Cigarette Smoking, Suicidal Behavior, and Serotonin Function in Major Psychiatric Disorders
Abstract
OBJECTIVE: Cigarette smoking is associated with a higher risk for suicide and attempted suicide, but psychopathological or biological explanations for this association have not been explored. Lower serotonin function and impulsive/aggressive traits are associated with suicidal acts, including completed suicide. The authors hypothesized that the relationship that may exist between cigarette smoking and suicidal behavior may be associated with lower serotonin function and the presence of impulsive/aggressive traits. METHOD: Study subjects were 347 patients with a psychiatric disorder (175 with depression, 127 with schizophrenia, and 45 with other disorders). Fifty-three percent of the subjects (N=184) had a lifetime history of suicide attempt, and 47% (N=163) had never attempted suicide. Smoking behavior, lifetime suicidal behavior, and psychopathology were assessed. Serotonin function was assessed in a subgroup of patients with depression (N=162) by using a fenfluramine challenge test and/or measurement of CSF levels of 5-hydroxyindoleacetic acid. RESULTS: Among all patients, smokers were more likely to have made a suicide attempt (adjusted odds ratio=2.60, 95% confidence interval=1.60–4.23) and had higher suicidal ideation and lifetime aggression scores, compared with nonsmokers. An inverse relationship was observed between amount of cigarette smoking and both indices of serotonin function. CONCLUSIONS: The association between cigarette smoking and the presence and severity of suicidal behavior across major psychiatric disorders may be related to lower brain serotonin function in smokers with depression. Further investigation is required to replicate these findings, to measure serotonin function in patients with disorders other than depression, and to test potential therapeutic effects of serotonin-enhancing treatments on both smoking behavior and suicide risk.
Epidemiologic studies have reported an association between cigarette smoking and suicide of a magnitude similar to that of the association between smoking and coronary heart disease (1–7). Prospective studies have reported a similar association, including a dose-dependent relationship between smoking and increased risk for suicide (8–11). Relative rates of suicide in smokers are elevated when adjusted for potential confounding factors such as income, race, previous myocardial infarction, diabetes, and alcohol intake. An association between smoking and suicidal ideation has also been reported for psychiatric patients, among whom smokers had a 43% greater risk of experiencing mild to severe suicidal ideation, compared with nonsmokers (8). To our knowledge, this association has no satisfactory explanation, other than the observation that patients with psychiatric disorders are more likely to smoke (12). The lack of an explanation led one group of researchers to dismiss the association as an example of a chance finding generated in observational epidemiology (13). However, important biologic and psychopathologic explanatory factors may have been overlooked.
Cigarette smoking is associated with major depression, schizophrenia, and alcoholism (14–19) (for review see reference 20). Successful treatment of depression with fluoxetine, a selective serotonin reuptake inhibitor, improves depressive symptoms (14) and may improve the likelihood of smoking cessation (21), perhaps by reducing the risk of developing a new episode of depression during abstinence (22). These findings suggest a link between smoking, major depression, and serotonin function.
Acute administration of nicotine may result in release of serotonin as well as dopamine (23), whereas chronic nicotine administration to rats has been shown to decrease the concentration and biosynthesis of serotonin (24, 25). Consistent with these results, one postmortem human study comparing smokers and nonsmokers found that smokers had significantly lower concentrations of serotonin and the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the hippocampal formation, a lower concentration of 5-HIAA in the median raphe, and higher serotonin 1A (5-HT1A) receptor density (26). Thus, smoking may eventually impair serotonin function.
Altered indices of brain serotonin function, including lower serotonin transporter binding in the ventral prefrontal cortex and lower serotonin and/or 5-HIAA levels in the brainstem, have been found in postmortem studies of suicide completers, compared with subjects who died of other causes (27–29). Serotonin hypofunction, as indicated by a lower level of 5-HIAA in the CSF or a blunted prolactin response to serotonergic challenge with fenfluramine, has been reported in patients with major depression or schizophrenia and a history of serious suicide attempt, compared with psychiatric patients without a history of serious suicide attempt (30–32). Lower levels of CSF 5-HIAA predicted future suicide or suicide attempts in patients with mood disorders or schizophrenia (33, 34) and may reflect a vulnerability or predisposition for suicidal behavior independent of a specific psychiatric disorder.
We hypothesized that among patients with major psychiatric disorder: 1) cigarette smokers would be more likely to have a history of attempted suicide, independent of psychiatric diagnosis; 2) smoking would be associated with aggressive/impulsive traits; 3) the amount of daily cigarette consumption would correlate with severity of suicidal behavior (number and severity [lethality] of suicide attempts); and 4) cigarette smoking would be associated with lower serotonin function in a subgroup of depressed subjects for whom data on serotonin function were available.
Method
Subjects
The study subjects were recruited as inpatients as part of a longitudinal study on the neurobiology of suicidal behavior conducted by the Conte Center for the Neuroscience of Mental Disorders at New York State Psychiatric Institute in New York City and previously at the Center for the Study of Suicidal Behavior at the University of Pittsburgh Medical Center in Pittsburgh. All subjects were age 18–80 years; had an IQ >80; had a current DSM-III-R axis I psychiatric disorder of major depressive episode, bipolar disorder (depressed), dysthymia, schizophrenia or other psychotic disorder, or adjustment disorder, with or without a comorbid axis II (personality disorder) diagnosis; were without current substance abuse or alcohol dependence; and were free of all medication and psychoactive substances for at least 2 weeks before entry into the study. All patients had a negative urine toxicology screen before biological testing. The required drug-free period was at least 3 weeks for tricyclic antidepressants, 4 weeks for oral antipsychotics, and 6 weeks for fluoxetine. Women who were pregnant were excluded, and phase of menstrual cycle was documented. All patients gave written informed consent for the protocol as approved by the Institutional Review Board.
Clinical Assessment
Subjects’ DSM-III-R axis I diagnoses were based on the Structured Clinical Interview for DSM-III-R and were reviewed at a consensus conference involving two research psychiatrists (K.M.M., J.J.M.). Clinical symptoms at index hospitalization/evaluation were rated by using the Brief Psychiatric Rating Scale (BPRS) (35), the Hamilton Depression Rating Scale (36), the Beck Depression Inventory (37), and the Beck Hopelessness Scale (38). Lifetime history of aggression was assessed with the Brown-Goodwin life history of aggression interview (39, 40).
Assessment of Suicide Attempts
A lifetime history of all suicide attempts, including number of attempts and the method and degree of medical damage for each attempt, was obtained. A lethality scale was used to measure the degree of medical damage caused by each suicide attempt (41). The scale was scored from 0 to 8 (0=no medical damage, 8=death), with different anchor points for various suicide attempt methods. A suicide attempt was defined as a self-destructive act that was committed with some intent to end one’s life and that caused sufficient injury to require medical evaluation. The degree of suicide intent was rated with the Suicide Intent Scale (42). The Scale for Suicide Ideation (43) was used to measure the severity of suicidal ideation during the week before the index hospitalization.
Assessment of Cigarette Smoking
Patients were asked if they ever smoked cigarettes and, if they were presently smoking cigarettes, how many cigarettes they smoked per day. Smoking status was scored as follows: nonsmoker (0 cigarettes/day), light smoker (1–20 cigarettes/day), moderate smoker (21–39 cigarettes/day), and heavy smoker (≥40 cigarettes/day). Patients did not smoke cigarettes for at least 12 hours before biological testing (as biological testing was routinely performed at 8:00 a.m.). Lifetime duration of cigarette smoking was not documented, and former smokers were not identified as such.
Indices of Serotonin Function
Studies of serotonin function were conducted in a subgroup of depressed patients (N=162). Patients with schizophrenia were excluded from these analyses.
Fenfluramine challenge test
The method has been described in detail previously (44). Patients fasted from midnight on the day of the test. An intravenous cannula was inserted into a forearm vein, and an infusion of 5% dextrose and N/5 saline was begun. The dextrose was administered to reduce the effects of fasting-induced hypoglycemia, including its potential effect on prolactin response. The patients were given about 1 hour to become accustomed to the intravenous infusion and the testing environment. During the procedure, they remained awake while seated or in a supine position. At about 8:45 a.m. and 9:00 a.m., blood was drawn for assay of plasma prolactin. At 9:00 a.m., 60 mg of d, l-fenfluramine was given orally, and blood was drawn hourly thereafter for 5 hours for measurement of plasma prolactin, as well as plasma levels of fenfluramine and norfenfluramine. Plasma prolactin and cortisol levels were determined by radioimmunometric assay, as previously described (44). The sensitivity of the procedure was improved to 1 ng/ml by using a sequential 37°C incubation of reagents. Patients whose duplicate measurements had a coefficient of variation exceeding 5% were retested. The range for intraassay variation of individual patients’ samples over the study was 1.08% to 2.86% (mean=1.96%). Plasma levels of fenfluramine and norfenfluramine were measured by a gas-liquid chromatographic method (44). The minimum detectable concentrations of fenfluramine and norfenfluramine were 2.0 ng/ml and 5.0 ng/ml, respectively. The outcome measure was the net maximal change in prolactin level, defined as the difference between the baseline prolactin level (the mean of the two prefenfluramine levels) and the highest postfenfluramine level.
Lumbar puncture and CSF 5-HIAA measurement
The lumbar puncture was performed at about 8:00 a.m., after the patient had been restricted to bed rest overnight and had fasted since midnight. CSF was drawn from the interspace between the third and fourth lumbar vertebrae while the patient was in the left decubitus position. After the removal of 1 ml of CSF, a further 15 ml of CSF was removed and immediately transferred on ice water to be centrifuged at 4°C and aliquoted into 1-ml samples for storage at –70°C until assay. The CSF 5-HIAA was assayed by high-performance liquid chromatography with electrochemical detection modified from the method described by Scheinin et al. (45). The within-run and between-run coefficients of variation of the assay method were less than 10%. The level of sensitivity of the assay for 5-HIAA was 0.5 pmol per injection. Assays were done by laboratory staff who were blind to the clinical data.
Statistical Analysis
We examined the association between lifetime history of suicide attempt and cigarette smoking (yes/no) using crude and adjusted odds ratios computed with logistic regression (95% confidence intervals). Multivariate initial statistical analyses tested a priori hypotheses. For example, analysis of variance (ANOVA) and logistic regression models controlled for the effects of severity of depression and comorbid alcohol abuse on the association between suicide attempt and smoking. Spearman rank correlations were used to examine the relationships between 1) degree of smoking and degree of medical damage resulting from the most medically damaging suicide attempt and 2) degree of smoking and both the level of 5-HIAA in CSF and the prolactin response to fenfluramine. With CSF 5-HIAA or net maximal change in prolactin levels as the dependent variable, the ANOVA and regression analyses controlled for the effects of potentially confounding demographic and clinical variables on smoking behavior. The potentially confounding variables included age, sex, race, DSM-III-R axis I diagnosis, severity of depression, and past alcohol and substance abuse.
Results
Patient Characteristics
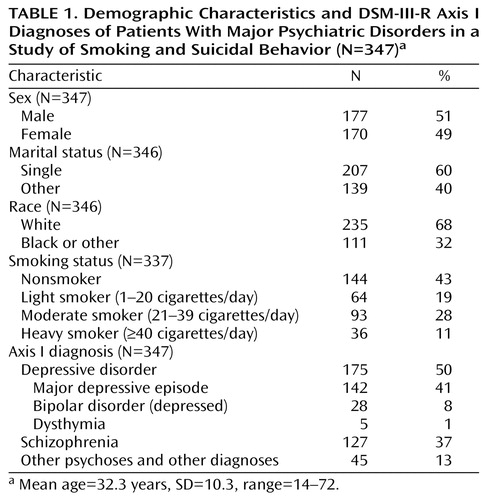
The study group consisted of 347 patients with an axis I psychiatric disorder (177 male subjects and 170 female subjects), including 184 patients (53%) who had made at least one previous suicide attempt. Eighty-nine patients (26%) had a lumbar puncture and CSF 5-HIAA assay, and 143 patients (41%) underwent a fenfluramine challenge test. Fifty-six patients underwent both biological procedures. All 162 patients who underwent serotonin function testing had a depressive disorder (major depressive episode, bipolar disorder [depressed], or dysthymia). Ninety-nine of the 162 patients (61%) had a lifetime history of suicide attempt. The demographic and clinical characteristics of the overall study group are summarized in Table 1.
Smoking Patterns in Suicide Attempters Versus Nonattempters
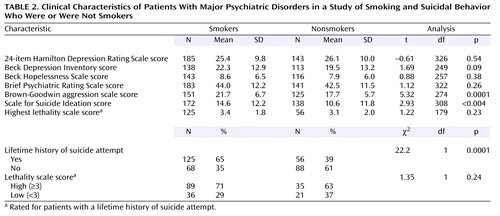
Cigarette smoking was similarly prevalent in the groups with schizophrenia (N=70 of 122 patients, 57%) and depression (N=90 of 170 patients, 53%). Proportionally more patients with other diagnoses smoked (N=33 of 45 patients, 73%). (This category included patients with a past history of alcohol or substance abuse.) Smoking was more common among suicide attempters (N=125 of 181, 69%) than among patients who had not made a suicide attempt (N=68 of 156, 44%) (χ2=22.2, df=1, p=0.001) (Data on smoking were available for 337 patients.)
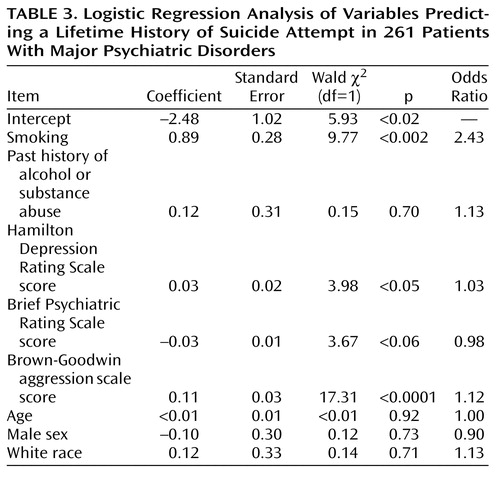
The association between smoking and suicide attempt status (Table 2) was significant after the analysis controlled for the effects of 1) demographic characteristics such as age, sex, and race; 2) DSM-III-R axis I psychopathology; and 3) clinical features such as severity of depression (Hamilton depression scale score), severity of psychiatric symptoms (BPRS score), past history of alcohol or substance abuse, and aggression scores (Table 3).
In the overall study group, the likelihood of being a suicide attempter was correlated with the amount of smoking (number of cigarettes smoked per day) (rs=0.24, N=337, p=0.0001). A linear relationship was not found between the lethality of the most medically damaging lifetime suicide attempt and amount of smoking in the overall study group, but such a relationship was found for male patients (r=0.22, N=91, p=0.03).
Cigarette Smoking and Indices of Serotonin Function
Biological assessments (measurement of level of 5-HIAA in CSF and the fenfluramine challenge) were completed for a subgroup of depressed patients (N=162). This subgroup did not differ from the patients who did not have biological assays on demographic variables such as race, age, or marital status. However, Hamilton depression scores were predictably higher in the group who underwent the biological assessments (mean=27.4, SD=9.56, versus mean=23.8, SD=10.6) (t=3.29, df=333, p<0.001), as was the level of suicidal ideation (Scale for Suicide Ideation score: mean=16.9, SD=11.2, versus mean=8.3, SD=11.6) (t=6.73, df=313, p<0.001).
CSF 5-HIAA and cigarette smoking in depressed subjects
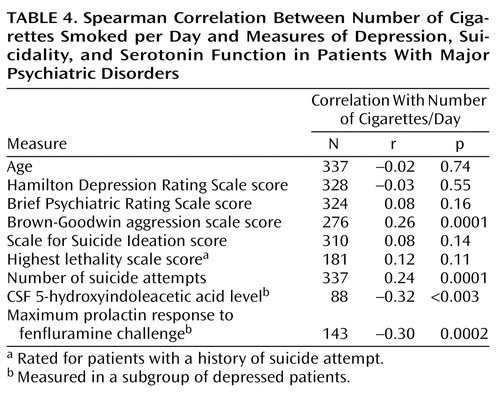
CSF 5-HIAA level was negatively correlated with the amount of cigarette smoking (rs=–0.32, N=88, p<0.003) (Figure 1 and Table 4). Moreover, a regression analysis that controlled for key clinical variables confirmed a significant adjusted effect of amount of smoking on CSF 5-HIAA level (t=–2.89, residual df=78, p<0.005, N=86). This analysis showed that age also contributed weakly as a predictor of CSF 5-HIAA level (t=–1.68, residual df=78, p<0.10). The CSF 5-HIAA level did not predict suicide attempter status (CSF 5-HIAA level: mean=93.7, SD=34.4 for attempters; mean=94.4, SD=42.1 for nonattempters) (t=0.09, df=87, p<0.93). Aggression scores contributed significantly to the model (Table 4).
Fenfluramine challenge test and cigarette smoking in depressed subjects
The maximum prolactin response to fenfluramine above baseline prolactin was used as the measure of outcome of the fenfluramine challenge test in the depressed subgroup. As with CSF 5-HIAA level, the maximum prolactin response to fenfluramine correlated inversely with the amount of cigarette smoking (rs=–0.30, N=143, p=0.0002) (Table 4, Figure 2). The association between the maximum prolactin response to fenfluramine and the amount of smoking remained statistically significant in a linear regression analysis that controlled for the effects of age, sex, severity of depression, aggression, past history of alcohol or substance abuse, and current BPRS scores (F=4.78, df=7, 128, p<0.001, adjusted t=–2.95, residual df=128, adjusted p=0.003). Smoking status (yes/no) was similarly associated with the maximum prolactin response to fenfluramine above baseline after the effects of these demographic and clinical variables were controlled. Supplementary analyses that used data for only those subjects with major depressive episode (excluding those with depressed symptoms but not major depressive episode) did not significantly alter the inverse correlations between number of cigarettes smoked per day and either CSF 5-HIAA level (rs=–0.31, N=55, p<0.02) or maximum prolactin response to fenfluramine above baseline prolactin (rs=–0.25, N=120, p<0.006).
Cigarette smoking and cortisol
We examined for possible stress effects and found no difference in baseline cortisol level (ng/ml) between nonsmokers and smokers in the group that underwent fenfluramine challenge (nonsmokers: N=82, mean=12.44, SD=4.9; smokers: N=64, mean=12.93, SD=5.4) (t=–0.57, df=144, p=0.57).
Discussion
This study extends previous findings (1–3, 8) by demonstrating that the seriousness of suicidal behavior is correlated with the amount of smoking, after the effects of axis I psychiatric diagnosis are controlled (14–17, 19, 20). Our results also extend previous epidemiologic findings (14–17, 19, 20) by demonstrating that the association between cigarette smoking and the presence and severity of suicidal behavior is not confined to a specific psychiatric disorder.
To our knowledge, this study is the first to report a correlation between cigarette smoking and impaired serotonin function (as measured by CSF 5-HIAA level and prolactin response to fenfluramine) in depressed patients in vivo. Serotonergic hypofunction appears to be related to more lethal suicidal behavior in several major psychiatric disorders (30–32, 46). Moreover, severity of suicidal behavior and lifetime aggression are related to level of serotonergic function (39, 40). Our results suggest that lower serotonin function and smoking are also associated in depressed patients. Thus, lower serotonergic function may be related to cigarette smoking, suicidal behavior, and aggressive behaviors.
An association between impaired serotonergic function and smoking or between suicidal behavior and smoking does not imply causality. Nicotine may have both biological and behavioral effects in the brain that increase the probability of suicidal behavior in depressed patients. Alternatively, low serotonin function may predispose the patient with psychiatric disorder to both smoking and to suicidal acts. Finally, both mechanisms could combine to increase the risk for suicidal behavior. Low serotonin function could increase the probability of developing a smoking habit, and, after the onset of a depressive disorder, further depletion of serotonin by smoking may heighten the risk for suicidal acts.
Acute nicotine administration has been shown to promote serotonin release (47), whereas chronic nicotine administration results in serotonin depletion in brain areas such as the hippocampal formation and reduces firing of serotonergic neurons arising in the midbrain raphe (48). These effects may trigger depression and enhance the predisposition to suicidal behavior. A lower serotonin level brought about by acute tryptophan depletion in patients with a past history of depression can trigger depression and, depending on preexisting traits and temperament, can increase aggressive and impulsive behaviors (49–51).
Lower serotonin function has previously been reported in primates and humans with higher levels of impulsive, novelty-seeking behavior (52–54). It is possible that lower serotonin turnover in the brain may render the subject more susceptible to the serotonin-lowering effects of nicotine and thus may enhance suicidal behavior during depression by reducing the restraining influences exerted over unwanted suicidal thoughts mediated through central serotonin pathways (55). Smokers in our study had higher aggression scores than nonsmokers, consistent with this biological hypothesis. There may be a common factor predisposing individuals to smoking and suicidal behavior that is manifested in a variety of psychiatric disorders. In this study, both lifetime aggression scores and current suicidal ideation scores were significantly higher in smokers, suggesting a common diathesis. Higher levels of lifetime aggression reported to be associated with both substance abuse and suicidal behavior may also reflect this common diathesis (56). A predisposition to both major depression and cigarette smoking has been proposed on the basis of findings from twin studies suggesting that the relationship between lifetime smoking and depression resulted “solely from genes that predispose to both conditions” (57). Rates of suicidal behavior were not reported in that study. Serotonergic function, aggression, and suicidal behavior also have genetic causal factors. Thus, further research is required to identify the disease-susceptibility genes associated with smoking, aggression, and suicidal behavior and to determine whether low serotonin function is a common predisposing factor with a genetic basis.
Our study had some limitations. We did not document the lifetime duration or age at onset of smoking or the history of ex-smokers. We did not study serotonin function and smoking in the diagnostic groups without depression. We did not find a relationship between either measure of serotonin function and history of suicide attempt in the subgroup we studied. That result was consistent with our previous reports, in which low serotonin function was detected only in patients who had made suicide attempts with a high level of lethality and was negatively correlated with lifetime severity of aggressive behaviors (30–32).
In conclusion, our study suggests a biological explanation for a relationship between suicidal behavior and smoking. Enhancement of serotonergic function may reduce suicide risk and assist smoking cessation.
 |
 |
 |
 |
Received Aug. 13, 2001; revision received Nov. 15, 2002; accepted Nov. 21, 2002. From the Division of Neuroscience, Department of Psychiatry, College of Physicians and Surgeons of Columbia University, New York; Department of Adult Psychiatry, University College Dublin, Dublin, Ireland; and Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, Pittsburgh. Address reprint requests to Dr. Malone, Department of Psychiatry and Mental Health Research, Conway Institute for Biomolecular & Biomedical Research, University College Dublin, and Dublin Molecular Medicine Centre (DMMC), St. Vincent’s University Hospital, Elm Park, Dublin 4, Ireland; [email protected] (e-mail). Supported by NIMH grants MH-46745, MH-48514, and MH-62185; NIH grant RR-00645; and a Young Investigator Award to Dr. Malone from the National Alliance for Research on Schizophrenia and Depression. The authors thank Donna Abbondanza, R.N., for assistance with the biological procedures, Thomas Kelly, M.S.W., and Diane Dolata, R.N., for assistance in the clinical assessments, and Steven Ellis, Ph.D., for statistical assistance.
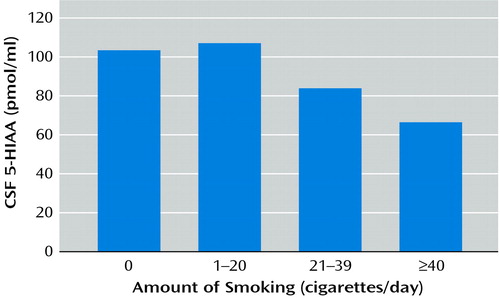
Figure 1. Relation of CSF 5-Hydroxyindoleacetic Acid (5-HIAA) Level and Number of Cigarettes Smoked per Day in 88 Depressed Subjectsa
ars=–0.32, p<0.003.
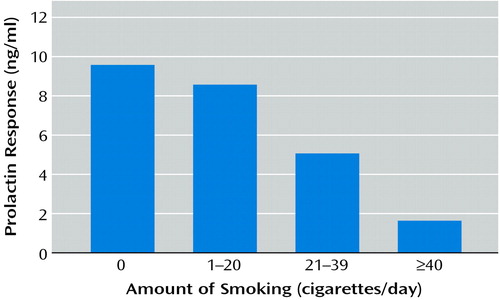
Figure 2. Relation of Prolactin Response to Fenfluramine and Number of Cigarettes Smoked per Day in 143 Depressed Subjectsa
ars=–0.30, p=0.0002.
1. Doll R, Peto R: Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J 1976; 2:1525-1536Crossref, Medline, Google Scholar
2. Neaton JD, Kuller LH, Wentworth D, Borhani NO: Total and cardiovascular mortality in relation to cigarette smoking, serum cholesterol concentration, and diastolic blood pressure among black and white males followed up for five years. Am Heart J 1984; 108:759-769Crossref, Medline, Google Scholar
3. Ross RK, Bernstein L, Trent L, Henderson BE, Paganini-Hill A: A prospective study of risk factors for traumatic deaths in a retirement community. Prev Med 1990; 19:323-334Crossref, Medline, Google Scholar
4. Tverdal A, Thelle D, Stensvold I, Leren P, Bjartveit K: Mortality in relation to smoking history: 13 years’ follow-up of 68,000 Norwegian men and women 35-49 years. J Clin Epidemiol 1993; 46:475-487Crossref, Medline, Google Scholar
5. Paffenbarger RS Jr, Lee I-M, Leung R: Physical activity and personal characteristics associated with depression and suicide in American college men. Acta Psychiatr Scand Suppl 1994; 377:16-22Crossref, Medline, Google Scholar
6. Hemenway D, Solnick SJ, Colditz GA: Smoking and suicide among nurses. Am J Public Health 1993; 83:249-251Crossref, Medline, Google Scholar
7. Angst J, Clayton PJ: Personality, smoking and suicide: a prospective study. J Affect Disord 1998; 51:55-62Crossref, Medline, Google Scholar
8. Tanskanen A, Viinamäki H, Hintikka J, Koivumaa-Honkanen H-T, Lehtonen J: Smoking and suicidality among psychiatric patients. Am J Psychiatry 1998; 155:129-130Link, Google Scholar
9. Leistikow BN, Martin DC, Samuels SJ: Injury death excesses in smokers: a 1990-95 United States national cohort study. Inj Prev 2000; 6:277-280Crossref, Medline, Google Scholar
10. Miller M, Hemenway D, Rimm E: Cigarettes and suicide: a prospective study of 50,000 men. Am J Public Health 2000; 90:768-773Crossref, Medline, Google Scholar
11. Miller M, Hemenway D, Bell NS, Yore MM, Amoroso PJ: Cigarette smoking and suicide: a prospective study of 300,000 male active-duty army soldiers. Am J Epidemiol 2000; 151:1060-1063Crossref, Medline, Google Scholar
12. Clayton P: Smoking and suicide (editorial). J Affect Disord 1998; 50:1-2Crossref, Medline, Google Scholar
13. Smith GD, Phillips AN, Neaton JD: Smoking as “independent” risk factor for suicide: illustration of an artifact from observational epidemiology? Lancet 1992; 340:709-712Crossref, Medline, Google Scholar
14. Dalack GW, Glassman AH, Rivelli S, Covey L, Stetner F: Mood, major depression, and fluoxetine response in cigarette smokers. Am J Psychiatry 1995; 152:398-403Link, Google Scholar
15. Breslau N, Kilbey MM, Andreski P: Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry 1991; 48:1069-1074Crossref, Medline, Google Scholar
16. Breslau N, Kilbey MM, Andreski P: Nicotine dependence and major depression: new evidence from a prospective investigation. Arch Gen Psychiatry 1993; 50:31-35Crossref, Medline, Google Scholar
17. Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J: Smoking, smoking cessation, and major depression. JAMA 1990; 254:1546-1549Crossref, Google Scholar
18. Goff DC, Henderson DC, Amico E: Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry 1992; 149:1189-1194Link, Google Scholar
19. Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA: Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 1986; 143:993-997Link, Google Scholar
20. Glassman AH: Cigarette smoking: implications for psychiatric illness. Am J Psychiatry 1993; 150:546-553Link, Google Scholar
21. Glassman AH, Covey LS, Stetner F: Smoking cessation, depression, and antidepressants, in 1989 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1989, number 122Google Scholar
22. Glassman AH, Covey LS, Stetner F, Rivelli S: Smoking cessation and the course of major depression: a follow-up study. Lancet 2001; 357:1929-1932Crossref, Medline, Google Scholar
23. Rosecrans JA: The psychopharmacological basis of nicotine’s differential effects on behavior: individual subject variability in the rat. Behav Genet 1995; 25:187-196Crossref, Medline, Google Scholar
24. Benwell ME, Balfour DJ: Effects of nicotine administration and its withdrawal on plasma corticosterone and brain 5-hydroxyindoles. Psychopharmacology (Berl) 1979; 63:7-11Crossref, Medline, Google Scholar
25. Benwell ME, Balfour DJ: Effects of chronic nicotine administration on the response and adaptation to stress. Psychopharmacology (Berl) 1982; 76:160-162Crossref, Medline, Google Scholar
26. Benwell ME, Balfour DJ, Anderson JM: Smoking-associated changes in the serotonergic systems of discrete regions of human brain. Psychopharmacology (Berl) 1990; 102:68-72Crossref, Medline, Google Scholar
27. Stanley M, Mann JJ: Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet 1983; 1:214-216Crossref, Medline, Google Scholar
28. Arango V, Ernsberger P, Marzuk PM, Chen J-S, Tierney H, Stanley M, Reis DJ, Mann JJ: Autoradiographic demonstration of increased serotonin 5-HT2 and β-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry 1990; 47:1038-1047Crossref, Medline, Google Scholar
29. Arango V, Underwood MD, Gubbi AV, Mann JJ: Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res 1995; 688:121-133Crossref, Medline, Google Scholar
30. Malone KM: Psychobiology of suicidal behavior in major depression (thesis). Galway, National University of Ireland, Department of Psychiatry, 1994, pp 1-152Google Scholar
31. Malone KM, Corbitt EM, Li S, Mann JJ: Prolactin response to fenfluramine and suicide attempt lethality in major depression. Br J Psychiatry 1996; 168:324-329Crossref, Medline, Google Scholar
32. Mann JJ, Malone KM: Cerebrospinal fluid amines and higher lethality suicide attempts in depressed inpatients. Biol Psychiatry 1997; 41:162-171Crossref, Medline, Google Scholar
33. Nordström P, Samuelsson M, Åsberg M, Träskman-Bendz L, Aberg-Wistedt A, Nordin C, Bertilsson L: CSF 5-HIAA predicts suicide risk after attempted suicide. Suicide Life Threat Behav 1994; 24:1-9Medline, Google Scholar
34. Cooper SJ, Kelly CB, King DJ: 5-Hydroxyindoleacetic acid in cerebrospinal fluid and prediction of suicidal behaviour in schizophrenia. Lancet 1992; 340:940-941Crossref, Medline, Google Scholar
35. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799-812Crossref, Google Scholar
36. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56-62Crossref, Medline, Google Scholar
37. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561-571Crossref, Medline, Google Scholar
38. Beck AT, Weissman A, Lester D, Trexler L: The measurement of pessimism: the Hopelessness Scale. J Consult Clin Psychol 1974; 42:861-865Crossref, Medline, Google Scholar
39. Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF: Aggression in human correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1979; 1:131-139Crossref, Medline, Google Scholar
40. Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE Jr, Goodwin FK: Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am J Psychiatry 1982; 139:741-746Link, Google Scholar
41. Beck AT, Beck R, Kovacs M: Classification of suicidal behaviors, I: quantifying intent and medical lethality. Am J Psychiatry 1975; 132:285-287Link, Google Scholar
42. Beck AT, Herman I, Schuyler D: Development of suicidal intent scales, in The Prediction of Suicide. Edited by Beck AT, Risnik HLP, Lettieri D. Bowie, Md, Charles Press, 1974, pp 45-56Google Scholar
43. Beck AT, Kovacs M, Weissman A: Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol 1979; 47:343-352Crossref, Medline, Google Scholar
44. Malone KM, Thase ME, Mieczkowski T, Myers JE, Stull SD, Cooper TB, Mann JJ: Fenfluramine challenge test as a predictor of outcome in major depression. Psychopharmacol Bull 1993; 29:155-161Medline, Google Scholar
45. Scheinin M, Chang W-H, Kirk KL, Linnoila M: Simultaneous determination of 3-methoxy-4-hydroxphenylglycol, 5-hydroxyindoleacetic acid, and homovanillic acid in cerebrospinal fluid with high-performance liquid chromatography using electrochemical detection. Anal Biochem 1983; 131:246-253Crossref, Medline, Google Scholar
46. Mann JJ, McBride PA, Brown RP, Linnoila M, Leon AC, DeMeo MD, Mieczkowski TA, Myers JE, Stanley M: Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Arch Gen Psychiatry 1992; 49:442-446Crossref, Medline, Google Scholar
47. Schwartz RD, Lehmann J, Kellar KJ: Presynaptic nicotinic cholinergic receptors labeled by [3H]acetylcholine on catecholamine and serotonin axons in brain. J Neurochem 1984; 42:1495-1498Crossref, Medline, Google Scholar
48. Bakalian MJ, Underwood MD, Mann JJ, Paykina N, Kassir SA, Douglass M, Gapon S, Brent DA, Ellis SP, Li S, Arango V: Cortical a1-adrenergic binding in alcoholism and suicide. Abstracts of the Society for Neuroscience 1996; 22:729Google Scholar
49. Salomon RM, Mazure CM, Delgado PL, Mendia P, Charney DS: Serotonin function in aggression: the effect of acute plasma tryptophan depletion in aggressive patients. Biol Psychiatry 1994; 35:570-572Crossref, Medline, Google Scholar
50. Pihl RO, Young SN, Harden P, Plotnick S, Chamberlain B, Ervin FR: Acute effect of altered tryptophan levels and alcohol on aggression in normal human males. Psychopharmacology (Berl) 1995; 119:353-360Crossref, Medline, Google Scholar
51. Chamberlain B, Ervin FR, Pihl RO, Young SN: The effect of raising or lowering tryptophan levels on aggression in vervet monkeys. Pharmacol Biochem Behav 1987; 28:503-510Crossref, Medline, Google Scholar
52. Higley JD, Mehlman PT, Taub DM, Higley SB, Suomi SJ, Linnoila M, Vickers JH: Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Arch Gen Psychiatry 1992; 49:436-441Crossref, Medline, Google Scholar
53. Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M: Correlation of CSF 5-HIAA concentration with sociality and the timing of emigration in free-ranging primates. Am J Psychiatry 1995; 152:907-913Link, Google Scholar
54. Kruesi MJP, Rapoport JL, Hamburger S, Hibbs E, Potter WZ, Lenane M, Brown GL: Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behavior disorders of children and adolescents. Arch Gen Psychiatry 1990; 47:419-426Crossref, Medline, Google Scholar
55. Mann JJ, Arango V: Integration of neurobiology and psychopathology in a unified model of suicidal behavior. J Clin Psychopharmacol 1992; 12(2 suppl):2S-7SGoogle Scholar
56. Mann JJ, Waternaux C, Haas GL, Malone KM: Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry 1999; 156:181-189Abstract, Google Scholar
57. Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC: Smoking and major depression: a causal analysis. Arch Gen Psychiatry 1993; 50:36-43Crossref, Medline, Google Scholar



