google Scholar
34. Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SCR, Giampietro V, Andrew CM, Taylor E: Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage 2001; 13:250–261Crossref, Medline, Google Scholar
35. Rubia K, Smith AB, Brammer MJ, Taylor E: Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 2003; 20:351–358Crossref, Medline, Google Scholar
36. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
37. Young RC, Biggs JT, Ziegler VE, Meyer DA: A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
38. Poznanski EO, Freeman LN, Mokros HB: Children’s Depression Rating Scale—Revised (September 1984). Psychopharmacol Bull 1985; 21:979–989Google Scholar
39. Wechsler D: Wechsler Abbreviated Scale of Intelligence. San Antonio, Tex, Psychological Corp, 1999Google Scholar
40. Carrow-Woolfolk E: Comprehensive Assessment of Spoken Language (CASL). Circle Pines, Minn, American Guidance Service, 1999Google Scholar
41. Nowicki S, Duke MP: Individual differences in the nonverbal communication of affect: the Diagnostic Analysis of Nonverbal Accuracy Scale. J Nonverbal Behavior 1994; 18:9–35Crossref, Google Scholar
42. Snodgrass J, Corwin J: Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 1988; 117:34–50Crossref, Medline, Google Scholar
43. Wiig E, Secord W: Test of Language Competence (TLC). San Antonio, Tex, Psychological Corp, 1989Google Scholar
44. Logan GD: On the ability to inhibit thought and action: a users’ guide to the stop signal paradigm, in Inhibitory Processes in Attention, Memory, and Language. Edited by Dagenbach D, Carr TH. San Diego, Academic Press, 1994, pp 189–239Google Scholar
45. Logan GD, Schachar RJ, Tannock R: Impulsivity and inhibitory control. Psychol Sci 1997; 8:60–64Crossref, Google Scholar
46. Williams BR, Pones JS, Schachar RJ, Logan GD, Tannock R: Development of inhibitory control across the life span. Dev Psychol 1999; 35:205–213Crossref, Medline, Google Scholar
47. Rosvold H, Mirsky A, Sarason I, Bransome EJ, Beck L: A continuous performance test of brain damage. J Consult Psychol 1956; 20:343–350Crossref, Medline, Google Scholar
48. Eriksen B, Eriksen C: Effects of noise letters upon the identification of target letter in a nonsearch task. Percept Psychophys 1974; 16:143–149Crossref, Google Scholar
49. Posner M: Orienting of attention: the 7th Sir FC Bartlett Lecture. Q J Exp Psychol 1980; 32:3–25Crossref, Medline, Google Scholar
50. Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L: The Continuous Performance Test, Identical Pairs Version (CPT-IP), I: new findings about sustained attention in normal families. Psychiatry Res 1988; 26:223–238Crossref, Medline, Google Scholar
51. National Institute of Mental Health research roundtable on prepubertal bipolar disorder. J Am Acad Child Adolesc Psychiatry 2001; 40:871–878Crossref, Medline, Google Scholar
52. Kringelbach ML, Rolls ET: Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage 2003; 20:1371–1383Crossref, Medline, Google Scholar



