Left Hemisphere Dysfunction During Verbal Dichotic Listening Tests in Patients Who Have Social Phobia With or Without Comorbid Depressive Disorder
Abstract
OBJECTIVE: Behavioral, electrophysiological, and imaging studies have found evidence that anxiety disorders are associated with left hemisphere dysfunction or higher than normal activation of right hemisphere regions. Few studies, however, have examined hemispheric asymmetries of function in social phobia, and the influence of comorbidity with depressive disorders is unknown. The present study used dichotic listening tests to assess lateralized cognitive processing in patients with social phobia, depression, or comorbid social phobia and depression. METHOD: The study used a two-by-two factorial design in which one factor was social phobia (present versus absent) and the second factor was depressive disorder (present versus absent). A total of 125 unmedicated patients with social phobia, depressive disorder, or comorbid social phobia and depressive disorder and 44 healthy comparison subjects were tested on dichotic fused-words, consonant-vowel syllable, and complex tone tests. RESULTS: Patients with social phobia with or without a comorbid depressive disorder had a smaller left hemisphere advantage for processing words and syllables, compared with subjects without social phobia, whereas no difference between groups was found in the right hemisphere advantage for processing complex tones. Depressed women had a larger left hemisphere advantage for processing words, compared with nondepressed women, but this difference was not seen among men. CONCLUSIONS: The results support the hypothesis that social phobia is associated with dysfunction of left hemisphere regions mediating verbal processing. Given the importance of verbal processes in social interactions, this dysfunction may contribute to the stress and difficulty experienced by patients with social phobia in social situations.
Neuroimaging and electrophysiological studies have found evidence that panic disorder is associated with lower than normal activation of the left parietal or superior temporal cortex and relatively greater activation of the right than of the left frontal or hippocampal regions (1–3). Although few studies have examined regional brain activation in social phobia, enhanced activation of the right frontal-temporal regions was found in the EEGs of patients with social phobia during provocation of fear or anxiety (4). Greater activation of the amygdala has been found in social phobia, but there is conflicting evidence about whether this activation involves primarily the right or left amygdala (5, 6).
Given the high rate of comorbidity of anxiety and depressive disorders (7), an important issue is the extent to which these abnormalities of regional hemispheric activation are specific to anxiety disorders or reflect the presence of comorbidity with depression. In a study measuring EEG in a resting state (8), patients with comorbidity of an anxiety disorder and major depressive disorder showed greater activity in the right than in the left hemisphere over frontal and more posterior sites. In contrast, patients with major depressive disorder alone showed less activity over the right than over the left posterior sites, and neither they nor the healthy comparison subjects in that study had asymmetry of activity in frontal sites.
Studies using behavioral laterality tests have found evidence of left hemisphere dysfunction (9, 10) or bias favoring right hemisphere processing (11, 12) in subjects with anxiety disorders or high levels of trait anxiety. We have used dichotic listening tests to examine lateralized cognitive processing in patients with comorbid anxiety and depressive disorders (13). In dichotic tests, paired stimuli (two different words or two different tones) are simultaneously presented to the two ears, and the difference in perceiving the stimulus in the right and left ear provides an index of perceptual asymmetry. On the Fused Rhymed Words Test (14), healthy adults are more likely to perceive the word in the right ear, i.e., to show a right-ear advantage, which reflects the dominance of contralateral left hemisphere regions for language-related processing. In contrast, the Complex Tone Test (15) yields a left-ear advantage in healthy adults that is indicative of right hemisphere dominance for processing of tonal stimuli. In our prior study, patients with comorbidity of an anxiety disorder and major depressive disorder differed from patients with major depressive disorder alone in having less left hemisphere advantage for fused words and a greater right hemisphere advantage for complex tones (13). It is not, however, clear whether this evidence of left hemisphere dysfunction or bias toward right hemispheric processing is associated with having an anxiety disorder or with comorbidity of anxiety and depressive disorders. Our prior study was limited by the lack of patients with an anxiety disorder alone and by inclusion of a heterogeneous mix of patients with different anxiety disorders.
The present study was designed to overcome these limitations by assessing dichotic listening measures of lateralized cognitive processing in groups of patients with social phobia alone, with a depressive disorder alone, or with comorbidity of social phobia and a depressive disorder. On the basis of prior findings, we predicted that patients with social phobia either with or without a comorbid depressive disorder would show a smaller left hemisphere advantage for fused words and a greater right hemisphere advantage for complex tones, compared to subjects without social phobia, i.e., patients with a depressive disorder alone and healthy comparison subjects. Moreover, the dichotic listening tests yield separate measures of accuracy for perceiving syllables or complex tones in each ear. Given the predominance of contralateral projections from ear to auditory cortex, these absolute accuracy measures provide additional information concerning the source of differences in perceptual asymmetry between groups. Thus, left hemisphere dysfunction in subjects with social phobia would be expected to result in poorer right-ear accuracy for perceiving syllables in that group, compared to subjects without social phobia, whereas right hemisphere hyperactivation would result in increased left-ear accuracy for perceiving complex tones in subjects with social phobia, compared to subjects without social phobia.
Method
Subjects
A factorial design with two grouping factors was used to contrast the dichotic listening performance of subjects with versus without social phobia as one factor and subjects with versus without a depressive disorder as the second factor. The four cells in this two-by-two design were 1) patients with social phobia but no depressive disorder (N=25), 2) patients with social phobia and a comorbid depressive disorder (N=18), 3) patients with a depressive disorder but no anxiety disorder (N=82), and 4) healthy comparison subjects with neither disorder (N=44). The patients were recruited from the Anxiety Disorders Clinic and Depression Evaluation Service at New York State Psychiatric Institute. Semistructured diagnostic interviews of the patients were carried out by research psychiatrists at these units before the dichotic listening tests. Patients in group 1 met the DSM-IV criteria for a principal diagnosis of social phobia (mostly generalized subtype) without a current depressive disorder, those in group 2 met the DSM-IV criteria for current social phobia and also major depressive disorder (N=10), dysthymic disorder (N=5), or both disorders (N=3), and those in group 3 met the DSM-IV criteria for current major depressive disorder (N=48), dysthymic disorder (N=20), or both disorders (N=14) without social phobia or other anxiety disorder. (Both patients with a major depressive disorder and those with dysthymic disorder were included because we have not found evidence of a difference in perceptual asymmetry between patients with those diagnoses [16]. Analysis of variance [ANOVA] comparing the dichotic listening of patients with major depressive disorder, patients with dysthymic disorder, and patients with both disorders did not reveal a significant difference among these groups in perceptual asymmetry in the fused-words, consonant-vowel, or complex tone tests.) The patients were drug-free for a minimum of 7 days before testing, although most patients had been unmedicated for a considerably longer period. We have not found any effects of antidepressants on the dichotic listening performance of depressed patients (17). Healthy comparison subjects were recruited through notices to hospital staff and college students and through advertisements in local newspapers. Potential comparison subjects were screened with a semistructured interview to exclude those with current or past psychopathology. Subjects were excluded from the study if they had a hearing loss greater than 30 dB in either ear at 500, 1,000, or 2,000 Hz or if they had a hearing difference between ears that was greater than 10 dB. Subjects were also excluded if they had current substance abuse or a history of head trauma or other neurological disorder. After a complete description of the study to the subjects, written informed consent was obtained.
Table 1 summarizes the characteristics of the subjects in the four groups. About two-thirds of the patients with social phobia alone were men, whereas two-thirds of those in the group with comorbid disorders were women. Gender was therefore included as a variable in the analysis of the dichotic listening data (see later discussion). ANOVAs comparing the four groups found no significant differences in age or education level. All subjects were right-handed, and no difference among groups was found in the Edinburgh Handedness Inventory (18) handedness laterality quotient. A laterality quotient of 100 signifies complete right-hand preference, and –100 indicates complete left-hand preference. Beck Depression Inventory (19) scores differed significantly among groups (F=56.73, df=3, 165, p<0.001). Newman-Keuls post hoc tests indicated that the comorbid and depressed groups had higher Beck Depression Inventory scores than the social phobia and comparison groups (p<0.05) and that the social phobia group had a higher Beck Depression Inventory score than the comparison group (p<0.05). State-anxiety scores on the State-Trait Anxiety Inventory (20) also differed significantly among groups (F=27.69, df=3, 163, p<0.001). Post hoc comparisons indicated that all three patient groups had significantly higher state anxiety scores than the comparison group (p<0.05) and that the comorbid group had a higher state anxiety score than the social phobia group (p<0.05). The difference in Beck Depression Inventory scores between the social phobia and comparison groups and the differences in state-anxiety scores between the depressed and comparison groups and between the comorbid and social phobia groups are likely to reflect the lack of specificity of these self-report scales for assessing depression as opposed to anxiety (21).
Procedure
Subjects were tested with the same dichotic fused-words and complex tone tests used in our prior study (13) and also with a consonant-vowel syllable test (22). The order of these tests was counterbalanced across subjects. The tests are described in detail in prior reports (13–15, 22), and a brief description of each is given here.
Fused-words test
The Fused Rhymed Words Test (14) consists of 15 different single-syllable word pairs, in which the paired words differ only in the initial consonant (e.g., coat, goat). All words begin with one of six stop consonants (b, d, p, t, g, k) and are presented in natural speech spoken by a male voice. When presented dichotically, paired words fuse into a single percept. Subjects indicate what word they heard by marking a line through it on a prepared answer sheet that has four possible responses, including both members of the dichotic pair and two other words differing from the dichotic stimuli only in the initial consonant. After practice trials, each subject received four 30-item blocks for a total of 120 trials. Orientation of headphones was reversed after the first and third quarters to control for channel differences and ear of presentation. The words were presented through a matched pair of headphones (TDH-49 headphones, Northeastern Technologies, Glen Cove, N.Y.) at a comfortable level of 75 dB (SPL).
Syllable test
In the syllable test, a different consonant-vowel syllable (ba, da, ga, ka, pa, or ta) is presented simultaneously to each ear (22). Unlike the stimuli in the fused-words test, the syllables in both the right and the left ear can sometimes be perceived by subjects. Subjects are therefore asked to report the two syllables that they think they heard on each trial by using a multiple-choice answer sheet that contains a random ordering of the six possible syllables. After 16 monaural and 14 dichotic practice trials, each subject was tested in two blocks of 30 trials. The orientation of headphones was reversed after the first block. The tones were presented at 75 dB (SPL).
Tone test
The Complex Tone Test (15) requires participants to compare the pitch of a binaural complex tone to the pitches of a dichotic pair of complex tones presented 1 second earlier. Subjects point to a response card labeled “Yes” when the probe tone is the same as either member of the previous dichotic pair or to a card labeled “No” when it differs from both. The complex tones are square waves with fundamental frequencies corresponding to eight notes in the octave between C4 and C5. After 14 binaural and 14 dichotic practice trials, participants were tested on four blocks of 28 trials in which half of the probe tones matched a member of the dichotic pair and half did not. Orientation of headphones was reversed after the first and third blocks. The tones were presented at 74 dB (SPL).
Data Analysis
The number of correct responses was scored for the right and left ear presentations for each test. These scores were used to compute a perceptual asymmetry score, i.e., 100(right – left)/(right + left). These scores were analyzed using a two-by-two-by-two-by-three ANOVA with three grouping factors, i.e., social phobia (present/absent), depressive disorder (present/absent), and gender (women/men), as well as one repeated measure variable, i.e., three dichotic tests. Given significant interactions of the group and test variables, separate two-by-two-by-two ANOVAs were performed to evaluate the significance of group differences on each test. A two-by-two-by-two-by-two ANOVA was also performed on the absolute accuracy scores for the right and left ear for the syllable and tone tests, with the variables being social phobia, depressive disorder, gender, and ear. This analysis was not performed on the data for the fused-words test because subjects’ accuracy was essentially 100% for the single response given on each trial, i.e., subjects correctly reported one of the words presented to the right or left ear on virtually every trial.
Results
An ANOVA confirmed the expected difference in perceptual asymmetry across tests (F=66.94, df=2, 322, p<0.001), with the word and syllable tests yielding a left hemisphere advantage (i.e., positive asymmetry scores) and the tonal test yielding a right hemisphere advantage (i.e., negative asymmetry scores). The ANOVA revealed a significant main effect for social phobia (F=8.04, df=1, 161, p=0.005), which reflects the generally smaller left hemisphere advantage for words or syllables in subjects with social phobia (i.e., subjects with social phobia alone and those with comorbid social phobia and depressive disorder), compared to subjects without social phobia (i.e., those with depression alone and the healthy comparison subjects). Most important, a significant social phobia-by-test interaction was found (F=4.06, df=2, 322, p<0.05), indicating that the difference in perceptual asymmetry between the subjects with versus without social phobia was dependent on the test. This interaction is illustrated in Figure 1, which shows the mean perceptual asymmetry scores on the word, syllable, and tone tests for subjects with versus without social phobia. A separate analysis of asymmetry scores on each test yielded a main effect of social phobia for both the fused-words test (F=7.33, df=1, 161, p<0.01) and the syllable test (F=6.82, df=1, 161, p=0.01), with a smaller left hemisphere advantage in patients with social phobia. In contrast, there was no significant difference among groups in right hemisphere advantage for complex tones. The difference in perceptual asymmetry between subjects with versus without social phobia was not modulated by the presence of a depressive disorder or by gender, as indicated by the absence of significant interactions of social phobia with these factors.
These findings were based on a design in which social phobia (present/absent) and depressive disorder (present/absent) were treated as independent variables. An alternative, less powerful, statistical design would treat the four groups as a single independent variable. Using this design, there were significant differences among groups in left hemisphere advantage for words (F=4.22, df=3, 161, p<0.01) and syllables (F=2.85, df=3, 161, p<0.05) but no difference in right hemisphere advantage for tones (F=1.36, df=3, 161, n.s.). For words, Newman-Keuls post hoc tests revealed a significant difference in left hemisphere advantage between the patients with social phobia alone and those with depression alone (p<0.05) but not between the groups in the remaining comparisons. Nor did the post hoc comparisons for the syllable test achieve statistical significance.
The only significant effect of depression in the ANOVA of asymmetry scores for the three dichotic tests was a depression-by-test-by-gender interaction (F=4.66, df=1, 322, p<0.05). The nature of that interaction is illustrated in Figure 2, which shows the mean perceptual asymmetry scores for women and men with versus without a depressive disorder. Depressed women had a markedly larger left hemisphere advantage for words, compared to nondepressed women (t=2.88, df=85, p=0.005), but this difference was not evident for men, which was reflected in a depression-by-gender interaction in the ANOVA of the asymmetry scores for this test (F=4.64, df=1, 161, p<0.05). A similar depression-by-gender interaction was present in the ANOVA of the asymmetry scores for the syllable test (F=3.98, df=1, 161, p<0.05) but not for the tone test. However, in a result that approached significance, depressed men had a smaller right hemisphere advantage for tones, compared to nondepressed men (t=1.82, df=80, p=0.07). This difference was not evident for women.
The syllable and complex tone tests also permit analyses of the absolute accuracy scores for each ear. These analyses provide important information about whether the smaller left hemisphere advantage for syllables in social phobia was due to poorer performance for items presented to the right ear or better left ear performance. An ANOVA of the accuracy scores for the syllable test yielded a significant interaction of social phobia and ear (F=6.97, df=1, 161, p<0.01). Figure 3 shows the mean percentage of correct responses for subjects with versus without social phobia for syllables presented to the right or left ear. The smaller left hemisphere advantage for patients with social phobia was clearly due to their poorer right ear performance. Separate analyses of the accuracy scores for each ear revealed that subjects with social phobia had significantly poorer right ear accuracy, compared to subjects without social phobia (F=11.00, df=1, 161, p=0.001). In contrast, there was no significant difference between subjects with versus without social phobia in accuracy for perceiving syllables presented to the left ear. ANOVA of the accuracy scores for the complex tones test did not reveal any significant group difference for tones presented to the right or the left ear.
Correlational analyses examined whether the smaller left hemisphere advantage for words and syllables in social phobia was related to self-ratings of state anxiety on the State-Trait Anxiety Inventory. Perceptual asymmetry scores for words were not significantly associated with state-anxiety scores for the patients with social phobia either with or without a comorbid depressive disorder (r=0.001, df=42, n.s.), the patients with a depressive disorder alone (r=0.03, df=79, n.s.), or the comparison subjects (r=–0.02, df=43, n.s.). The only significant correlation between perceptual asymmetry for syllables and state-anxiety scores was seen for the comparison subjects (r=0.35, df=43, p<0.05), with greater state anxiety being associated with a larger left hemisphere advantage. When partial correlations were computed with the scores on the Beck Depression Inventory controlled, the correlations between perceptual asymmetry and state-anxiety scores remained essentially the same as those reported earlier.
Discussion
Patients with social phobia had a smaller left hemisphere advantage for perceiving dichotic words or consonant-vowel syllables, compared with subjects without social phobia. Their poorer accuracy for perceiving syllables in the right ear, compared to that for subjects without social phobia, as well as the absence of a group difference in perceptual asymmetry for complex tones, supports the hypothesis that social phobia is associated with dysfunction of left hemisphere regions mediating verbal processing. This finding is consistent with evidence that anxiety disorders are in general associated with left hemisphere dysfunction on behavioral laterality tests (9, 10) and with lower than normal metabolism or cerebral blood flow in left temporoparietal regions (1, 2) that are thought to mediate phonetic processing of dichotic words or syllables (23, 24).
The reduced left hemisphere processing of verbal stimuli in social phobia is not specific to this disorder but has been found for obsessive-compulsive disorder (10) and schizophrenia (24, 25). Patients with comorbid anxiety and depressive disorders have also shown a smaller left hemisphere advantage for dichotic fused words, compared to those with a depressive disorder alone (13). The findings of the present study support the conclusion that the smaller left hemisphere advantage in the patients with comorbid disorders was primarily due to the presence of an anxiety disorder.
Although the patients with social phobia showed less left hemisphere processing of verbal stimuli than the subjects without social phobia, they did not show the predicted enhancement of right hemisphere advantage for processing complex tones. In our prior study (13), patients with comorbid anxiety and depressive disorders showed a greater right hemisphere advantage for perceiving complex tones, compared to depressed patients without an anxiety disorder and healthy comparison subjects. However, this difference was not due to better left-ear accuracy but to poorer right-ear accuracy. Given the predominantly contralateral projections from ear to auditory cortex, this finding is more suggestive of left hemisphere dysfunction than of right hemisphere hyperactivation. Patients with social phobia in the present study had poorer right-ear accuracy for perceiving consonant-vowel syllables, compared to subjects without social phobia, which is again supportive of the hypothesis of left hemisphere dysfunction in anxiety disorders.
One could argue that the smaller left hemisphere advantage for verbal processing in social phobia is due to greater performance anxiety during the tests in those patients. Previous research has found evidence that aversive arousal can reduce the left hemisphere advantage for dichotic consonant-vowels (26). Although the lack of a correlation of state-anxiety and perceptual asymmetry scores provides no support for the effects of state anxiety on dichotic listening in patients with social phobia, self-ratings on the State-Trait Anxiety Inventory may not provide an accurate measure of performance anxiety during the dichotic tests. A study comparing dichotic listening performance of patients with social phobia before treatment and after clinical remission is needed to further examine the state-versus-trait issue.
Given the importance of verbal processing in social situations, the deficit in left hemispheric processing of verbal input in social phobia may be intrinsic to the disorder and may contribute to the stress and compound the anxiety experienced by patients with social phobia in these situations. Reports that persons with social phobia have difficulty taking in information when they are anxious and self-conscious could reflect reduced verbal processing resources. Cognitive behavior therapy techniques that enhance shifting patients’ focus of attention from self to the social situation (27) may help patients allocate additional resources to improve processing of verbal input.
Adults and adolescents with a depressive disorder without an anxiety disorder have been found to show an abnormally large left hemisphere advantage for dichotic fused words (13, 28). In the present study, depressed women had a larger left hemisphere advantage for words, compared to nondepressed women, but this difference was not seen among men. Depressed women had about twice the left hemisphere advantage, compared to nondepressed women. The enhanced left hemisphere advantage in depressed women is opposite the direction of the perceptual asymmetry seen in social phobia, and gender did not modulate perceptual asymmetry in social phobia. These findings underscore the importance of taking comorbidity and gender into account in studies of hemispheric asymmetry of function in depressive disorders.
A limitation of this study was the reliance on dichotic listening tests that provide only indirect measures of lateralized hemispheric function. The findings are, however, consistent with neuroimaging (1, 2) and electrophysiological (29) evidence of left temporoparietal hypoactivation in anxiety disorders. Further studies using neuroimaging and electrophysiological measures are needed to better understand the basis for the left-lateralized deficit in verbal processing in social phobia.
 |
Presented at the 22nd national conference of the Anxiety Disorders Association of America, Austin, Tex., March 21–24, 2002. Received May 2, 2002; revision received April 16, 2003; accepted April 29, 2003. From the New York State Psychiatric Institute; and the Department of Psychiatry, College of Physicians and Surgeons, Columbia University, New York. Address reprint requests to Dr. Bruder, Department of Biopsychology, New York State Psychiatric Institute, 1051 Riverside Dr., New York, NY 10032; [email protected] (e-mail). Supported by NIMH grant MH-36295. The authors thank Dr. Deborah Deliyannides and members of the New York State Psychiatric Institute Depression Evaluation Service and Anxiety Disorders Clinic for assistance with patient evaluations and Paul Leite and Barbara Stuart for assistance with data collection and analysis.
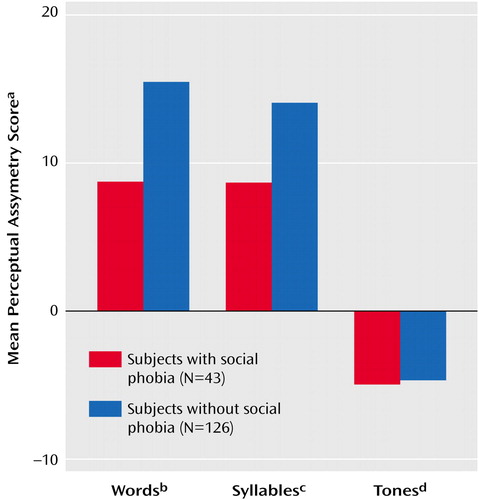
Figure 1. Mean Perceptual Asymmetry Scores on the Fused-Words, Consonant-Vowel Syllable, and Complex Tone Dichotic Listening Tests for Subjects With and Without Social Phobia
aPerceptual asymmetry score = 100 (right – left)/(right + left), based on the number of correct responses for right- and left-ear presentations. Scores greater than zero indicate a right-ear (left brain) advantage; scores less than zero indicate a left-ear (right brain) advantage.
bScores for the Fused Rhymed Words Test, in which 15 different single-syllable word pairs differing only in the initial consonant (e.g., coat, goat) are presented dichotically and subjects are asked to select which word they heard from four possible responses, including both members of the dichotic pair and two other words differing from the dichotic stimuli only in the initial consonant. Significant main effect of social phobia (F=7.33, df=1, 161, p<0.01).
cScores for the consonant-vowel syllable test, in which a different consonant-vowel syllable (ba, da, ga, ka, pa, or ta) is presented simultaneously to each ear and subjects are asked to report the two syllables they heard from among the six possible syllables. Significant main effect of social phobia (F=6.82, df=1, 161, p=0.01).
dScores for the complex tone test, on which subjects are asked to compare the pitch of a binaural complex tone to the pitches of a dichotic pair of complex tones presented 1 second earlier. No significant main effect of social phobia.
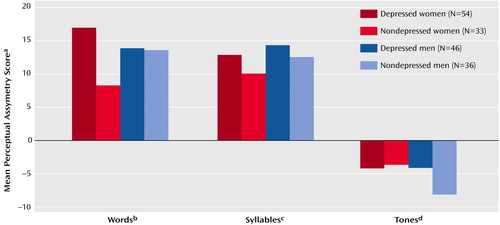
Figure 2. Mean Perceptual Asymmetry Scores on the Fused-Words, Consonant-Vowel Syllable, and Complex Tone Dichotic Listening Tests for Women and Men With or Without a Depressive Disorder
aPerceptual asymmetry score = 100 (right – left)/(right + left), based on the number of correct responses for right- and left-ear presentations. Scores greater than zero indicate a right-ear (left brain) advantage; scores less than zero indicate a left-ear (right brain) advantage.
bScores for the Fused Rhymed Words Test, in which 15 different single-syllable word pairs differing only in the initial consonant (e.g., coat, goat) are presented dichotically and subjects are asked to select which word they heard from four possible responses, including both members of the dichotic pair and two other words differing from the dichotic stimuli only in the initial consonant. Significant depression-by-gender interaction (F=4.64, df=1, 161, p<0.05); significant difference between depressed and nondepressed women (t=2.88, df=85, p=0.005); no significant difference between depressed and nondepressed men.
cScores for the consonant-vowel syllable test, in which a different consonant-vowel syllable (ba, da, ga, ka, pa, or ta) is presented simultaneously to each ear and subjects are asked to report the two syllables they heard from among the six possible syllables. Significant depression-by-gender interaction (F=3.98, df=1, 161, p<0.05).
dScores for the complex tone test, on which subjects are asked to compare the pitch of a binaural complex tone to the pitches of a dichotic pair of complex tones presented 1 second earlier. No significant depression-by-gender interaction.
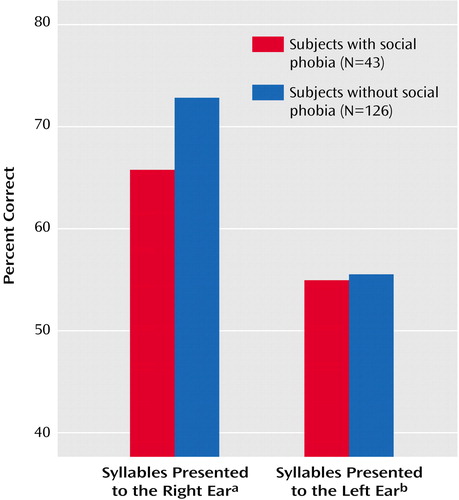
Figure 3. Mean Percentage of Correct Responses for Syllables Presented to the Right Ear and Left Ear in the Consonant-Vowel Syllable Test for Subjects With or Without Social Phobia
aSignificant main effect of social phobia (F=11.00, df=1, 161, p=0.001).
bNo significant main effect of social phobia.
1. Nordahl TE, Semple WE, Gross M, Mellman TA, Stein MB, Goyer P, King AC, Uhde TW, Cohen RM: Cerebral glucose metabolic differences in patients with panic disorder. Neuropsychopharmacology 1990; 3:261–272Medline, Google Scholar
2. Meyer JH, Swinson R, Kennedy SH, Houle S, Brown GM: Increased left posterior parietal-temporal cortex activation after d–fenfluramine in women with panic disorder. Psychiatry Res Neuroimaging 2000; 98:133–143Crossref, Medline, Google Scholar
3. Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G: Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Arch Gen Psychiatry 1999; 56:78–84Crossref, Medline, Google Scholar
4. Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB: While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biol Psychiatry 2000; 47:85–95Crossref, Medline, Google Scholar
5. Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Långström B, Fredrikson M: Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry 2001; 158:1220–1226Link, Google Scholar
6. Stein MB, Goldin PR, Sareen J, Eyler Zorrilla LT, Brown GG: Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 2002; 59:1027–1034Crossref, Medline, Google Scholar
7. Schneier FR, Johnson J, Hornig CD, Liebowitz MR, Weissman MM: Social phobia: comorbidity and morbidity in an epidemiologic sample. Arch Gen Psychiatry 1992; 49:282–288Crossref, Medline, Google Scholar
8. Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, McGrath PJ, Quitkin FM: Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biol Psychiatry 1997; 41:939–948Crossref, Medline, Google Scholar
9. Liotti M, Sava D, Rizzolatti G, Caffarra P: Differential hemispheric asymmetries in depression and anxiety: a reaction-time study. Biol Psychiatry 1991; 29:887–899Crossref, Medline, Google Scholar
10. Wexler BE, Goodman WK: Cerebral laterality, perception of emotion, and treatment response in obsessive-compulsive disorder. Biol Psychiatry 1991; 29:900–908Crossref, Medline, Google Scholar
11. Heller W, Etienne MA, Miller GA: Patterns of perceptual asymmetry in depression and anxiety: implications for neuropsychological models of emotion and psychopathology. J Abnorm Psychol 1995; 104:327–333Crossref, Medline, Google Scholar
12. Keller J, Nitschke JB, Bhargava T, Deldin PJ, Gergen JA, Miller GA, Heller W: Neuropsychological differentiation of depression and anxiety. J Abnorm Psychol 2000; 9:3–10Crossref, Google Scholar
13. Bruder GE, Wexler BE, Stewart JW, Price LH, Quitkin FM: Perceptual asymmetry differences between major depression with or without a comorbid anxiety disorder: a dichotic listening study. J Abnorm Psychol 1999; 108:233–239Crossref, Medline, Google Scholar
14. Wexler BE, Halwes T: Increasing the power of dichotic methods: the Fused Rhymed Words Test. Neuropsychologia 1983; 21:59–66Crossref, Medline, Google Scholar
15. Sidtis JJ: The Complex Tone Test: implications for the assessment of auditory laterality effects. Neuropsychologia 1981; 19:103–112Crossref, Medline, Google Scholar
16. Bruder GE, Stewart JW, McGrath PJ, Ma GJ, Wexler BE, Quitkin FM: Atypical depression: enhanced right hemispheric dominance for perceiving emotional chimeric faces. J Abnorm Psychol 2002; 111:446–454Crossref, Medline, Google Scholar
17. Bruder GE, Otto MW, McGrath PJ, Stewart JW, Fava M, Rosenbaum JF, Quitkin FM: Dichotic listening before and after fluoxetine treatment for major depression. Neuropsychopharmacology 1996; 15:171–179Crossref, Medline, Google Scholar
18. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
19. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
20. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA: Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
21. Clark LA, Watson D: Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol 1991; 100:316–336Crossref, Medline, Google Scholar
22. Bruder GE, Quitkin FM, Stewart JW, Martin C, Voglmaier M, Harrison WM: Cerebral laterality and depression: differences in perceptual asymmetry among diagnostic subtypes. J Abnorm Psychol 1989; 98:177–186Crossref, Medline, Google Scholar
23. Hugdahl K: Dichotic listening: probing temporal lobe functional integrity, in Brain Asymmetry. Edited by Davidson RJ, Hugdahl K. Cambridge, Mass, MIT Press, 1995, pp 123–156Google Scholar
24. Bruder GE, Kayser J, Tenke C, Amador X, Friedman M, Sharif Z, Gorman J: Left temporal lobe dysfunction in schizophrenia: event-related potential and behavioral evidence from phonetic and tonal dichotic listening tasks. Arch Gen Psychiatry 1999; 56:267–276Crossref, Medline, Google Scholar
25. Wexler BE, Giller EL Jr, Southwick S: Cerebral laterality, symptoms, and diagnosis in psychotic patients. Biol Psychiatry 1991; 29:103–116Crossref, Medline, Google Scholar
26. Asbjörnsen A, Hugdahl K, Bryden MP: Manipulations of subjects’ level of arousal in dichotic listening. Brain Cogn 1992; 19:183–194Crossref, Medline, Google Scholar
27. Roth DA, Heimberg RG: Cognitive-behavioral models of social anxiety disorder. Psychiatr Clin North Am 2001; 24:753–771Crossref, Medline, Google Scholar
28. Pine DS, Kentgen LM, Bruder GE, Leite P, Bearman K, Ma Y, Klein RG: Cerebral laterality in adolescent major depression. Psychiatry Res 2000; 93:135–144Crossref, Medline, Google Scholar
29. Bruder GE, Kayser J, Tenke CE, Leite P, Schneier FR, Stewart JW, McGrath PJ, Quitkin FM: Cognitive ERPs in depressive and anxiety disorders during tonal and phonetic oddball tasks. Clin Electroencephalogr 2002; 33:119–124Crossref, Medline, Google Scholar



