Equivalent Occupancy of Dopamine D1 and D2 Receptors With Clozapine: Differentiation From Other Atypical Antipsychotics
Abstract
OBJECTIVE: Clozapine, the prototype of atypical antipsychotics, remains unique in its efficacy in the treatment of refractory schizophrenia. Its affinity for dopamine D4 receptors, serotonin 5-HT2A receptor antagonism, effects on the noradrenergic system, and its relatively moderate occupancy of D2 receptors are unlikely to be the critical mechanism underlying its efficacy. In an attempt to elucidate the molecular/synaptic mechanism underlying clozapine’s distinctiveness in refractory schizophrenia, the authors studied the in vivo D1 and D2 receptor profile of clozapine compared with other atypical antipsychotics. METHOD: Positron emission tomography with the radioligands [11C]SCH23390 and [11C]raclopride was used to investigate D1 and D2 receptor occupancy in vivo in 25 schizophrenia patients receiving atypical antipsychotic treatment with clozapine, olanzapine, quetiapine, or risperidone. RESULTS: Mean striatal D1 occupancies ranged from 55% with clozapine to 12% with quetiapine (rank order: clozapine > olanzapine > risperidone > quetiapine). The striatal D2 occupancy ranged from 81% with risperidone to 30% with quetiapine (rank order: risperidone > olanzapine > clozapine > quetiapine). The ratio of striatal D1/D2 occupancy was significantly higher for clozapine (0.88) relative to olanzapine (0.54), quetiapine (0.41), or risperidone (0.31). CONCLUSIONS: Among the atypical antipsychotics, clozapine appears to have a simultaneous and equivalent occupancy of dopamine D1 and D2 receptors. Whether its effect on D1 receptors represents agonism or antagonism is not yet clear, as this issue is still unresolved in the preclinical arena. This distinctive effect on D1/D2 receptors may be responsible for clozapine’s unique effectiveness in patients with schizophrenia refractory to other typical and atypical antipsychotics.
Despite the introduction of several new antipsychotics such as olanzapine, risperidone, and quetiapine—which share clozapine’s advantageous profile of a relatively low risk for extrapyramidal symptoms and no prolactin elevation—clozapine remains unique in its efficacy in the treatment of refractory schizophrenia (1–5). It has been established that patients with treatment-resistant schizophrenia may still improve from clozapine after nonresponse to the novel antipsychotic olanzapine (6), although one study reported equal efficacy of olanzapine and clozapine in otherwise treatment-resistant schizophrenia (7). Clozapine has also been noted to be superior to risperidone in patients suffering from severe chronic schizophrenia with poor previous treatment response (8) and in chronically hospitalized patients with treatment-resistant schizophrenia (9). In a study that used less stringent criteria for treatment resistance and that had a surprisingly high response rate to both risperidone and clozapine, risperidone reportedly showed equal efficacy to clozapine (10). Up to now no study to our knowledge has been carried out to compare clozapine with quetiapine in patients with treatment-resistant schizophrenia—although the general impression is that quetiapine is not especially effective in treatment-resistant cases. Thus, clozapine still remains the most effective antipsychotic in cases of schizophrenia otherwise unresponsive to treatment.
The pharmacological basis of clozapine’s low propensity to induce extrapyramidal symptoms can be explained by its combined antagonism at 5-HT2A and D2 receptors (11) or its fast dissociation from the D2 receptor (12), but none of these factors satisfactorily explain its unique efficacy in the treatment of refractory schizophrenia.
Various explanations have been put forward in an attempt to characterize clozapine’s pharmacological uniqueness, which include its affinity for the dopamine D4 receptor (13), its potent serotonin 5-HT2A receptor antagonism (14), and its robust alterations of noradrenergic biochemistry (15, 16). In addition, a hypothesis has been formulated stating that moderate occupancy of D2 receptors might be clinically superior to a more complete D2 blockade (17). None of these theories have held so far. In a double-blind, controlled study of 97 patients, the potent D4 and 5-HT2A receptor antagonist fananserin proved to be an ineffective antipsychotic (18). Further, the moderate D2 occupancy hypothesis was rejected because patients who had been receiving oral neuroleptics and who were switched to clozapine did not differ from patients who had been receiving depot medications (19). In the oral discontinuation group, one could expect to find a faster decline in D2 occupancy, and if moderate D2 blockade were the key to clozapine’s uniqueness, this group should have shown a more rapid response, which was not the case.
Clozapine’s relatively high affinity for the dopamine D1 receptor may be related to its unique clinical efficacy, as D1 receptors mediate the reward function in animal models, a principle thought relevant for the therapeutic action of antipsychotics (20). In vitro, clozapine shows a relatively high affinity for D1 receptors together with a moderate affinity for D2 receptors (21, 22). In man, clozapine showed a distinctively lower D2 occupancy and a higher D1 receptor occupancy compared with the typical neuroleptics (23), but no comparisons with the newer atypical antipsychotics are available.
We used positron emission tomography (PET) with the radioligands [11C]SCH23390 and [11C]raclopride to investigate D1 and D2 receptor occupancy in vivo in 25 schizophrenia patients being treated with the atypical antipsychotics clozapine, olanzapine, quetiapine, or risperidone.
Method
We included 25 patients (18 men and seven women; mean age=35.4 years, range=18–58) with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder in the study. Their diagnosis of schizophrenia was ascertained with the Structured Clinical Interview for DSM-IV, which was administered by an experienced psychiatrist (J.T., N.P.L.G.V., or O.A.). We recruited the patients from the Schizophrenia and Continuing Care Program of the Centre for Addiction and Mental Health in Toronto, where each patient received ongoing antipsychotic medication either as an inpatient or outpatient. All subjects gave their written consent after the procedure had been fully explained. The study and recruitment procedures were approved by the Research Ethics Board of the Centre for Addiction and Mental Health and the University of Toronto.
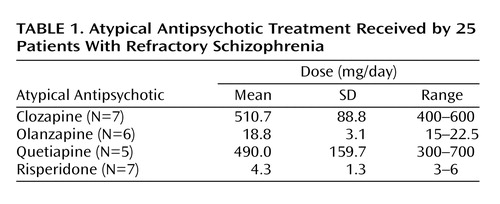
Patients had been receiving atypical antipsychotic treatment for at least 14 days before the PET study. A summary of the treatment regimens received by the patients is presented in Table 1. In general, medications were prescribed once daily, twice daily if that was not tolerated. At the day of the PET scans, all patients took their total daily dose of medication at once approximately 2 hours before the first PET scan, and their striatal D1 and D2 occupancy were measured subsequently.
PET Scanning Procedures
Striatal dopamine D2 receptor occupancy was determined by using 10.8 mCi (SD=2.7) of high-specific-activity [11C]raclopride (mean=692.9 Ci/mmol, SD=465.0) administered as a bolus plus continuous infusion. Striatal D1 receptor occupancies were determined by using 9.9 mCi (SD=0.6) of high-specific-activity [11C]SCH23390 (mean=875.7 Ci/mmol, SD=328.8) administered as a bolus. Imaging was performed with a Scanditronix/GEMS PC-2048–15B head scanner as described in previous publications (24–27). The PET scans were performed in a fixed order starting with the [11C]SCH23390 scan approximately 2 hours after the last dose and continuing with the [11C]raclopride scan approximately 5–6 hours postdose.
A magnetic resonance imaging (MRI) scan was obtained for each of the patients (GE Signa 1.5-T scanner, proton density maps) and was coregistered to the composite [11C]raclopride and [11C]SCH23390 PET scans by using RView8/mpr software (28). As described in previous publications (24–27), we drew the striatal (caudate plus putamen) and cerebellar regions of interest on two contiguous PET slices with reference to the overlapping coregistered MRI scan. The cerebellar time-activity curve was taken as an estimate of the free and nonspecific [11C]raclopride binding (29), while the striatal time-activity curve provided an estimate of specific binding to the D2 receptors plus free and nonspecific binding. Under these assumptions, it can be shown that the striatal-cerebellar ratio minus one, at the time when the binding is at equilibrium (30–75 minutes in the aforementioned scans), provides an index proportional to the Bmax/Kd ratio of [11C]raclopride for dopamine D2 receptors (referred to as the binding potential). In previous studies (30) we have demonstrated that this ratio method correlates very well (r>0.95) with analytically derived estimates of D2 binding potential, is highly reliable with a scan-rescan standard deviation of 6%, and has been standardized in our laboratory with excellent inter- and intrarater reliability (intraclass correlation coefficients >0.95). For estimation of specific binding to D1 receptors (plus free and nonspecific binding) in the striatum and frontal cortex, a ratio was calculated between those regions and the cerebellum as a reference region (27). Data from the left and right hemispheres were pooled for all subsequent calculations, since there was no significant asymmetry in D1 or D2 binding potentials.
Since we did not have baseline measures of D1 or D2 binding potentials for the patients, we used an age-corrected estimate from a comparison group of 29 untreated healthy volunteers who had no current or past axis I DSM-IV psychiatric disorder as determined with the Structured Clinical Interview for DSM-IV, nonpatient version, and had not taken any psychotropic medication in the 3 months preceding this study. Twelve subjects (five men and seven women) with a mean age of 32 years (SD=11, range=20–49) served as the comparison group for D1 binding potential, and 17 subjects (nine men and eight women) with a mean age of 29 years (SD=6, range=20–40) served as baseline D2 binding potential values.
Determination of Drug and Prolactin Plasma Levels
At the time of the PET scans, blood was drawn for a plasma drug level and prolactin level analysis. We determined clozapine, olanzapine, quetiapine, and risperidone levels in heparinized plasma using a liquid chromatography/mass spectroscopy method (31, 32). Prolactin levels were determined by using a two-site chemiluminometric immunoassay with a minimum detectable limit of 0.3 ng/ml and a coefficient of variance of 3.6% to 4.5% (ACS, Ciba-Corning Diagnostics, Corning, N.Y.).
Statistical Analysis
Statistical analyses were performed with SPSS for Windows 11.0.1 (SPSS, Inc., Chicago, 2001). Dopamine D1 and D2 receptor binding indices of different antipsychotics were compared by using a general linear model (univariate analysis of variance [ANOVA]) with post hoc Scheffé tests for differences between clozapine and other atypical antipsychotics. Putative relations between plasma prolactin levels and D1 or D2 occupancies, respectively, were calculated by using Pearson’s product-moment correlation coefficients. All tests were performed two-tailed with an alpha level of p<0.05 set as the threshold for statistical significance.
Results
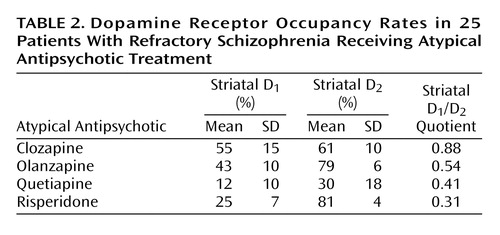
The mean striatal D1 occupancies ranged from 55% with clozapine to 12% with quetiapine, with the following rank order: clozapine > olanzapine > risperidone > quetiapine (Table 2). An ANOVA revealed that the differences in striatal D1 occupancy between groups were statistically significant (F=17.122, df=3, p<0.001). Post hoc Scheffé tests showed that the differences in D1 occupancies of clozapine versus risperidone (p=0.001) and versus quetiapine (p<0.001) were statistically significant, whereas the difference between clozapine and olanzapine did not reach statistical significance (p<0.36).
In the striatum, the D2 occupancy ranged from 81% for risperidone to 30% for quetiapine, with the following rank order: risperidone > olanzapine > clozapine > quetiapine. The differences in striatal D2 occupancies were statistically significant between groups (F=28.770, df=3, p<0.001). Post hoc tests revealed that clozapine showed a significantly lower D2 occupancy than risperidone (p<0.02) and olanzapine (p<0.04), but significantly higher D2 occupancy than quetiapine (p=0.001). The ratio of striatal D1/D2 occupancy as an index for a “balanced” or equivalent occupancy of D1 and D2 receptors was significantly higher for clozapine versus olanzapine (F=23.174, df=1, p=0.001), quetiapine (F=7.365, df=1, p<0.03), or risperidone (F=87.736, df=1, p<0.001) (Figure 1, Table 2).
Mean prolactin plasma levels were significantly different among the groups (F=8.421, df=3, p=0.001) and ranged from 9.0 μg/liter with clozapine to 15.3 μg/liter with olanzapine and quetiapine, and 39.2 μg/liter with risperidone. Post hoc tests revealed that plasma prolactin levels with risperidone were significantly higher than with any of the other three antipsychotics (p=0.001). However, there was no statistically significant difference between clozapine, olanzapine, and quetiapine. Furthermore, there was no significant correlation between plasma prolactin levels and striatal D1 (r=–0.36, df=23, p<0.09) or striatal D2 (r=0.30, df=23, p<0.20) occupancy.
Discussion
Clozapine occupies dopamine D1 and D2 receptors in vivo, which is in line with previous studies that used PET and [11C]SCH23390 (23) or [11C]raclopride (11). Our study represents the first attempt to compare clozapine’s action on D1 and D2 receptors to that of other novel antipsychotics—and we found that it is unique in this regard.
Our receptor occupancy results correspond with the D1 and D2 receptor affinity values found in preclinical studies: the Ki values for clozapine were 290–540 nM for D1 and 130–150 nM for D2, suggesting relatively similar affinity for both receptors (22, 33). Corresponding to the comparatively higher D2 than D1 occupancy with quetiapine and risperidone in our in vivo PET study, preclinical data suggest a 10- to 100-fold higher affinity for D2 receptors than for D1 receptors for both quetiapine and risperidone (22, 33). We found that olanzapine came closest to clozapine with regard to the “balanced” or equivalent occupancy of D1 and D2 receptors, still showing a D1/D2 occupancy ratio of 0.54. In line with that, preclinical data showed olanzapine’s Ki values at D1 of 52 nM and at D2 of 20 nM (33).
While we observed occupancy of D1 receptors, there is no broad consensus on clozapine’s intrinsic efficacy at these receptors. In a number of in vivo assays, clozapine has some preferential, although not selective, action to antagonize D1 receptor-mediated function (34). However, D1 antagonism by itself has not been an effective antipsychotic principle: studies with the selective D1 antagonists SCH23390 (35), SCH39166 (36–38), and NNC 01–0687 (39) by themselves were ineffective as antipsychotics. In addition, relatively brief treatment with SCH39166 in doses inducing a more than 70% occupancy of striatal D1 receptors failed to induce antipsychotic action (40).
On the other hand, there is some evidence that clozapine behaves as a D1 agonist: hypothermia produced by clozapine in rats was fully antagonized by either of the selective D1 receptor antagonists SCH23390 or NNC 01–687 (41). This aspect could be interesting given the clinical and laboratory observations implicating D1 receptor agonism in the prefrontal cortex in cognitive functions (41, 42). Finally, regardless of its agonist/antagonist action, a recent [18F]fluorodeoxyglucose PET study in patients suffering from treatment-resistant schizophrenia showed that brain metabolic and clinical responses to clozapine were related to D1 receptor genotype (43). After 5 weeks of treatment with clozapine, brain metabolic decreases were found in patients with the 2,2 but not the 1,2 D1 receptor genotype. Moreover, patients with the 2,2 D1 genotype significantly improved with clozapine, whereas those with a 1,2 D1 genotype did not (43).
We did not observe a simple relationship between prolactin plasma levels and D1 or D2 occupancy rates. This can be explained by the differential blood-brain disposition of the atypical antipsychotics under investigation. In line with our findings, risperidone has been shown to lead to higher prolactin levels than clozapine, olanzapine, or quetiapine (44). This fact is not directly related to dopamine receptor occupancy in the brain but is mainly due to differential blood-brain barrier penetration of atypical antipsychotics. It has been shown that risperidone has a comparably higher central to peripheral potency for prolactin elevation than olanzapine (44). Compounds with a higher peripheral potency bring about higher prolactin levels for a given level of functional central antagonism, and thus one cannot expect a simple linear relationship between plasma prolactin and dopamine receptor occupancy with different antipsychotics.
There are several limitations of the current study that suggest caution in how these results are interpreted. We compared D1 and D2 receptor binding potential values of patients treated with clozapine and other atypical antipsychotics to that of healthy subjects, since the patients were already receiving treatment and it is very difficult to find neuroleptic-naive patients with similar demographic characteristics. However, this is unlikely to induce a systematic bias in our results, since there is no clear evidence for alterations of striatal D1 or D2 receptor number in schizophrenia (45–50). Moreover, the main intent of the study was to compare antipsychotics. Since the same baseline was used for all of the agents, it is unlikely to have given rise to differences among antipsychotics.
Plasma drug levels were positively correlated with striatal D2 receptor occupancies in clozapine- and olanzapine-treated patients but not with quetiapine or risperidone, nor was there such a correlation between plasma drug levels and D1 occupancy. This is surprising but may be due to the fact that subjects were not randomly assigned to different doses. The apparent difference in the relationships between drug plasma levels and receptor occupancies on D1 and D2 receptors can partly be explained by differences in the central and plasma kinetics of the four antipsychotics. The [11C]SCH23390 PET scans to determine D1 occupancy were performed at around peak plasma levels for all antipsychotics, while the PET scan with [11C]raclopride was performed 3–4 hours later. Different time points of PET scans with regard to intake of the last dose of the medication do not make a difference with antipsychotics that show a sustained high blockade of dopamine receptors, such as olanzapine and risperidone (51). However, it is conceivable that with clozapine and quetiapine, given their more rapid decline from peak plasma concentration (26, 52), the second scan may have underestimated the peak occupancy values for D2 receptors.
In summary, this PET study in schizophrenia patients is consistent with the idea that clozapine has a unique interaction with the D1/D2 system as suggested by animal models. The relatively equivalent D1/D2 occupancy may explain the clinical uniqueness of clozapine in patients with refractory symptoms. These cross-sectional data provide a strong impetus for prospective clinical studies focusing on the role of dopamine D1 receptors, with the caveat that it is still unclear whether agonistic or antagonistic properties are desirable, along with moderate D2 antagonism as a means for enhanced therapeutic efficacy against psychosis.
 |
 |
Received April 22, 2003; revision received Nov. 4, 2003; accepted Nov. 12, 2003. From the University of Toronto Department of Psychiatry, Schizophrenia Program, and the Centre for Addiction and Mental Health PET Centre, Toronto; the Department of General Psychiatry, Medical University of Vienna, Austria; and the Kunin-Lunenfeld Applied Research Unit, Baycrest Centre for Geriatric Care, Toronto. Address reprint requests to Dr. Tauscher, Department of General Psychiatry, Medical University of Vienna, Austria, Währinger Gürtel 18–20, A-1090 Vienna, Austria; [email protected] (e-mail). Supported in part by a grant from Eli Lilly Canada. The authors thank the patients and the volunteers for their participation and Doug Hussey, Kevin Cheung, and Penny Barsoum for technical assistance.
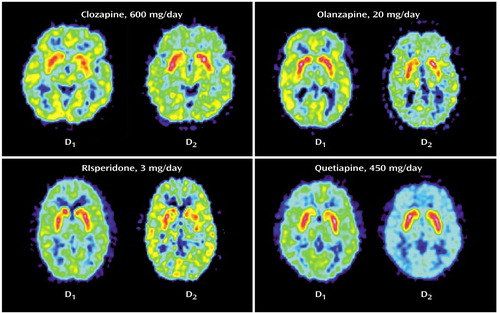
Figure 1. Summation PET Scan Images of D1 and D2 Receptor Occupancy in Patients With Refractory Schizophrenia Receiving Atypical Antipsychotic Treatment
1. Kane J, Honigfeld G, Singer J, Meltzer H (Clozaril Collaborative Study Group): Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
2. Kane JM, Marder SR, Schooler NR, Wirshing WC, Umbricht D, Baker RW, Wirshing DA, Safferman A, Ganguli R, McMeniman M, Borenstein M: Clozapine and haloperidol in moderately refractory schizophrenia: a 6-month randomized and double-blind comparison. Arch Gen Psychiatry 2001; 58:965–972Crossref, Medline, Google Scholar
3. Claghorn J, Honigfeld G, Abuzzahab FS Sr, Wang R, Steinbook R, Tuason V, Klerman G: The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol 1987; 7:377–384Crossref, Medline, Google Scholar
4. Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH: Clinical and biologic response to clozapine in patients with schizophrenia: crossover comparison with fluphenazine. Arch Gen Psychiatry 1992; 49:345–353Crossref, Medline, Google Scholar
5. Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT Jr: Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry 1994; 151:20–26Link, Google Scholar
6. Conley RR, Tamminga CA, Kelly DL, Richardson CM: Treatment-resistant schizophrenic patients respond to clozapine after olanzapine non-response. Biol Psychiatry 1999; 46:73–77Crossref, Medline, Google Scholar
7. Tollefson GD, Birkett MA, Kiesler GM, Wood AJ: Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry 2001; 49:52–63Crossref, Medline, Google Scholar
8. Azorin J-M, Spiegel R, Remington G, Vanelle J-M, Péré J-J, Giguere M, Bourdeix I: A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry 2001; 158:1305–1313Link, Google Scholar
9. Sharif ZA, Raza A, Ratakonda SS: Comparative efficacy of risperidone and clozapine in the treatment of patients with refractory schizophrenia or schizoaffective disorder: a retrospective analysis. J Clin Psychiatry 2000; 61:498–504Crossref, Medline, Google Scholar
10. Bondolfi G, Dufour H, Patris M, May JP, Billeter U, Eap CB, Baumann P (Risperidone Study Group): Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. Am J Psychiatry 1998; 155:499–504Link, Google Scholar
11. Farde L, Nordstrom AL, Nyberg S, Halldin C, Sedvall G: D1-, D2-, and 5-HT2-receptor occupancy in clozapine-treated patients. J Clin Psychiatry 1994; 55(suppl B):67–69Google Scholar
12. Kapur S, Seeman P: Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? a new hypothesis. Am J Psychiatry 2001; 158:360–369Link, Google Scholar
13. Seeman P: Dopamine receptor sequences: therapeutic levels of neuroleptics occupy D2 receptors, clozapine occupies D4. Neuropsychopharmacology 1992; 7:261–284Medline, Google Scholar
14. Meltzer HY: An overview of the mechanism of action of clozapine. J Clin Psychiatry 1994; 55(suppl B):47–52Google Scholar
15. Breier A, Buchanan RW, Waltrip RW II, Listwak S, Holmes C, Goldstein DS: The effect of clozapine on plasma norepinephrine: relationship to clinical efficacy. Neuropsychopharmacology 1994; 10:1–7Crossref, Medline, Google Scholar
16. Breier A: Clozapine and noradrenergic function: support for a novel hypothesis for superior efficacy. J Clin Psychiatry 1994; 55(suppl B):122–125Google Scholar
17. Meltzer HY, Gudelsky GA: Dopaminergic and serotonergic effects of clozapine: implications for a unique clinical profile. Arzneimittelforschung 1992; 42:268–272Medline, Google Scholar
18. Truffinet P, Tamminga CA, Fabre LF, Meltzer HY, Rivière ME, Papillon-Downey C: Placebo-controlled study of the D4/5-HT2A antagonist fananserin in the treatment of schizophrenia. Am J Psychiatry 1999; 156:419–425Abstract, Google Scholar
19. Carpenter WT Jr, Zito JM, Vitrai J, Volavka J: Hypothesis testing: is clozapine’s superior efficacy dependent on moderate D2 receptor occupancy? Biol Psychiatry 1998; 43:79–83Crossref, Medline, Google Scholar
20. Miller R, Wickens JR, Beninger RJ: Dopamine D-1 and D-2 receptors in relation to reward and performance: a case for the D-1 receptor as a primary site of therapeutic action of neuroleptic drugs. Prog Neurobiol 1990; 34:143–183Crossref, Medline, Google Scholar
21. Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT: Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996; 14:87–96Crossref, Medline, Google Scholar
22. Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996; 124:57–73Crossref, Medline, Google Scholar
23. Nordström A-L, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G: D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry 1995; 152:1444–1449Link, Google Scholar
24. Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA, Houle S: 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry 1998; 155:921–928Link, Google Scholar
25. Kapur SJ, Zipursky R, Jones C, Remington G, Houle S: Relationship between dopamine D2 occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 2000; 157:514–520Link, Google Scholar
26. Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P: A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry 2000; 57:553–559Crossref, Medline, Google Scholar
27. Verhoeff NP, Hussey D, Lee M, Tauscher J, Papatheodorou G, Wilson AA, Houle S, Kapur S: Dopamine depletion results in increased neostriatal D, but not D(1), receptor binding in humans. Mol Psychiatry 2002; 7:322–328Crossref, Google Scholar
28. Studholme C, Hill DL, Hawkes DJ: Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys 1997; 24:25–35Crossref, Medline, Google Scholar
29. Farde L, Hall H, Ehrin E, Sedvall G: Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 1986; 231:258–261Crossref, Medline, Google Scholar
30. Kapur S, Zipursky RB, Jones C, Remington GJ, Wilson AA, DaSilva J, Houle S: The D2 receptor occupancy profile of loxapine determined using PET. Neuropsychopharmacology 1996; 15:562–566Crossref, Medline, Google Scholar
31. Woestenborghs R, Lorreyne W, Van Rompaey F, Heykants J: Determination of risperidone and 9-hydroxyrisperidone in plasma, urine and animal tissues by high-performance liquid chromatography. J Chromatogr 1992; 583:223–230Crossref, Medline, Google Scholar
32. Berna M, Shugert R, Mullen J: Determination of olanzapine in human plasma and serum by liquid chromatography/tandem mass spectrometry. J Mass Spectrom 1998; 33:1003–1008Crossref, Medline, Google Scholar
33. Duncan GE, Zorn S, Lieberman JA: Mechanisms of typical and atypical antipsychotic drug action in relation to dopamine and NMDA receptor hypofunction hypotheses of schizophrenia. Mol Psychiatry 1999; 4:418–428Crossref, Medline, Google Scholar
34. Murray AM, Waddington JL: The interaction of clozapine with dopamine D1 versus dopamine D2 receptor-mediated function: behavioural indices. Eur J Pharmacol 1990; 186:79–86Crossref, Medline, Google Scholar
35. Gessa GL, Canu A, Del Zompo M, Burrai C, Serra G: Lack of acute antipsychotic effect of Sch 23390, a selective dopamine D1 receptor antagonist (letter). Lancet 1991; 337:854–855Crossref, Medline, Google Scholar
36. de Beaurepaire R, Labelle A, Naber D, Jones BD, Barnes TR: An open trial of the D1 antagonist SCH 39166 in six cases of acute psychotic states. Psychopharmacology (Berl) 1995; 121:323–327Crossref, Medline, Google Scholar
37. Den Boer JA, van Megen HJ, Fleischhacker WW, Louwerens JW, Slaap BR, Westenberg HG, Burrows GD, Srivastava ON: Differential effects of the D1-DA receptor antagonist SCH39166 on positive and negative symptoms of schizophrenia. Psychopharmacology (Berl) 1995; 121:317–322Crossref, Medline, Google Scholar
38. Karlsson P, Smith L, Farde L, Harnryd C, Sedvall G, Wiesel FA: Lack of apparent antipsychotic effect of the D1-dopamine receptor antagonist SCH39166 in acutely ill schizophrenic patients. Psychopharmacology (Berl) 1995; 121:309–316Crossref, Medline, Google Scholar
39. Karle J, Clemmesen L, Hansen L, Andersen M, Andersen J, Fensbo C, Sloth-Nielsen M, Skrumsager BK, Lublin H, Gerlach J: NNC 01–0687, a selective dopamine D1 receptor antagonist, in the treatment of schizophrenia. Psychopharmacology (Berl) 1995; 121:328–329Crossref, Medline, Google Scholar
40. Sedvall G, Farde L, Hall H, Halldin C, Karlsson P, Nordstrom AL, Nyberg S, Pauli S: Utilization of radioligands in schizophrenia research. Clin Neurosci 1995; 3:112–121Medline, Google Scholar
41. Ahlenius S: Clozapine: dopamine D1 receptor agonism in the prefrontal cortex as the code to decipher a Rosetta stone of antipsychotic drugs. Pharmacol Toxicol 1999; 84:193–196Crossref, Medline, Google Scholar
42. Goldman-Rakic PS, Muly EC III, Williams GV: D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev 2000; 31:295–301Crossref, Medline, Google Scholar
43. Potkin SG, Basile VS, Jin Y, Masellis M, Badri F, Keator D, Wu JC, Alva G, Carreon DT, Bunney WE, Fallon JH, Kennedy JL: D1 receptor alleles predict PET metabolic correlates of clinical response to clozapine. Mol Psychiatry 2003; 8:109–113Crossref, Medline, Google Scholar
44. Kapur S, Langlois X, Vinken P, Megens AA, De Coster R, Andrews JS: The differential effects of atypical antipsychotics on prolactin elevation are explained by their differential blood-brain disposition: a pharmacological analysis in rats. J Pharmacol Exp Ther 2002; 302:1129–1134Crossref, Medline, Google Scholar
45. Domyo T, Kurumaji A, Toru M: An increase in [3H]SCH23390 binding in the cerebral cortex of postmortem brains of chronic schizophrenics. J Neural Transm 2001; 108:1475–1484Crossref, Medline, Google Scholar
46. Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M: Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature 1997; 385:634–636Crossref, Medline, Google Scholar
47. Karlsson P, Farde L, Halldin C, Sedvall G: PET study of D1 dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry 2002; 159:761–767Link, Google Scholar
48. Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M: Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 2002; 22:3708–3719Crossref, Medline, Google Scholar
49. Farde L, Wiesel FA, Stone-Elander S, Halldin C, Nordstrom AL, Hall H, Sedvall G: D2 dopamine receptors in neuroleptic-naive schizophrenic patients: a positron emission tomography study with [11C]raclopride. Arch Gen Psychiatry 1990; 47:213–219Crossref, Medline, Google Scholar
50. Wong DF, Wagner HN Jr, Tune LE, Dannals RF, Pearlson GD, Links JM, Tamminga CA, Broussolle EP, Ravert HT, Wilson AA, et al: Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science 1986; 234:1558–1563; correction, 1987; 235:6230Google Scholar
51. Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S: Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry 2002; 7:317–321Crossref, Medline, Google Scholar
52. Tauscher-Wisniewski S, Kapur S, Tauscher J, Jones C, Daskalakis ZJ, Papatheodorou G, Epstein I, Christensen BK, Zipursky RB: Quetiapine: an effective antipsychotic in first-episode schizophrenia despite only transiently high dopamine-2 receptor blockade. J Clin Psychiatry 2002; 63:992–997Crossref, Medline, Google Scholar



