PET Study of D1 Dopamine Receptor Binding in Neuroleptic-Naive Patients With Schizophrenia
Abstract
OBJECTIVE: Postmortem studies and a positron emission tomography (PET) study have suggested that there is a disturbance of central D1 dopamine receptor function in schizophrenia. The objective of the present PET study was to compare D1 receptor binding in first-admission, neuroleptic-naive patients with schizophrenia and in healthy subjects. METHOD: Ten healthy comparison subjects and 10 neuroleptic-naive patients with schizophrenia (diagnosed according to DSM-III-R) were examined twice by PET using 11C-labeled SCH 23390 ([11C]SCH 23390) with high and low specific radioactivity, respectively. The binding potential, receptor density (Bmax), and affinity (Kd) were determined for the caudate nucleus, the putamen, and several neocortical regions during both PET examinations. Scatchard plots from the two measurements were used to calculate regional D1 Bmax and Kd. The regional binding values were tested for hemispheric asymmetry and for correlation to clinical symptoms measured by the Brief Psychiatric Rating Scale (BPRS). RESULTS: [11C]SCH 23390 binding to D1 receptors did not differ significantly between subjects with schizophrenia and healthy subjects in any of the brain regions or for any of the binding measures studied. Asymmetry of the regional binding values did not differ significantly between the two groups. Scores on the BPRS negative symptom subscale correlated significantly with the Bmax in the right frontal cortex. CONCLUSIONS: These results do not replicate previous postmortem and PET findings of altered central dopamine D1 receptor binding in schizophrenia. The finding of a positive correlation between frontal D1 binding and scores on the negative symptom subscale of the BPRS is contrary to a previously reported finding of a negative correlation. These discrepancies motivate further studies using D1 ligands with higher signals for cortical regions.
A disturbance of central dopaminergic neurotransmission in schizophrenia was postulated more than 30 years ago (1). Each of the five currently identified dopamine receptor subtypes (D1–D5) has been proposed to be related to the pathophysiological mechanisms of schizophrenia (2–5).
The D1-like receptors are more abundant than the D2-like receptors in the human brain because there is a high concentration of D1-like receptors not only in the striatum but also in the neocortex (6). The family of D1-like receptors includes the D1 and D5 dopamine receptor subtypes, which have similar biochemical and pharmacological characteristics but different distributions in the brain (for reviews see Sibley and Monsma [7], Sokoloff and Schwartz [8], and Missale et al. [9]). D1 dopamine receptor mRNA has been found in the striatum, in the neocortex, and in all limbic regions (10), but D5 dopamine receptor mRNA is found to a lesser degree in neocortical and limbic regions and very little in the striatum (11).
Several postmortem and experimental studies suggest a role for the central D1-like receptors in schizophrenia (for reviews see Knable and Weinberger [12] and Willner [13]). Reduced radioligand binding to striatal D1-like receptors in postmortem tissue from patients with schizophrenia has been reported in one study (14) but not all studies (15–17). A more intricate disturbance of D1 receptor function in the striatum has been proposed by Seeman et al. (18), who found evidence for the absence of a postulated link between D1 and D2 receptors in postmortem brain tissue.
Among the regions containing D1-like receptors, the prefrontal cortex has been suggested to have a critical role for some cognitive functions (19). Of particular interest is that animal models indicate that D1-like receptors are crucial for working memory (20–23), which is usually impaired in schizophrenia (24, 25).
11C-Labeled SCH 23390 ([11C]SCH 23390) is the first of several receptor ligands that bind to D1-like receptors and has been extensively used by positron emission tomography (PET) (26–32). At present no ligands are available that bind selectively to either the D1 or D5 subtype (33). In a preliminary PET study (34), we reported on D1 receptor binding in five of the patients initially recruited for the present study. There was no change in striatal or cortical D1 receptor density (Bmax) and affinity (Kd) in these five patients. The observation of unchanged striatal D1 binding was later supported by Okubo et al. (35), who reported reduced [11C]SCH 23390 binding in the prefrontal cortex of patients with schizophrenia. They also found that the frontal binding correlated inversely to negative symptoms and positively to cognitive performance.
In the current study, we used PET to determine D1 receptor binding in the caudate nucleus, the putamen, and five neocortical brain regions of 10 healthy volunteers and 10 first-admission, neuroleptic-naive patients with schizophrenia. Each subject participated in two PET measurements using [11C]SCH 23390 with high and low specific radioactivity, respectively. D1 dopamine receptor binding potential, Bmax, and Kd were calculated by using Scatchard analysis.
Method
Subjects
The study was performed in compliance with principles of the Declaration of Helsinki and was approved by the Ethics Committee and Isotope Committee of the Karolinska Hospital. After complete description of the study, all participating subjects provided written informed consent regarding the medical risks of the study. Ten healthy subjects (eight men and two women) were recruited. Their mean age was 26.3 years (SD=3.6), and their mean weight was 74.3 kg (SD=8.6). They were healthy according to history, physical examination, blood and urine chemistry, ECG, and MRI examination of the brain.
Exclusion criteria for the healthy volunteers and the patients were organic brain disease, other somatic disorder, history of head injury with loss of consciousness for more than 5 minutes, cranial fracture, previous treatment with antipsychotic drugs, clinically significant abnormal laboratory test results, pregnancy, and history of alcohol or drug abuse according to DSM-III-R criteria. Further exclusion criteria for the healthy volunteers were history or presence of any psychiatric disorder and history of psychiatric disorder in a first-degree relative.
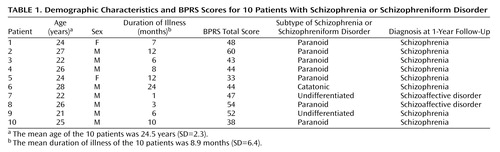
Patients were recruited from a consecutive series of 14 neuroleptic-naive patients with schizophrenia or schizophreniform psychosis diagnosed according to DSM-III-R who were admitted for the first time to the Psychiatric Clinic at the Karolinska Hospital (Table 1). Four of the original 14 patients were excluded for hostility or uncooperativeness. The remaining 10 patients (eight men and two women) were included in the study. Their mean age was 24.5 years (SD=2.3), and their mean body weight was 65.8 kg (SD=9.3).
The patient and comparison groups were matched for age (t=1.34, df=18, p=0.20). The patients had a lower mean body weight than the comparison subjects (t=2.13, df=18, p=0.05), a difference that should not have an effect on the PET measurements (36). The time period from appearance of the first prodromal symptom to the PET examination varied from 1 to 24 months. Patient records and at least one family member confirmed this time period, the case history, and the fact that the patient had never received neuroleptics. Each patient was followed prospectively for 1 year after inclusion in the study.
For sedation, a benzodiazepine was chosen because it has been reported that the benzodiazepine lorazepam has no effect on [11C]SCH 23390 binding in the brain (37). Oxazepam was selected because it has a relatively short half-life and lack of active metabolites. Shortly before the PET, three patients (numbers 2, 7, and 8) received 10, 50, and 25 mg of oxazepam orally, respectively, for anxiety.
Clinical Ratings
Patients’ clinical symptoms were rated by using the 18-item Brief Psychiatric Rating Scale (BPRS) (each item rated on a 0–6 scale) (38). The overall total rating and scores on positive and negative symptom clusters were used (39). The positive symptom cluster consists of conceptual disorganization, suspiciousness, hallucinatory behavior, and unusual thought content (BPRS items 4, 11, 12, and 15). The negative symptom cluster consists of emotional withdrawal, motor retardation, and blunted affect (BPRS items 3, 13 and 16).
Head Fixation System and MRI Examination
A previously described head fixation system (40) was used during both MRI and PET. MRI scans were performed on a 1.5-T Signa unit (General Electric, Milwaukee). A standard spin-echo sequence with a 256 × 256 matrix was used with a repetition time of 4 seconds. Echo times were 17 msec for proton-density-weighted images and 85 msec for T2-weighted images.
Radiochemistry
[11C]SCH 23390 with high specific radioactivity (range=321–2061 Ci/mmol) was prepared by methylation of the corresponding desmethyl precursor analog (SCH 24518) by using [11C]methyl iodide (27). [11C]SCH 23390 with low specific radioactivity (range=1.7–4.1 Ci/mmol) was prepared by the addition of unlabeled SCH 23390 according to a procedure described previously (30).
PET Measurements
All subjects participated in two PET measurements. The first was after bolus intravenous injection of [11C]SCH 23390 with high specific radioactivity (200–327 MBq) at about 10:00 a.m., and the second was after bolus intravenous injection of [11C]SCH 23390 with low specific radioactivity (196–361 MBq) at about 3:00 p.m. on the same day.
The Scanditronix PC2048-15B PET system (Uppsala, Sweden) measures radioactivity in 15 horizontal sections covering the whole extension of the brain (41). The system has a resolution of 4.5 mm (full width at half maximum) in the image plane; the section thickness is 6.2 mm. To control for attenuation, a transmission scan was performed before each measurement. In each PET measurement, data were acquired in a series of 16 consecutive time frames for 33 minutes (1–9: 20 seconds each; 10–13: 3 minutes each, and 14–16: 6 minutes each). The acquisition time of 33 minutes assured that peak equilibrium was reached during the PET measurement (20 minutes) (30, 32).
Regions of Interest
Regions of interest were delineated manually on the proton-weighted MRI images for the caudate nucleus and the putamen on all of the sections in which they appeared (four to five sections). The regions of interest were transferred to the corresponding PET images. For the frontal, lateral temporal, medial temporal, and occipital cortices and the anterior cingulate gyrus, 10-mm-wide elongated regions of interest were delineated directly on the PET images on the four mid-sections. Regions of interest for the cerebellum were drawn on the two mid-PET sections.
Each region of interest was pooled ipsilaterally from the set of sections into a volume of interest. The radioactivity concentration in each volume of interest was calculated for each sequential time frame and corrected for carbon-11 decay.
Scatchard Analysis
D1 Bmax and Kd were determined by using a two-point Scatchard analysis (30). The cerebellum has negligible density of D1 receptors (6, 42, 43), and the radioactivity in this region was used as an estimate for the free radioligand concentration in the brain (29). Radioactivity representing specific [11C]SCH 23390 binding to D1 receptors (CB) was defined as the difference between the total radioactivity in a brain region (CROI[t]) and the free radioligand concentration in the brain (CF[t]) according to the following equation:
A sum of three exponential functions was fitted to the time curves for the total radioactivity in a brain region and radioligand concentration in the brain. Peak equilibrium was defined as occurring when [11C]SCH 23390 binding to D1 receptors was maximal (44, 45). The values for the two components of binding potential—bound radioligand (B) and free radioligand (F)—were obtained at equilibrium by dividing the radioactivity concentration (nCi/ml) by the specific radioactivity (Ci/mmol) of [11C]SCH 23390. The two sets of binding potential (B/F) and B values obtained from the two PET experiments, with high and low specific radioactivity respectively, were used to solve for Bmax (nmol/liter) and Kd (nmol/liter) in the Scatchard equation:
The binding potential at high specific radioactivity was defined as the binding potential of [11C]SCH 23390 (44, 45). Hemispheric asymmetry of the binding potential and Bmax values was calculated for each region with the asymmetry index ([right–left]/[right+left]).
Adjustment for Age Effect
Okubo et al. (35) found a significant age decline for the [11C]SCH 23390 binding potential in the striatum and the frontal cortex in 40 human subjects between the ages of 21 and 40. Another PET study has reported a similar age effect for the [11C]SCH 23390 binding potential (46). In the current study, 28 human subjects who were 22 to 34 years old showed no significant age decline in any regional binding potential, and age did not differ significantly between healthy subjects and patients with schizophrenia. Therefore, the binding potential was not corrected for age. Neither were the Kd values corrected for age, because a postmortem study (47) did not find age-related changes in D1 receptor binding affinity.
Statistical Analysis
The statistical analyses were performed by using the software JMP (SAS Institute, Cary, N.C.) implemented on a Macintosh computer. The comparison and patient groups were compared regarding MRI volumes, regional binding measures, and asymmetry index by using a two-tailed t test. Alpha incorporating a Bonferroni correction for multiple comparisons was set at 0.007. The association between the patients’ BPRS scores and the binding potential and Bmax values, respectively, as well as between the binding potential and Bmax values, was tested with linear regression analysis. The reliability for each binding measure was evaluated by assessing the association between the hemispheres for each value with linear regression analysis.
Results
All of the subjects completed the study according to the protocol. At inclusion, eight patients satisfied DSM-III-R criteria for schizophrenia and two patients (numbers 7 and 8) satisfied DSM-III-R criteria for schizophreniform disorder (Table 1). After 1-year follow-up, these two patients satisfied DSM-III-R criteria for schizoaffective disorder.
MRI
At clinical evaluation of the T2-weighted MRI images by a neuroradiologist, one patient (number 3) had borderline enlargement of the lateral ventricles. No brain abnormalities were reported for the other patients. There were no statistically significant differences between comparison subjects and patients regarding the MRI volumes of the caudate (t=0.18, df=18, p=0.86) and putamen (t=–0.79, df=18, p=0.44), indicating similar recovery of radioactivity in the two groups.
Scatchard Analysis
After injection of [11C]SCH 23390 with high specific radioactivity, there was a rapid uptake of radioactivity in the brain of all subjects. The time of peak equilibrium in the caudate and putamen was 22 minutes (SD=3) after injection. The corresponding time for the cortical regions was 19 minutes (SD=4). The time of equilibrium correlated between the hemispheres for each region (r=0.48–0.74, N=20, p<0.05) except for the anterior cingulate (r=36, N=20, p=0.12).
The binding potential was calculated for each region (Table 2). There was no significant difference between the patients with schizophrenia and the normal subjects in any of the regions. In the right putamen, the binding potential for three of the patients (numbers 2, 6, and 7) was more than two standard deviations below that of the rest of the subjects (Figure 1). To account for the different variances between the groups, a nonparametric test (Wilcoxon rank order test) was performed for this region; this test did not reveal any differences between the groups (S=87, Z=–1.32, p=0.19).
After injection of [11C]SCH 23390 with low specific radioactivity, the radioactivity uptake was markedly lower than it was after high specific radioactivity in all brain regions except in the cerebellum, where the activity was on a similar level as after high specific radioactivity. There were no significant differences in the Bmax and Kd values, obtained from the Scatchard analysis, between the patients and the healthy comparison subjects for any of the brain regions (Table 2).
The asymmetry index did not differ between the groups for any of the regional binding measures (t=–2.1 to 1.0, df=18, p>0.05). The binding potential correlated significantly between the hemispheres in most of the regions (r=0.48–0.74, N=20, p<0.05) but not in the anterior cingulate (r=0.36, N=20, p=0.12). The binding potential and Bmax values correlated for most regions (r=0.80–0.97, N=20, p<0.05) but not for the right putamen (r=0.22, N=20, p=0.37) because of a patient outlier (number 7). The Bmax correlated significantly between the hemispheres in the putamen (r=0.74, N=20, p=0.0002), frontal cortex (r=0.71, N=20, p=0.0005), and lateral temporal cortex (r=0.52, N=20, p=0.02) but not in the caudate (r=0.36, N=20, p=0.11), anterior cingulate (r=0.34, N=20, p=0.14), medial temporal cortex (r=0.31, N=20, p=0.19), or occipital cortex (r=0.08, N=20, p=0.75).
D1 Receptor Binding and BPRS Scores
The patients’ total BPRS scores varied between 33 and 60 (there were 18 items, each rated on a scale from 0 to 6) (38). There was no significant correlation between the total BPRS scores and the binding potential or Bmax values in any of the brain regions. The score for negative symptoms (emotional withdrawal, motor retardation, and blunted affect) correlated significantly with the Bmax in the right frontal cortex (r=0.64, N=10, p=0.05). No such correlation was found in any of the other brain regions. After Bonferroni correction for multiple comparisons, there was no significant correlation between negative symptom scores and Bmax.
Discussion
To our knowledge, this is the first PET study in which an in vivo saturation analysis was applied to quantify central D1 dopamine receptor binding in patients with schizophrenia. Okubo et al. (35), using a less elaborate design with one measurement, reported reduced D1 receptor binding potential (Bmax/Kd) in schizophrenia. In the current study, PET examinations using [11C]SCH 23390 with high and low specific radioactivity were used to determine the Bmax and Kd by a Scatchard analysis in 10 neuroleptic-naive patients with schizophrenia and 10 healthy comparison subjects.
There were no significant differences in binding potential, Bmax, and Kd values between the comparison subjects and the patients for the caudate, the putamen, or any of the cortical regions (Table 2). This was also the case without correction for multiple comparisons. The results from this extended study are thus consistent with our preliminary report based on five early-recruited patients (34). Our current results are also consistent with most postmortem saturation studies, which show no change in striatal and frontal D1 receptor density in the striatum of patients with schizophrenia (15–17). A single postmortem report of reduced striatal D1 receptor density was thus not supported (14).
For the striatum our current results are consistent with the report by Okubo et al. (35) of no deviant D1 receptor binding in a PET study of patients with schizophrenia; however, our data do not confirm their finding of reduced binding potential in the frontal cortex. Major differences between our current study and that of Okubo et al. are shorter disease duration of our patients (1 month to 2 years versus 4 months to 18 years) and higher resolution of our PET system (full width at half maximum spatial 4.5 mm versus 8 mm; axial 5.9 mm versus 12 mm) (41).
One explanation for the discrepant findings may be differences in statistical power. The coefficient of variance (sigma) for the frontal binding potentials was similar in the study of Okubo et al. and our current study (about 15%), suggesting similar statistical power. In the study of Okubo et al., the statistical power was 72% for the finding of 15% lower frontal binding potential (delta) in 10 neuroleptic-naive patients compared with 18 normal subjects based on a two-sided test with a type I error of 5% (alpha) (48). The number of comparison subjects in the present study was lower (N=10), which reduces our statistical power to 56%. To increase the power to that of the study of Okubo et al., we made an additional analysis that was not included in the original protocol. We added data for eight healthy comparison subjects from previous studies with an identical experimental protocol with the exception that only high specific radioactivity was used (31). With 18 comparison subjects we still failed to demonstrate any differences in the frontal binding potential between the groups (t=–0.46, df=26, p=0.65). Hence, in spite of a similar coefficient of variance, the same number of neuroleptic-naive patients (N=10), and improved resolution, our current study failed to replicate the finding by Okubo et al. (35) of lower frontal binding potential in schizophrenia.
An explanation for the discrepancy may be the small number of neuroleptic-naive patients in both studies (N=10), which may not have been sufficiently large to constitute a representative sample of the schizophrenia population. The small number of subjects may also be a reason for the contradictory results for correlation between negative symptom scores and frontal D1 binding, which was negative for Bmax and Kd in the study of Okubo et al. (35) and positive for Bmax in the right frontal cortex in our current study.
High reliability for binding potential has been demonstrated in test-retest studies of [11C]SCH 23390 binding in the striatum (32) and the neocortex (49). The additional computational steps for Bmax and Kd imply error of propagation and subsequent decreased reliability, which is reflected in a higher coefficient of variance than that of binding potential (Table 2). However, the correlation for Bmax between the hemispheres in the putamen and the frontal and lateral temporal cortices suggests a relatively high reliability of the Scatchard analysis in these regions.
Three patients had binding potential values in the right putamen that were two to three standard deviations below those of the remaining seven patients, resulting in a coefficient of variance of 30%, which was higher than in the comparison subjects (12%). In another PET study (32), the coefficient of variance for the binding potential in the putamen of healthy comparison subjects was similar to that of the healthy comparison subjects in the present study (11%). Moreover, the test-retest variability in the other study was 6%. Thus, the greater variability of the binding measures in the right putamen may reflect heterogeneity regarding D1 receptor function and the disease process in schizophrenia.
The Kd had a high variability in both groups, which may reflect interindividual differences in endogenous dopamine levels (44). However, PET studies with a number of D1 radioligands in monkeys have failed to show amphetamine-induced reduction (50, 51) or reserpine-induced elevation (51) of binding potential. Thus, it appears that PET-measured [11C]SCH 23390 binding is not sensitive to endogenous dopamine release.
Conclusions
In the current study of central D1 receptors, Scatchard analysis did not reveal any significant differences in any of the regional binding potential, Bmax, and Kd values between 10 neuroleptic-naive patients with schizophrenia and 10 healthy subjects. There was no support for the difference between subjects with schizophrenia and normal subjects in the D1 binding potential for the frontal cortex reported by Okubo et al. (35). To improve reliability and statistical power, future studies on D1-like receptors in cortical regions should be done with ligands providing higher signals (52, 53).
 |
 |
Presented in part at the 1993 Journal of Cerebral Blood Flow and Metabolism meeting (JCBF Brain ’93) in Sendai, Japan, May 22–28, 1993. Received Sept. 15, 2000; revision received July 9, 2001; accepted Oct. 19, 2001. From the Karolinska Institutet. Address reprint requests to Dr. Karlsson, Karolinska Institutet, Department of Clinical Neuroscience, Psychiatry Section, Karolinska Hospital, SE-171 76 Stockholm, Sweden; [email protected] (e-mail). Supported by NIMH grant MH-44814, grants B93-21X-03560 and B93-21X-09114 from the Swedish Medical Research Council, grant K-KU 9973-305 from the Swedish Natural Science Research Council, and by the Söderström-König Foundation and Karolinska Institutet.
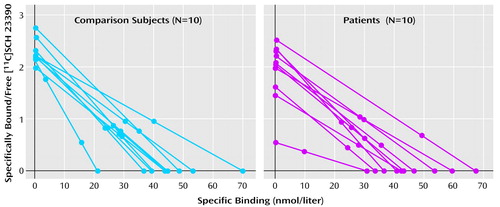
Figure 1. Scatchard Plots of [11C]SCH 23390 Binding in the Right Putamen of Healthy Comparison Subjects and Patients With Schizophrenia or Schizophreniform Disorder
1. van Rossum JM: The significance of dopamine receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther 1966; 160:492-494Medline, Google Scholar
2. Schwartz JC, Levesque D, Martres MP, Sokoloff P: Dopamine D3 receptor: basic and clinical aspects. Clin Neuropharmacol 1993; 16:295-314Crossref, Medline, Google Scholar
3. Seeman P, Van Tol HH: Dopamine receptor pharmacology. Trends Pharmacol Sci 1994; 15:264-270Crossref, Medline, Google Scholar
4. Meador-Woodruff JH: Update on dopamine receptors. Ann Clin Psychiatry 1994; 6:79-90Crossref, Medline, Google Scholar
5. Farde L: Brain imaging of schizophrenia—the dopamine hypothesis. Schizophr Res 1997; 28:157-162Crossref, Medline, Google Scholar
6. Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L: Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 1994; 11:245-256Crossref, Medline, Google Scholar
7. Sibley DR, Monsma FJ Jr: Molecular biology of dopamine receptors. Trends Pharmacol Sci 1992; 13:61-69Crossref, Medline, Google Scholar
8. Sokoloff P, Schwartz JC: Novel dopamine receptors half a decade later. Trends Pharmacol Sci 1995; 16:270-275Crossref, Medline, Google Scholar
9. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG: Dopamine receptors: from structure to function. Physiol Rev 1998; 78:189-225Crossref, Medline, Google Scholar
10. Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, Watson SJ: Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology 1996; 15:17-29Crossref, Medline, Google Scholar
11. Beischlag TV, Marchese A, Meador-Woodruff JH, Damask SP, O’Dowd BF, Tyndale RF, van Tol HH, Seeman P, Niznik HB: The human dopamine D5 receptor gene: cloning and characterization of the 5′-flanking and promoter region. Biochemistry 1995; 34:5960-5970Crossref, Medline, Google Scholar
12. Knable MB, Weinberger DR: Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol 1997; 11:123-131Crossref, Medline, Google Scholar
13. Willner P: The dopamine hypothesis of schizophrenia: current status, future prospects. Int Clin Psychopharmacol 1997; 12:297-308Crossref, Medline, Google Scholar
14. Hess EJ, Bracha HS, Kleinman JE, Creese I: Dopamine receptor subtype imbalance in schizophrenia. Life Sci 1987; 40:1487-1497Crossref, Medline, Google Scholar
15. Seeman P, Bzowej NH, Guan HC, Bergeron C, Reynolds GP, Bird ED, Riederer P, Jellinger K, Tourtellotte WW: Human brain D1 and D2 dopamine receptors in schizophrenia, Alzheimer’s, Parkinson’s, and Huntington’s diseases. Neuropsychopharmacology 1987; 1:5-15Crossref, Medline, Google Scholar
16. Czudek C, Reynolds G: Binding of [11C]SCH 23390 to post-mortem brain tissue in schizophrenia. Br J Pharmacol 1988; 93(suppl):166PGoogle Scholar
17. Knable MB, Hyde TM, Herman MM, Carter JM, Bigelow L, Kleinman JE: Quantitative autoradiography of dopamine-D1 receptors, D2 receptors, and dopamine uptake sites in postmortem striatal specimens from schizophrenic patients. Biol Psychiatry 1994; 36:827-835Crossref, Medline, Google Scholar
18. Seeman P, Niznik HB, Guan HC, Booth G, Ulpian C: Link between D1 and D2 dopamine receptors is reduced in schizophrenia and Huntington diseased brain. Proc Natl Acad Sci USA 1989; 86:10156-10160Crossref, Medline, Google Scholar
19. Goldman-Rakic PS: Working memory and the mind. Sci Am 1992; 267:110-117Crossref, Medline, Google Scholar
20. Sawaguchi T, Goldman-Rakic PS: D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 1991; 251:947-950Crossref, Medline, Google Scholar
21. Sawaguchi T, Goldman-Rakic PS: The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol 1994; 71:515-528Medline, Google Scholar
22. Castner SA, Williams GV, Goldman-Rakic PS: Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science 2000; 287:2020-2022Crossref, Medline, Google Scholar
23. Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW: Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci 2000; 20:1208-1215Medline, Google Scholar
24. Goldman-Rakic PS: Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 1994; 6:348-357Crossref, Medline, Google Scholar
25. Park S, Holzman PS, Goldman-Rakic PS: Spatial working memory deficits in the relatives of schizophrenic patients. Arch Gen Psychiatry 1995; 52:821-828Crossref, Medline, Google Scholar
26. Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba CA: SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther 1983; 226:462-468Medline, Google Scholar
27. Halldin C, Stone-Elander S, Farde L, Ehrin E, Fasth KJ, Langstrom B, Sedvall G: Preparation of 11C-labelled SCH 23390 for the in vivo study of dopamine D-1 receptors using positron emission tomography. Int J Rad Appl Instrum A 1986; 37:1039-1043Crossref, Medline, Google Scholar
28. Sedvall G, Farde L, Stone-Elander S, Halldin C: Dopamine D1-receptor binding in the living human brain. Adv Exp Med Biol 1986; 204:119-124Crossref, Medline, Google Scholar
29. Farde L, Halldin C, Stone-Elander S, Sedvall G: PET analysis of human dopamine receptor subtypes using 11C-SCH 23390 and 11C-raclopride. Psychopharmacology (Berl) 1987; 92:278-284Crossref, Medline, Google Scholar
30. Farde L: Selective D1- and D2-dopamine receptor blockade both induces akathisia in humans—a PET study with [11C]SCH 23390 and [11C]raclopride. Psychopharmacology (Berl) 1992; 107:23-29Crossref, Medline, Google Scholar
31. Karlsson P, Farde L, Halldin C, Sedvall G, Ynddal L, Sloth-Nielsen M: Oral administration of NNC 756—a placebo controlled PET study of D1-dopamine receptor occupancy and pharmacodynamics in man. Psychopharmacology (Berl) 1995; 119:1-8Crossref, Medline, Google Scholar
32. Chan GL, Holden JE, Stoessl AJ, Doudet DJ, Wang Y, Dobko T, Morrison KS, Huser JM, English C, Legg B, Schulzer M, Calne DB, Ruth TJ: Reproducibility of the distribution of carbon-11-SCH 23390, a dopamine D1 receptor tracer, in normal subjects. J Nucl Med 1998; 39:792-797Medline, Google Scholar
33. Halldin C: Dopamine receptor radioligands. Med Chem Res 1994; 5:127-149Google Scholar
34. Karlsson P, Farde L, Halldin C, Nordström A, Sedvall G: D1 dopamine receptor binding in drug naive schizophrenic patients measured by PET (abstract). J Cereb Blood Flood Flow Metab 1993; 13:S556Google Scholar
35. Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M: Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature 1997; 385:634-636Crossref, Medline, Google Scholar
36. Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ: A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol 1984; 15:217-227Crossref, Medline, Google Scholar
37. Hietala J, Kuoppamaki M, Nagren K, Lehikoinen P, Syvalahti E: Effects of lorazepam administration on striatal dopamine D2 receptor binding characteristics in man—a positron emission tomography study. Psychopharmacology (Berl) 1997; 132:361-365Crossref, Medline, Google Scholar
38. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
39. Bech P, Kastrup M, Rafaelsen OJ: Mini-compendium of rating scales for states of anxiety depression mania schizophrenia with corresponding DSM-III syndromes. Acta Psychiatr Scand Suppl 1986; 326:1-37Medline, Google Scholar
40. Bergstrom M, Boethius J, Eriksson L, Greitz T, Ribbe T, Widen L: Head fixation device for reproducible position alignment in transmission CT and positron emission tomography. J Comput Assist Tomogr 1981; 5:136-141Crossref, Medline, Google Scholar
41. Litton JE, Holte S, Eriksson L: Evaluation of the Karolinska new positron camera system: the Scanditronix PC2048-15B. IEEE Trans Nucl Sci 1990; 37:743-748Crossref, Google Scholar
42. Hall H, Farde L, Sedvall G: Human dopamine receptor subtypes—in vitro binding analysis using 3H-SCH 23390 and 3H-raclopride. J Neural Transm 1988; 73:7-21Crossref, Medline, Google Scholar
43. Cortes R, Gueye B, Pazos A, Probst A, Palacios JM: Dopamine receptors in human brain: autoradiographic distribution of D1 sites. Neuroscience 1989; 28:263-273Crossref, Medline, Google Scholar
44. Farde L, Eriksson L, Blomquist G, Halldin C: Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET—a comparison to the equilibrium analysis. J Cereb Blood Flow Metab 1989; 9:696-708Crossref, Medline, Google Scholar
45. Ito H, Hietala J, Blomqvist G, Halldin C, Farde L: Comparison of the transient equilibrium and continuous infusion method for quantitative PET analysis of [11C]raclopride binding. J Cereb Blood Flow Metab 1998; 18:941-950Crossref, Medline, Google Scholar
46. Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ: Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse 1998; 30:56-61Crossref, Medline, Google Scholar
47. Montague DM, Lawler CP, Mailman RB, Gilmore JH: Developmental regulation of the dopamine D1 receptor in human caudate and putamen. Neuropsychopharmacology 1999; 21:641-649Crossref, Medline, Google Scholar
48. Cohen J: Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
49. Hirvonen J, Någren K, Kajander J, Hietala J: Measurement of cortical dopamine D1 receptor binding with [11C]SCH 23390: a test-retest analysis. J Cereb Blood Flow Metab 2001; 21:1133-1145Medline, Google Scholar
50. Abi-Dargham A, Simpson N, Kegeles L, Parsey R, Hwang DR, Anjilvel S, Zea-Ponce Y, Lombardo I, Van Heertum R, Mann JJ, Foged C, Halldin C, Laruelle M: PET studies of binding competition between endogenous dopamine and the D1 radiotracer [11C]NNC 756. Synapse 1999; 32:93-109Crossref, Medline, Google Scholar
51. Chou YH, Karlsson P, Halldin C, Olsson H, Farde L: A PET study of D(1)-like dopamine receptor ligand binding during altered endogenous dopamine levels in the primate brain. Psychopharmacology (Berl) 1999; 146:220-227Crossref, Medline, Google Scholar
52. Halldin C, Foged C, Chou YH, Karlsson P, Swahn CG, Sandell J, Sedvall G, Farde L: Carbon-11-NNCNNC 112: a radioligand for PET examination of striatal and neocortical D1-dopamine receptors. J Nucl Med 1998; 39:2061-2068Medline, Google Scholar
53. Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, Anjilvel S, Pidcock J, Guo NN, Lombardo I, Mann JJ, Van Heertum R, Foged C, Halldin C, Laruelle M: Measurement of striatal and extrastriatal dopamine D-1 receptor binding potential with [C-11]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab 2000; 20:225-243Crossref, Medline, Google Scholar



