Smaller Nasal Volumes as Stigmata of Aberrant Neurodevelopment in Schizophrenia
Abstract
OBJECTIVE: Anatomical and functional deficits of the olfactory neural system have been identified in patients with schizophrenia. Since olfactory structures develop in conjunction with both the palate and ventral forebrain, the authors hypothesized that schizophrenia patients might have structural abnormalities of the nasal cavity, which could represent specific markers of embryological dysmorphogenesis underlying schizophrenia. METHOD: A measurement of nasal volume was acquired by acoustic rhinometry for 40 male schizophrenia patients and 24 healthy male comparison subjects. RESULTS: The patients had smaller posterior nasal volumes than the comparison subjects but did not differ in anterior nasal volumes. This difference persisted after covarying for height and smoking history. CONCLUSIONS: The lower observed posterior nasal volume likely reflects a specific developmental craniofacial abnormality. This finding confirms an early disruption in embryological development in males with schizophrenia and may represent a genetic or environmental “first hit” that leaves the individual vulnerable to subsequent pathology.
Schizophrenia is thought to be a neurodevelopmental brain disorder but without a clear etiology or pathognomonic biological markers. Neurodevelopmental disorders (e.g., Down’s syndrome, velocardiofacial syndrome) often include abnormalities of both cerebral and craniofacial morphogenesis, as these are intimately linked during embryological development. In schizophrenia, there is a somewhat higher rate of gross midline abnormalities, such as cleft palate and cavum septum pellucidum, and inconsistent evidence of subtle craniofacial dysmorphogenesis (1). Studies in our laboratory have identified anatomical and functional deficits in the olfactory neural system in patients with schizophrenia (2–4). Since olfactory structures develop in conjunction with both the palate and ventral forebrain, we hypothesized that there might be structural abnormalities of the nasal cavity, which could represent specific markers of embryological dysmorphogenesis underlying schizophrenia.
Method
We acquired volumetric and morphologic measurements of each nasal cavity, using a Hood Laboratories Eccovision acoustic rhinometer (Pembroke, Mass.) (Figure 1). Disposable silastic nosepieces were used and selected for each individual on the basis of the size of the nasal aperture. A sterile water-soluble lubricating jelly was swabbed around the edge of the nosepiece to create a good seal, decreasing any potential interference of the acoustic signal. The participant was asked to sit in a comfortable position while the trained examiner placed the acoustic rhinometer wave tube into the tip of the nostril, while being careful not to place any unnecessary pressure on the nasal aperture. The wave tube was aligned near the midline and about 45° off vertical; this angle was minimally adjusted to produce a stable trace. Two separate measurements were collected from each nostril, and the mean was computed for each of four output variables: nasal volume, airflow resistance, minimum cross-sectional area, and location of this minimum point. Total nasal volume was divided into anterior (0.0–3.5 mm) and posterior (3.5–5.5 mm) compartments. This boundary marks the beginning of the inferior turbinate (5). The anterior compartment is a dynamic structure, subject to changes in cross-sectional dimension and airflow resistance in response to vascular engorgement; the posterior compartment is relatively immune to such dynamic changes (6).
The study group included 40 men with a DSM-IV diagnosis of schizophrenia (14 Caucasian, 26 African American) and 24 healthy male comparison subjects without a family history of axis I psychiatric illness (17 Caucasian, seven African American). The difference in racial composition was significant (Fisher’s exact test, p<0.01). The patients’ ages ranged from 19 to 53 years (mean=32.7, SD=8.6); the comparison subjects’ ages ranged from 19 to 48 years (mean=32.2, SD=8.7). The patients were slightly smaller than the comparison subjects; the mean heights were 69.4 inches (SD=3.3) for the patients and 71.5 inches (SD=2.5) for the comparison subjects (t=2.79, df=62, p<0.01). The patients also smoked more; the mean number of pack-years was 5.2 (SD=8.2) for the patients and 1.2 (SD=2.8) for the comparison subjects (t=2.81, df=62, p<0.01). Subjects were excluded for obvious craniofacial trauma or abnormality, including septal deviation, and for any acute respiratory condition, cold, or allergy. Written informed consent was obtained after the procedures had been fully explained.
Results
Differences between the patients and comparison subjects were assessed through multivariate analyses of variance, with diagnosis and race as between-subject factors, nostril (left/right) as a within-subjects factor, and height and smoking status (pack-years) as covariates. For nasal volume, compartment (anterior/posterior) was an additional factor. There were no significant main or interaction effects of diagnosis on airflow resistance, minimum cross-sectional area, or location of the minimum cross-section. There was a significant main effect of diagnosis on nasal volume (lower in patients than comparison subjects) (F=7.96, df=1, 58, p<0.01) but no effect of race (F=3.12, df=1, 58, p=0.08) and no interaction between diagnosis and race (F=0.18, df=1, 58, p=0.68). There was a significant interaction between diagnosis and nasal compartment (F=9.72, df=1, 58, p<0.01). Post hoc contrasts indicated that the patients had smaller posterior nasal volumes than the comparison subjects (F=10.64, df=1, 58, p<0.01) but did not differ in anterior nasal volume (F=2.64, df=1, 58, p=0.11). The difference in posterior volume was present for both the left (F=8.10, df=1, 58, p<0.01) and right (F=7.31, df=1, 58, p<0.01) nostrils. Mean group volumes for each compartment by nostril are presented in Figure 2. On a percentage basis, the posterior nasal volume was 31% smaller in the patients, compared to an 11% smaller anterior volume.
Discussion
The presence of a smaller posterior nasal volume likely reflects a specific developmental craniofacial abnormality. The absence of any difference in nasal airflow resistance or minimum cross-sectional area, along with the normal anterior volume, argues that this is not a state-dependent effect of increased nasal engorgement or congestion. Embryologically, the nasal cavities develop through invagination of the nasal placodes, which are derived from neural crest tissue. This occurs between the 6th and 11th weeks of development, in conjunction with palatal and nasal septal fusion. While subsequent development results in overall enlargement, there is no substantial reshaping of the cavities (7).
This finding confirms an early disruption in embryological development in males with schizophrenia. This may represent a genetic or environmental “first hit” that leaves the individual vulnerable to subsequent pathology (8). The presence of similar olfactory and craniofacial abnormalities in otherwise healthy first-degree relatives of patients supports the genetic etiology (9).
We do not yet know whether nasal cavity abnormalities are also present in female patients, in unaffected family members of patients, or in selected individuals at risk for developing schizophrenia. However, this may be a simple vulnerability marker implicating both a specific process and a critical time period of neurodevelopment.
Received Dec. 30, 2003; revision received March 21, 2004; accepted April 5, 2004. From the Schizophrenia Research Center, Department of Psychiatry, and the Smell and Taste Center, Department of Otorhinolaryngology: Head and Neck Surgery, University of Pennsylvania School of Medicine. Address reprint requests to Dr. Moberg, Brain-Behavior Laboratory, Department of Psychiatry, 10th Floor, Gates Building, University of Pennsylvania School of Medicine, 3400 Spruce St., Philadelphia, PA 19104; [email protected] (e-mail). Supported by NIMH grants MH-63381 (Dr. Moberg) and MH-59852 (Dr. Turetsky), by an Independent Investigator Award from the National Alliance for Research on Schizophrenia and Depression (Dr. Moberg), and by Ms. Janice Lieber and Ms. Constance Lieber.
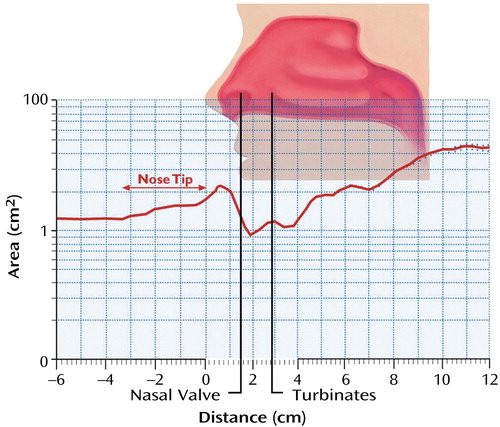
Figure 1. Rhinogram Showing the Cross-Sectional Area of the Nasal Cavity Versus Distance Into the Nasal Cavitya, b
aFrom the operator manual for the Hood Laboratories Eccovision acoustic rhinometer. Used with permission from Hood Laboratories, Pembroke, Mass.
bThe vertical axis is the cross-sectional area, and the horizontal axis is the distance into the nasal cavity, with 0.0 cm corresponding to the tip of the nose. The notation of “nose tip” on the rhinogram refers to potential individual variance in nose size, suggesting the tip of the nose may extend further or less than pictured.
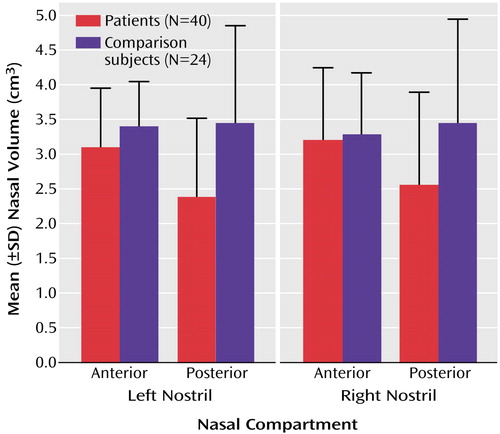
Figure 2. Volumes for Anterior and Posterior Nasal Compartments of Left and Right Nostrils in Male Patients With Schizophrenia and Healthy Comparison Subjects
1. Hennessy RJ, Lane A, Kinsella A, Larkin C, O’Callaghan E, Waddington JL: 3D morphometrics of craniofacial dysmorphology reveals sex-specific asymmetries in schizophrenia. Schizophr Res 2004; 67:261–268Crossref, Medline, Google Scholar
2. Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL: Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology 1999; 21:325–340Crossref, Medline, Google Scholar
3. Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE: Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry 2000; 157:828–830Link, Google Scholar
4. Turetsky BI, Moberg PJ, Owzar K, Johnson SC, Doty RL, Gur RE: Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol Psychiatry 2003; 53:403–411Crossref, Medline, Google Scholar
5. Grymer LF, Hilberg O, Elbrond O, Pedersen OF: Acoustic rhinometry: evaluation of the nasal cavity with septal deviations, before and after septoplasty. Laryngoscope 1989; 99:1180–1187Medline, Google Scholar
6. Brugel-Ribere L, Fodil R, Coste A, Larger C, Isabey D, Harf A, Louis B: Segmental analysis of nasal cavity compliance by acoustic rhinometry. J Appl Physiol 2002; 93:304–310Crossref, Medline, Google Scholar
7. Larsen WJ: Human Embryology, 3rd ed. London, Churchill Livingstone, 2001, pp 352–378Google Scholar
8. Maynard TM, Sikich L, Lieberman JA, LaMantia AS: Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull 2001; 27:457–476Crossref, Medline, Google Scholar
9. Deutsch CK, Hobbs K, Price SFR, Gordon-Vaughn K: Skewing of the brain midline in schizophrenia. Neuroreport 2000; 11:3985–3988Crossref, Medline, Google Scholar



