Abnormalities of Thalamic Volume and Shape in Schizophrenia
Abstract
OBJECTIVE: Postmortem and neuroimaging studies of schizophrenia have reported deficits in the volume of the thalamus and its component nuclei. However, the pattern of shape change associated with such volume loss has not been investigated. In this study, alterations in thalamic volume, shape, and symmetry were compared in subjects with and without schizophrenia. METHOD: T1-weighted magnetic resonance scans were collected in 52 schizophrenia and 65 comparison subjects matched for age, gender, race, and parental socioeconomic status. High-dimensional (large-deformation) brain mapping was used to assess thalamic morphology. RESULTS: Significant differences in thalamic volume, deformities of thalamic shape at the anterior and posterior extremes of the structure, and a significant exaggeration of thalamic asymmetry (i.e., left smaller than right) were found in the schizophrenia subjects. After covarying for total cerebral volume, the difference in thalamic volume became insignificant. When information about thalamic shape was combined with previously collected information about hippocampal shape, the discrimination between schizophrenia patients and comparison subjects was improved. CONCLUSIONS: Thalamic volume was smaller than normal in schizophrenia patients, but only proportionate to reductions in reduced total cerebral volume. The presence of changes in thalamic shape and asymmetry suggest greater pathologic involvement of individual nuclei at its anterior and posterior extremes of the thalamic complex.
Recent theories about the neurobiology of schizophrenia have emphasizedinvolvement of the thalamus in the pathophysiology of schizophrenia (1, 2). A growing body of evidence indicates that the thalamus is smaller, has fewer neurons, and exhibits metabolic disturbances in schizophrenia patients relative to healthy comparison subjects (3–15). From a functional standpoint, the thalamus is multifaceted, as it mediates sensory perception and gating via ventrobasal sensory relay nuclei (16), coordinates motor activity through basal ganglia and cerebellar relays in the ventral anterior and ventral lateral nuclei (17–19), and contributes to other cognitive functions via extensive reciprocal connections with associational cortices, particularly the prefrontal cortex (20–23). Some postmortem studies have reported reductions in volume and neuronal number within nuclei that make major contributions to cortical input, such as the mediodorsal nucleus (9, 11, 24), anterior nucleus (9), pulvinar (11), and the ventral lateral nucleus (12). These findings suggest that thalamic abnormalities may contribute to the cognitive disturbances that are characteristic of schizophrenia (25).
Controversy continues to exist as to the severity and pattern of thalamic abnormalities in subjects with schizophrenia, since not all postmortem and in vivo neuroimaging investigations have found evidence of volume reduction (12, 26–29). A meta-analysis of thalamic volume in schizophrenia and healthy comparison subjects indicated that the effect size was modest relative to effect sizes for volume reductions in other brain structures (30). Moreover, volume reductions of the thalamic complex have been reported to be asymmetric (i.e., left-sided) (28, 29) or restricted to subregions such as the mediodorsal nucleus and pulvinar (7, 29). Also, Gur and colleagues reported thalamic volume reductions in neuroleptic-naive but not neuroleptic-treated patients, suggesting that drug treatment could increase thalamic volumes and mask otherwise significant differences between schizophrenia and healthy comparison subjects (31).
The tools of computational anatomy are being increasingly used to characterize neuroanatomical abnormalities associated with neuropsychiatric disease (32, 33). With these methods, assessment of neuroanatomical shapes as well as volumes is possible; thus, subtle abnormalities in the organization of a structure that may have little impact on volume can be assessed (34, 35). We have previously used one of these methods, high-dimensional (large-deformation) brain mapping (36–38), to characterize shape abnormalities in the hippocampus in patients with schizophrenia (34, 35), depression (39), and dementia of the Alzheimer type (40).
In the present study, we used high-dimensional brain mapping to compare the shape, symmetry, and volume of the thalamus in a group of schizophrenia subjects (in whom we previously characterized hippocampal abnormalities [34]) with a group of healthy subjects. Relationships between these neuroanatomical measures and the clinical and cognitive features of schizophrenia were also examined.
Method
Subjects
The study group consisted of 52 schizophrenia subjects and 65 comparison subjects, matched for age, gender, race, and parental socioeconomic status. All subjects gave written informed consent for participation in this study after the study’s risks and benefits were explained to them. The mean age of the 30 male and 22 female schizophrenia subjects was 38 years (SD=1.7), and their mean age at onset of illness was 23 years (SD=1.2). A more detailed summary of the clinical characteristics of the schizophrenia and comparison subjects can be found in a prior publication (34). The DSM-IV diagnosis of each subject was determined by the consensus of a research psychiatrist and a trained research assistant who used the Structured Clinical Interview for DSM-IV Axis I Disorders (41). Exclusion criteria were presence of an unstable medical condition, a neurologic disorder, head injury with loss of consciousness, or substance abuse or dependence in the 3 months preceding the study. Comparison subjects were also excluded if they had first-degree relatives with a psychotic disorder.
All schizophrenia subjects were clinically stable (i.e., their symptoms had remained unchanged for at least 2 weeks) (42), and all but three had been treated with antipsychotic drugs. The severity of three dimensions of residual psychopathology (i.e., negative symptoms, psychosis, and thought disorganization) was assessed in the schizophrenia subjects by using the Scale for the Assessment of Positive Symptoms (43) and the Scale for the Assessment of Negative Symptoms (44) as previously described (34). Forty-three of the schizophrenia subjects and 59 of the comparison subjects were assessed with neuropsychological tests of memory and general intelligence: the Wechsler Memory Scale (immediate and delayed), Benton Visual Retention (immediate and delayed), and WAIS-III verbal and performance IQ.
Image Collection and Generation of the Neuroanatomical Template
Magnetic resonance (MR) scans were collected by using a turbo-fast low-angle shots sequence (TR=20, TE=5.4, flip angle=30°, number of acquisitions=1, matrix=256×256, voxel size=1 mm3, scanning time=13.5 minutes) (45). MR data were reformatted by using Analyze software (Rochester, Minn.). Signed 16-bit data sets were compressed to unsigned 8-bit data sets to maximize tissue contrast. In all MR scans, landmarks were placed at the anterior, posterior, superior, inferior, and lateral brain boundaries and at points where the anterior and posterior commissures intersected the midsagittal plane. Points at the anterior and posterior boundaries of the thalamus were also demarcated, and a line connecting them was used to create an anterior/posterior axis. The thalamus was then divided into seven equally distanced slices along this axis, and five landmarks surrounding the thalamus were placed in each slice.
The template for the human thalamus was generated by using a T1-weighted MR sequence collected from another healthy comparison subject that was not otherwise included in the analysis. The thalamus was manually outlined in this MR scan by three authors (M.K.S., L.W., and M.G.) according to atlas guidelines (46).
High-Dimensional Brain Mapping
Transformation of the template onto the target MR scans occurred in two steps (33, 36, 37) First, it was coarsely aligned to each target scan by using the previously placed landmarks, and then a high-dimensional transformation was applied to achieve an optimal voxel-by-voxel match (36). During the transformation, the movement and deformation of template voxels were constrained by assigning them the physical properties of a fluid (47). The reliability and validity of high-dimensional brain mapping for segmenting closed volume brain structures with respect to expert manual outlining has been previously demonstrated (37). To check the validity of using high-dimensional brain mapping for mapping the thalamic template to the target scans in this study, we compared the segmentations generated by this process to those produced by an expert (M.G.) in the MR scans of three of the comparison subjects. The average overlap between the segmentations generated by high-dimensional brain mapping and the segmentations generated by an expert was 84.80% (minimum value was 72.81%).
To quantify thalamic shape and volume, a triangulated graph was superimposed onto the surface of the thalamus within the template; this graphic surface was then carried along as the template was transformed onto the target scans. Left and right thalamic volumes in the target scans were determined by calculating the volumes enclosed by the transformed surfaces. Fields of vectors were also derived from the displacements of the triangulated graph points during the transformations. To compare thalamic shapes between subject groups, a pooled within-group covariance matrix was derived from the transformation vectors, and the dimensionality of this matrix was reduced by using singular value decomposition to identify the major dimensions of shape variation (i.e., eigenvectors) (35, 36).
To examine thalamic asymmetry, vector fields were also generated by reflecting the transformed graphic surfaces from the thalamus in one hemisphere across the midsagittal plane (i.e., the plane yielding the least sum of squared errors between the thalamic surface points in the two hemispheres) onto the thalamus in the opposite hemisphere. An asymmetry covariance matrix was then computed using these vector fields, and the principal dimensions of thalamic asymmetry (i.e., eigenvectors) were determined (38).
Total cerebral volumes (excluding the brainstem and cerebellum) were derived by using a landmark-based elastic transformation (36) of the template scan, with total cerebral volume as a covariate in volume analyses. During these transformations, the entire template scan was globally registered with each target scan using scalar points at the boundaries of the brain and in the midline.
Statistical Analyses
Thalamic volumes were compared using two-way, repeated-measures analysis of variance, with diagnostic group as a between-subject factor and hemisphere as a within-subject factor. To test for a group difference in thalamic shape, the first 10 eigenvectors derived from the transformation vector field covariance matrix (explaining 78% of the variance) were selected a priori, and the statistical significance of the group difference was tested using Wilks’s lambda. A logistic regression model was then generated to select eigenvectors among the first 10 that yielded maximal discrimination between the groups, and log-likelihood ratio values for each subject were calculated. The eigenvectors selected by the logistic regression model were used in a “leave-one-out” discriminant function analysis to determine the percentage of correctly classified subjects in each group. For the comparison of thalamic asymmetry between groups, eigenvectors were developed and selected using the asymmetry vector fields and used in similar statistical analyses.
To visualize the physical patterns of thalamic deformity represented by the eigenvectors, maps of the composite thalamic surfaces in the schizophrenia and comparison subjects were reconstructed using the selected eigenvectors. The patterns of these deformities were then compared to “point-by-point” surface displacement maps. These displacements were calculated at each surface point as the difference between the means of the group vectors in magnitude.
Results
Thalamic Volume
Mean thalamic volumes were 7307 mm3 (SD=790) on the left and 7350 mm3 (SD=846) on the right in schizophrenia subjects, and 7702 mm3 (SD=851) on the left and 7734 mm3 (SD=837) on the right in comparison subjects (Figure 1). The group difference in thalamic volume was approximately 7% (effect size for left and right thalamus combined=0.48). The effect of diagnosis was significant (F=6.59, df=1, 115, p<0.02), and this effect remained significant after covarying thalamic volumes for age and gender. However, there was also a significant diagnosis effect for total cerebral volume (F=5.73, df=1, 115, p<0.02), and the effect of diagnosis on thalamic volume became nonsignificant after covarying thalamic volumes for total cerebral volume (F=1.30, df=1, 115, p=0.26). Post hoc correlations between the combined left and right thalamic volumes and total cerebral volumes were significant in the schizophrenia subjects (r=0.75, p<0.0001) and comparison subjects (r=0.75, p<0.0001). There was no significant effect of hemisphere (F=1.35, df=1, 115, p=0.25) nor was there a group-by-hemisphere interaction (F=0.03, df=1, 115, p=0.87). Including gender as a covariate in the analysis and the exclusion of left-handed subjects did not alter the results.
Thalamic Shape
Using the first 10 shape eigenvectors, a significant difference was found between the two subject groups (F=2.82, df=10, 106, p<0.004). Eigenvectors 1, 8, and 10 were then selected in a logistic regression model (χ2=16.0, df=3) to maximally discriminate the two subject groups, and in a “leave one out” discriminant function analysis, 28 (53.4%) out of the 52 schizophrenia subjects and 49 (75.4%) out of the 65 comparison subjects were correctly categorized (Figure 1). To test the stability of this “leave one out” analysis, 10 alternate comparisons were run after excluding in turn approximately 10% of the subjects in both groups. The overall mean rate of correct subject classification was 66.7% (SD=8.8%), which was significantly higher than the 50% correct categorization rate obtainable by chance (z=6.0, p<0.001). Also, in all of these alternate models, the selected eigenvectors were similar to those selected using the entire population (i.e., 1, 8, 10 in seven models; eigenvectors 1, 7, 10 in two models; eigenvectors 4, 8, 10 in one model). Including thalamic volume in this analysis, even as a forced variable, did not further improve the classification of schizophrenia and comparison subjects.
Visual representations of thalamic shape deformation in the subjects with schizophrenia suggested a loss of volume in the extreme anterior and posterior regions of the thalamus (Figure 2). This pattern of deformity was similar regardless of whether the maps of thalamic surface deformation were reconstructed from the eigenvector solution (i.e., 1, 8, and 10) or generated using point-by-point maps of the thalamic surface.
Combining Thalamic and Hippocampal Shape Information
In a previous study of the same subjects (34), 15 eigenvectors representing the shape of the surface of the hippocampus deformity were found to significantly distinguish the two groups of subjects (F=2.66, df=15, 101, p=0.002). From these hippocampal shape eigenvectors, eigenvectors 1, 5, and 14 (χ2=21.6, df=3) were then selected in a logistic regression model, which allowed for the successful classification of 59.6% of the schizophrenia subjects and 80.0% of the comparison subjects.
Combining information about the shape of the thalamus with previously collected information about the shape of the hippocampus improved the proportion of subjects correctly classified. Using all 15 hippocampal eigenvectors and all 10 thalamic eigenvectors together, the two groups of subjects were again found to be significantly different (F=2.83, df=25, 91, p=0.0002). A logistic regression model was then developed using both the hippocampal eigenvectors 1, 5, 14 and thalamic eigenvectors 8 and 10 (χ2=32.5, df=5), and in a “leave one out” discriminant function analysis, 73.1% of the schizophrenia subjects (N=38 of 52) and 83.1% of the comparison subjects (N=54 of 65) were correctly classified. Also, correlations between the left and right hippocampal or thalamic volumes in both groups of subjects were very high (r=0.86–0.92), while correlations between the hippocampal and thalamic volumes on the left or right were lower in both groups of subjects (r=0.52–0.64).
Thalamic Asymmetries
Development of a logistic regression model comparing patterns of thalamic asymmetry in the schizophrenia and comparison subjects resulted in the selection of eigenvectors 4, 6, 12, and 20 (χ2=12.4, df=4), and the log-likelihood ratio values derived using these eigenvectors indicated a significant group difference in the pattern of thalamic asymmetry (F=3.31, df=4, 112, p=0.01). Visual representations of this group difference (Figure 3) suggested that the subjects with schizophrenia showed an exaggeration of the normal pattern of asymmetry on the medial surface of the thalamus. This group difference in this pattern of thalamic asymmetry was more apparent in the point-by-point maps than in the maps generated from the eigenvector analysis, which suggests that the eigenvector-based analysis captured only a portion of the group difference in thalamic asymmetry.
Clinical Relationships
There were no significant correlations between thalamic volume or shape measures and the severity of any symptom dimension (i.e., negative symptoms, psychosis, or thought disorganization) in the schizophrenia subjects. Duration of illness in the schizophrenia subjects tended to be inversely correlated with the volume of the right thalamus (r=–0.26, df=47, p=0.07) but not the left thalamus (r=–0.13, df=47, p=0.38).
As previously reported (34), the schizophrenia subjects demonstrated significant and substantial (approximately two standard deviations) deficits in performance on several cognitive tests (i.e., Wechsler Memory Scale immediate and delayed, Benton Visual Retention immediate and delayed, and WAIS-III verbal and performance IQ). Significant correlations were found between performance on the Benton Visual Retention test (delayed) and left (r=0.38, p=0.01) and right (r=0.30, p=0.05) thalamic volume. No other significant correlations were found between measures of thalamic volume or shape and performance on any neuropsychological test nor between thalamic volume and shape measures and performance on neuropsychological tests in the healthy comparison subjects.
Discussion
In this study, a small but significant reduction in thalamic volume (approximately 7%) was found in schizophrenia subjects relative to healthy comparison subjects, which is consistent with the results of prior neuroimaging studies (5, 7, 13–15). While this difference became nonsignificant when thalamic volumes were covaried for total cerebral volumes, this result should not be taken to infer that the reduction in thalamic volumes was not of neurobiological significance. Indeed, given the robust connectivity between the thalamus and the cerebral cortex (16–22), alterations of thalamic structure would be expected to impact the cortex and vice versa. Moreover, the thalamic volume reduction was accompanied by significant deformities of thalamic shape and asymmetry, which suggests that schizophrenia may be characterized by abnormalities of specific thalamic nuclei.
The pattern of thalamic shape deformity observed in the schizophrenia subjects suggests involvement of nuclei in both the anterior and posterior extremes of the complex. In the case of the anterior extreme, this would implicate both the anterior and the dorsomedial nuclei, which have been previously shown to be smaller in volume and to contain fewer neurons in schizophrenia subjects (4, 8, 9, 11, 12, 24, 29). The pulvinar nucleus at the posterior extreme of the complex has also been shown to have a reduced volume and neuronal population in schizophrenia subjects (29). Involvement of these nuclei in schizophrenia is of particular interest because of their connections with the association cortices, particularly the prefrontal cortex. The anterior and mediodorsal nuclei of the thalamus have dense reciprocal connections with the prefrontal cortex (20–22), while the pulvinar has connections with parieto-occipital association cortices (23, 48), the prefrontal cortex (23, 48), and entorhinal cortex (49). The involvement of these association relay nuclei in schizophrenia is consistent with recent reports of gray matter reductions in the prefrontal cortex (50) and other heteromodal association cortices (51). However, while inward deformations of the anterior and posterior extremes of the thalamic surface could have resulted from volume losses of nuclei at those extremes, such deformations could also have been the result of volume losses of other nuclei that shrank the overall anterior-posterior dimension of the structure. Discriminating between these two possibilities would require application of high-dimensional brain mapping to the individual thalamic nuclei and MR scans that offer more detailed resolution of such structures than the ones available for this study.
Our results also lend support to hypotheses that the pathogenesis of schizophrenia involves disturbances in the development of neuroanatomical asymmetries (52). The disturbance of thalamic asymmetry (i.e., an exaggeration of the normal left > right pattern) observed in this study was similar to the disturbance of hippocampal asymmetry previously observed in the same subjects (34), suggesting that a common mechanism may underlie both disturbances. Such disturbances of neurodevelopment could occur as the result of genetic factors (53) or environmental insult (54, 55). Discriminating between these two possibilities could be achieved by the study of populations of subjects at genetic risk for developing schizophrenia (i.e., relatives of patients with schizophrenia) and in whom there is a detailed gestational and developmental history.
There were few functional correlates of thalamic volume and shape measures in the schizophrenia subjects. The lack of any substantial correlation between duration of illness and thalamic neuroanatomic measures suggests that the deformities of thalamic structure observed were probably not the result of chronicity or long-term treatment factors. However, there were few untreated patients in this study, and the possibility that relatively short treatment periods can alter the structure of the thalamus cannot be excluded. The small but significant correlation between poor performance on a test of visual memory (i.e., Benton visual retention test, delayed type) is intriguing and may be related to the functional relationships that exist between the thalamus and association cortices involved in pattern recognition and retention.
The elucidation of abnormalities in neuroanatomical volume, shape, and asymmetry in schizophrenia using MR imaging and computational anatomy may help direct investigators who perform postmortem studies to particular subregions of neuroanatomical structures. Moreover, measures of neuroanatomical shape and asymmetry may one day be useful for improving clinical diagnosis or for selecting and monitoring the effects of treatment. However, before such measures can be used for clinical purposes, the degree to which abnormalities that appear during the illness are specific to schizophrenia must be determined, and how they are affected by treatment.
Received May 9, 2003; revision received Sept. 2, 2003; accepted Sept. 5, 2003. From the Department of Psychiatry, Department of Anatomy and Neurobiology, Department of Pathology, and the Division of Biostatistics, Washington University School of Medicine; the Metropolitan St. Louis Psychiatric Center, St. Louis; the Department of Neurobiology, Yale University School of Medicine, New Haven, Conn.; and the Department of Biomedical Engineering, Johns Hopkins University, Baltimore. Address reprint requests to Dr. Csernansky, Department of Psychiatry (Box 8134), Washington University School of Medicine, 660 S. Euclid Ave., St. Louis, MO 63110; [email protected] (e-mail). Supported by an NIMH research grant (MH-56584) and a grant for the Silvio Conte Center at Washington University School of Medicine (MH-62130). Surgical Navigation Technologies (Louisville, Colo.) provided the thalamic template scan.
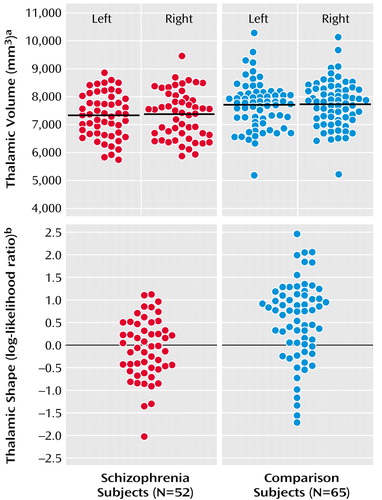
Figure 1. Thalamic Volume and Shape in Subjects With Schizophrenia (N=52) and Healthy Comparison Subjects (N=65)
aSignificant effect of diagnosis (F=6.59, df=1, 115, p<0.02). No asymmetry of left and right thalamic volumes was observed.
bLog-likelihood ratio values derived from the linear combination of eigenvectors 1, 8, and 10, which yielded the maximal between-group discrimination in a logistic regression model (28 [53.4%] of the schizophrenia subjects and 49 [75.4%] of the healthy subjects were correctly classified). Comparison of the log-likelihood ratio values revealed a significant difference between groups (F=2.82, df=10, 106, p<0.004).
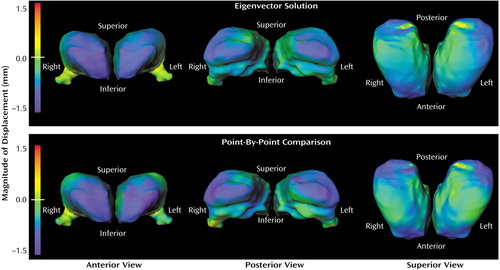
Figure 2. Surface Maps Depicting Thalamic Shape Differences in Subjects With Schizophrenia (N=52) and Healthy Comparison Subjects (N=65)a
aBlue-to-purple shading denotes regions of inward deformity of the thalamic surface in the schizophrenia versus the comparison subjects. The similarity of the representations in the two rows illustrates the physical basis of the eigenvector-based comparison of subject groups.
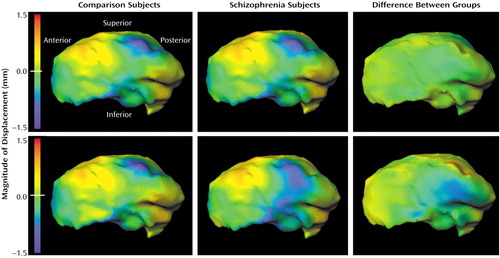
Figure 3. Surface Maps Depicting Thalamic Asymmetry in Subjects With Schizophrenia (N=52) and Healthy Comparison Subjects (N=65)a
aThe top row of panels shows the pattern of asymmetry using eigenvectors derived from mapping the right hemisphere onto the left in both groups of subjects. In the bottom row of panels, point-by-point maps are shown. Medial views of the thalamic complex are shown in all cases. Blue-to-purple shading denotes regions of inward deformity of the thalamic surface on the left side versus the right side.
1. Jones EG: Cortical development and thalamic pathology in schizophrenia. Schizophr Bull 1997; 23:483–502Crossref, Medline, Google Scholar
2. Andreasen NC, Paradiso S, O’Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203–218Crossref, Medline, Google Scholar
3. Pakkenberg B: Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 1990; 47:1023–1028Crossref, Medline, Google Scholar
4. Flaum M, Swayze VW II, O’Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC: Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 1995; 152:704–714Link, Google Scholar
5. Andreasen NC, Arndt S, Swayze V II, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WTC: Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994; 266:294–298Crossref, Medline, Google Scholar
6. Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Boles Ponto LL, Watkins GL, Hichwa RD: Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA 1996; 93:9985–9990Crossref, Medline, Google Scholar
7. Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE Jr: PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 1996; 153:191–199Link, Google Scholar
8. Popken GJ, Bunney WE, Potkin SG, Jones EG: Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA 2000; 97:9276–9280Crossref, Medline, Google Scholar
9. Young KA, Manaye KF, Liang C, Hicks PB, German DC: Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry 2000; 47:944–953Crossref, Medline, Google Scholar
10. Heckers S, Curan T, Goff D, Rauch SL, Fischman AJ, Albert NM, Schacter DL: Abnormalities in the thalamus and prefrontal cortex during episodic object recognition in schizophrenia. Biol Psychiatry 2000; 48:651–657Crossref, Medline, Google Scholar
11. Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L: Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 2002; 159:59–65Link, Google Scholar
12. Danos P, Baumann B, Bernstein H-G, Stauch R, Krell D, Falkai P, Bogerts B: The ventral lateral posterior nucleus of the thalamus in schizophrenia: a post-mortem study. Psychiatry Res Neuroimaging 2002; 114:1–9Crossref, Medline, Google Scholar
13. Ettinger U, Chitnis XA, Kumari V, Fannon DG, Sumich AL, O’Ceallaigh S, Doku VC, Sharma T: Magnetic resonance imaging of the thalamus in first-episode psychosis. Am J Psychiatry 2001; 158:116–118Link, Google Scholar
14. Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS: Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry 2001; 158:618–624Link, Google Scholar
15. Staal WG, Hulshoff Pol HE, Schnack HG, van Haren NEM, Seifert N, Kahn RS: Structural brain abnormalities in chronic schizophrenia at the extremes of the outcome spectrum. Am J Psychiatry 2001; 158:1140–1142Link, Google Scholar
16. Jones EG, Friedman DP: Projection pattern of functional components of thalamic ventrobasal complex on monkey somatosensory cortex. J Neurophysiol 1982; 48:521–544Crossref, Medline, Google Scholar
17. Kievit J, Kuypers HGJM: Organization of thalamo-cortical connexions to the frontal lobe in the rhesus monkey. Exp Brain Res 1977; 29:299–322Medline, Google Scholar
18. Schell GR, Strick P: The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci 1984; 4:539–560Crossref, Medline, Google Scholar
19. Ilinsky IA, Kultas-Ilinsky K: Sagittal cytoarchitectonic maps of the Macaca mulatta thalamus with a revised nomenclature of the motor-related nuclei validated by observations on their connectivity. J Comp Neurol 1987; 262:331–364Crossref, Medline, Google Scholar
20. Goldman-Rakic PS, Porrino LJ: The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol 1985; 242:535–560Crossref, Medline, Google Scholar
21. Giguere M, Goldman-Rakic P: Mediodorsal nucleus areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol 1988; 277:195–213Crossref, Medline, Google Scholar
22. Barbas H, Henion TH, Dermon CR: Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol 1991; 313:65–94Crossref, Medline, Google Scholar
23. Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS: Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol 1997; 379:313–332Crossref, Medline, Google Scholar
24. Pakkenberg B: The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr Res 1992; 7:95–100Crossref, Medline, Google Scholar
25. Goldman-Rakic PS, Selemon LD: Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997; 23:437–458Crossref, Medline, Google Scholar
26. Pierri JN, Dorph-Petersen KA, Lewis DA: Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia and affective illness. Abstracts of the Society for Neuroscience 2001; 27:446.17Google Scholar
27. Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG: Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry 1998; 43:649–659Crossref, Medline, Google Scholar
28. Hazlett EA, Buchsbaum MS, Byne W, Wei T-C, Spiegel-Cohen J, Geneve C, Kinderlehrer R, Haznedar MM, Shihabuddin L, Siever LJ: Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry 1999; 156:1190–1199Abstract, Google Scholar
29. Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulos V, Siever LJ: Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry 2001; 58:133–140Crossref, Medline, Google Scholar
30. Konick LC, Friedman L: Meta-analysis of thalamic size in schizophrenia: Biol Psychiatry 2001; 49:28–39Google Scholar
31. Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC: Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711–1717Link, Google Scholar
32. Thompson PM, MacDonald D, Mega MS, Holmes CJ, Evans AC, Toga AW: Detection and mapping of abnormal brain structure with a probabilistic atlas of cortical surfaces. J Comput Assist Tomogr 1997; 21:567–581Crossref, Medline, Google Scholar
33. Grenander U, Miller MI: Computational anatomy: an emerging discipline. Q Applied Mathematics 1998; 4:617–694Google Scholar
34. Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G, Miller JP, Miller MI: Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry 2002; 159:2000–2006Link, Google Scholar
35. Csernansky JG, Joshi S, Wang L, Haller J, Gado M, Miller JP, Grenander U, Miller MI: Hippocampal morphometry in schizophrenia via high dimensional brain mapping. Proc Natl Acad Sci USA 1998; 95:11406–11411Crossref, Medline, Google Scholar
36. Miller MI, Banerjee A, Christensen G, Joshi S, Khaneja N, Grenander U, Matejic L: Statistical methods in computational anatomy. Stat Methods Med Res 1997; 6:267–299Crossref, Medline, Google Scholar
37. Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, Sheline YI, Vannier MW, Csernansky JG: Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology 1997; 202:504–510Crossref, Medline, Google Scholar
38. Wang L, Joshi SC, Miller MI, Grenander U, Csernansky JG: Statistical analysis of hippocampal asymmetry. Neuroimage 2001; 14:531–545Crossref, Medline, Google Scholar
39. Posener JA, Wang L, Price JL, Gado MH, Province MA, Miller MI, Babb CM, Csernansky JG: High-dimensional mapping of the hippocampus in depression. Am J Psychiatry 2003; 160:83–89Link, Google Scholar
40. Csernansky JG, Wang L, Joshi S, Miller JP, Gado M, Kido D, McKeel D, Morris JC, Miller MI: Early DAT is distinguished from aging by high dimensional mapping of the hippocampus. Neurology 2000; 55:1636–1643Crossref, Medline, Google Scholar
41. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
42. Rastogi-Cruz D, Csernansky JG: Clinical rating scales, in Adult Psychiatry. Edited by Guze SB. St Louis, Mosby, 1997Google Scholar
43. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
44. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
45. Venkatesan R, Haacke EM: Role of high resolution in magnetic resonance (MR) imaging: applications of MR angiography, intracranial T1-weighted imaging, and image interpolation. Int J Imaging Systems and Technology 1997; 8:529–543Crossref, Google Scholar
46. Mai JK, Assheuer J, Paxinos G: Atlas of the Human Brain. San Diego, Academic Press, 1997Google Scholar
47. Miller MI, Christensen GE, Amit Y, Grenander U: Mathematical textbook of deformable neuroanatomies. Proc Natl Acad Sci USA 1993; 90:11944–11948Crossref, Medline, Google Scholar
48. Yeterian EH, Pandya DN: Corticothalamic connections of the posterior parietal cortex in the rhesus monkey. J Comp Neurol 1985; 237:408–426Crossref, Medline, Google Scholar
49. Insausti R, Amaral DG, Cowan WM: The entorhinal cortex of the monkey, III: subcortical afferents. J Comp Neurol 1987; 264:396–408Crossref, Medline, Google Scholar
50. Selemon LD, Kleinman JE, Herman MM, Goldman-Rakic PS: Smaller frontal gray matter volume in postmortem schizophrenic brains. Am J Psychiatry 2002; 159:1983–1991Link, Google Scholar
51. Pearlson GD, Petty RG, Ross CA, Tien AY: Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology 1996; 14:1–17Crossref, Medline, Google Scholar
52. Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC, Owens DGC, Roberts GW: Schizophrenia as an anomaly of development of cerebral asymmetry. Arch Gen Psychiatry 1989; 46:1145–1150Crossref, Medline, Google Scholar
53. Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen V-P, Huttunen M, Lonnqvist J, Standertskojd-Nordenstam C-G, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW: Genetic influences on brain structure. Nat Neurosci 2001; 4:1253–1258Crossref, Medline, Google Scholar
54. Algan O, Rakic P: Radiation-induced, lamina-specific deletion of neurons in the primate visual cortex. J Comp Neurol 1997; 381:335–352Crossref, Medline, Google Scholar
55. Schindler M, Wang L, Selemon LD, Goldman-Rakic PS, Rakic P, Csernansky JG: Abnormalities of thalamic volume and shape detected in fetally-irradiated Rhesus monkeys with high dimensional brain mapping. Biol Psychiatry 2002; 51:827–837Crossref, Medline, Google Scholar



