Reduced Olfactory Bulb Volume in Patients With Schizophrenia
Abstract
OBJECTIVE: The authors’ goal in this study was to compare the size of olfactory bulbs of patients with schizophrenia and those of healthy subjects.METHOD: Magnetic resonance imaging scans of olfactory bulbs were obtained from 26 patients with schizophrenia and 22 healthy comparison subjects. A reliable region of interest procedure was used to measure olfactory bulb volume.RESULTS: Patients exhibited 23% smaller bilateral bulb volume than comparison subjects, independent of acute clinical, demographic, or treatment measures. Bulb volume correlated with odor detection sensitivity in healthy subjects but not in patients with schizophrenia.CONCLUSIONS: Patients with schizophrenia exhibit structural olfactory deficits as well as functional olfactory deficits. The olfactory system may be a model system in which to study the neurobiology of the disorder.
Schizophrenia is characterized by abnormalities in frontotemporal limbic brain regions. Efforts to delineate the precise structural and functional impairments underlying schizophrenia have employed a variety of neurobehavioral and neuroimaging procedures. The olfactory system, which seems to be an ideal area for examination of this frontotemporal limbic pathology, has received limited attention. Olfactory processing is mediated by structures implicated in schizophrenia, including the ventromedial temporal lobe, basal forebrain, prefrontal cortex, and diencephalon. The olfactory system is also unique in that a single synapse in the olfactory bulb lies between peripheral olfactory receptors and the primary olfactory cortex, providing one of the most direct links between brain and environment.
There have been several reports of olfactory dysfunction in patients with schizophrenia (1). Abnormalities include impairments in odor sensitivity (ability to detect the presence of an odor), odor identification, and odor memory. The evidence suggests that these deficits are present early in the course of the disorder, are unrelated to illness severity, neuroleptic use, or smoking, and reflect, in part, a genetic vulnerability factor. These investigations have been limited, almost exclusively, to psychophysical assessments of behavioral responses, and there have been no neuroanatomical assessments of primary olfactory structures in patients with schizophrenia. We report here the results of an initial magnetic resonance imaging study of olfactory bulb size in patients with schizophrenia.
Method
Twenty-six patients meeting DSM-IV criteria for schizophrenia (15 men, 11 women) were recruited by the University of Pennsylvania Mental Health Clinical Research Center. Twenty-two healthy individuals (12 men, 10 women), without a family history of schizophrenia or affective illness were recruited from the community. On the basis of medical record review, physician interview, and physical examination, subjects were excluded for any history of neurological disorder, head trauma, loss of consciousness, substance abuse, medical conditions that might alter cerebral functioning, recent or current upper respiratory infection, or other conditions affecting olfactory functioning (e.g., acute or chronic allergies).
Patients ranged in age from 22 to 57 years (mean=37.8, SD=9.4); comparison subjects ranged in age from 19 to 55 years (mean=36.6, SD=11.8). Differences between patients and comparison subjects in gender (χ2=0.05, df=1, p=0.83) and age (t=0.41, df=46, p=0.68) were insignificant. After description of the study, written informed consent was obtained from all subjects.
Magnetic resonance images of the olfactory bulbs were acquired by using a 5-inch-round, general-purpose, receive-only surface coil placed over the nasion. After a sagittal localizer scan, 3-mm interleaved coronal images were acquired with a 256×256 matrix and a 12-cm field of view (TR=500, TE=15, two averages). In-plane voxel size was 0.47×0.47 mm. The olfactory bulbs could be clearly identified on the resulting images (Figure 1).
A trained operator manually traced the left and right olfactory bulbs on individual slices selected at the level of the anterior cribriform plate. The reliability and accuracy of olfactory bulb volume measurements were established for 55 subjects in an earlier study (2). Intraclass correlation coefficients for repeated measurements by a single operator were ³0.92; intraclass correlation coefficients for measurements across operators were ³0.92. Measurement accuracy was estimated by using a realistic phantom that simulated olfactory bulb placement and size. When two operators computed volumes from four different scans, their mean measurement error was 7.3%.
Analysis of variance was conducted, with diagnosis and gender as between-subjects factors and hemisphere (left versus right olfactory bulb) as a within-subjects factor. Relationships between olfactory bulb volumes and psychophysical, demographic, and clinical symptom measures were assessed by using Pearson correlation coefficients.
Results
There was a significant effect of diagnosis on olfactory bulb volume (F=15.75, df=1, 44, p<0.001), which persisted after covarying for cranial volume, age, and smoking status, either as a quantitative variable (number of pack-years) (F=4.58, df=1, 44, p<0.05) or categorically (current smoker or nonsmoker) (F=4.50, df=1, 44, p<0.05). There were no effects of gender or hemisphere and no interactions. Total mean olfactory bulb volume for healthy subjects was 104.3 mm3 (SD=19.6), and the mean for patients was 79.8 mm3 (SD=22.9), equivalent to a 23% reduction in patients (95% confidence interval=7%–38%). This reduction was not attributable to a subset of patients with especially small olfactory bulbs; 21 of the 26 patients had olfactory bulb volumes below the comparison group mean, but the volumes of only two healthy subjects fell below the patient mean.
There was a strong association between olfactory bulb volume and odor threshold sensitivity in healthy individuals (r=–0.86, N=22, p<0.001). Larger bulb size correlated with greater odor detection sensitivity. This association was not observed in patients (r=0.30, N=26, p=0.14). Olfactory bulb volume was unrelated to odor identification in either group, and, unlike our finding for odor identification (3), it did not correlate with illness duration (r=0.11, N=26, p=0.59). It was also unrelated to age at onset (r=–0.13, N=26, p=0.53), medication use (t=0.66, df=24, p=0.52), or total ratings on the Scale for the Assessment of Negative Symptoms (r=–0.19, N=26, p=0.35) and the Scale for the Assessment of Positive Symptoms (r=0.06, N=26, p=0.77).
Discussion
To our knowledge, this is the first demonstration of reduced olfactory bulb volume in patients with schizophrenia. This abnormality is independent of demographic, behavioral, clinical, or treatment-related measures, and its magnitude appears to exceed the 2%–10% reduction in total gray matter that we have observed in patients (4). This suggests that the olfactory bulb may be particularly vulnerable to those disease processes which produce structural brain changes.
Several potential methodological confounds must be noted. Susceptibility artifacts make ventromedial brain regions, including the olfactory bulb, especially difficult to image. As a small area of tissue surrounded by CSF, the olfactory bulb is also prone to volume-averaging errors with the 3-mm slice thickness employed here. Since there are few studies of olfactory bulb volume in the literature and the range of normal variability has not been clearly established, the possibility of type I error must be considered.
There are several reasons to be confident of the validity of the data, however. Phantom studies showed relatively low measurement error, and there was a strong association, in healthy subjects, between olfactory bulb volume and olfactory sensitivity. In addition, there was no correlation between olfactory bulb volume and CSF volume (r=–0.01, N=48, p=0.98), suggesting that partial volume artifact from adjacent CSF did not influence these measurements.
Whether this abnormality reflects neurodevelopmental or neurodegenerative processes is unknown. However, the olfactory bulb, unlike other brain regions, remains highly plastic throughout adult life. Evidence of continuing synaptogenesis can be found even in human postmortem material from elderly individuals (5). The olfactory bulb, therefore, is relatively resistant to degenerative processes that affect other areas of the brain. Midline developmental abnormalities have been reported in schizophrenia (6), and, histopathologically, central olfactory pathways exhibit cytoarchitectural, morphometric, and cytoskeletal protein abnormalities that are consistent with aberrant development rather than degeneration (5, 7).
These findings require replication and extension, including studies that examine the olfactory bulb longitudinally in patients and at-risk individuals. They suggest, however, that the olfactory system may be an important one in which to study the neurobiology of schizophrenia.
Received June 28, 1999; revision received Nov. 19, 1999; accepted Nov. 30, 1999. From the Departments of Psychiatry, Radiology, and Otorhinolaryngology, University of Pennsylvania. Address reprint requests to Dr. Turetsky, Department of Psychiatry, University of Pennsylvania, Gates Bldg., 10th Floor, Philadelphia, PA 19104; turetsky@ bblmail.psycha.upenn.edu (e-mail). Supported by NIMH grants MH-59852 and MH-43880 and National Institute on Deafness and Other Communication Disorders grant DC-00161.
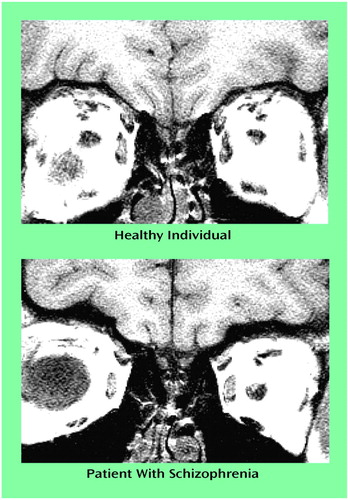
Figure 1. Images of the Olfactory Bulbs of a Healthy Subject and a Patient With Schizophreniaa
aMagnetic resonance images are oriented radiologically (left=right). The healthy subject was a 22-year-old woman with a total bulb volume of 116.1 mm3 (left=53.4 mm3, right=62.7 mm3). The patient was a 23-year-old woman with a total bulb volume of 83.8 mm3 (left=45.6 mm3, right=38.2 mm3).
1. Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL: Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology 1999; 21:325–340Crossref, Medline, Google Scholar
2. Yousem DM, Geckle RJ, Doty RL, Bilker WB: Reproducibility and reliability of volumetric measurements of olfactory eloquent structures. Acad Radiol 1997; 4:264–269Crossref, Medline, Google Scholar
3. Moberg PJ, Doty RL, Turetsky BI, Arnold SE, Mahr RN, Gur RC, Gur RE: Olfactory identification deficits in schizophrenia: correlation with duration of illness. Am J Psychiatry 1997; 154:1016–1018Google Scholar
4. Gur RE, Turetsky BI, Yan MX, Bilker W, Gur RC: Reduced gray matter volume in schizophrenia. Arch Gen Psychiatry 1999; 56:905–911Crossref, Medline, Google Scholar
5. Arnold SE, Israeli NE, Han L-Y, Smutzer GS, Rioux L, Leslie GJ: Olfactory neuroepithelium and bulb as a model system for studying developmental neuropathology in schizophrenia. Abstracts of the Society for Neuroscience 1998; 24:1274Google Scholar
6. Scott TF, Price TR, George MS, Brillman J, Rothfus W: Midline cerebral malformations and schizophrenia. J Neuropsychiatry Clin Neurosci 1993; 5:287–293Crossref, Medline, Google Scholar
7. Arnold SE, Smutzer GS, Trojanowski JQ, Moberg PJ: Cellular and molecular neuropathology of the olfactory epithelium and central olfactory pathways in Alzheimer’s disease and schizophrenia. Ann NY Acad Sci 1998; 855:762–775Crossref, Medline, Google Scholar



